Abstract
We investigated the pathophysiological mechanism by proteomic approach as a possible tool to detect the marker proteins to develop lower urinary tract symptoms following bladder outlet obstruction (BOO). Rats were randomized into 3 groups; control, sham operation and BOO groups. BOO group was divided into 1, 3, and 5 day-group. Conventional proteomics was performed with high resolution 2-D gel electrophoresis followed by computational image analysis and protein identification using mass spectrometry using rat urinary bladders. A comparison of bladder of BOO group with control bladder showed that three proteins of optineurin, thioredoxin and preprohaptoglobin were over-expressed in the bladder of BOO group. In addition, four proteins, such as peroxiredoxin 2, transgelin, hippocampal cholinergic neurostimulating peptide (HCNP) and beta-galactoside-binding lectin, were under-expressed in the bladder of BOO group. These data supported that down-regulation of HCNP might make detrusor muscle be supersensitive to acetylcholine, up-regulation of optineurin means the protection of nerve injury, and down-regulation of transgelin means the decreased contractility of detrusor muscle. Beside these proteins, other proteins are related to oxidative stress or have a nonspecific function in this study. However more information is needed in human bladder tissue for clinical usage.
Keywords: Bladder, Prostatic Hyperplasia, Proteomics, Urethral Obstruction
INTRODUCTION
Symptoms of benign prostatic hyperplasia (BPH) are called lower urinary tract symptoms (LUTS) and are categorized as voiding and storage symptoms. The voiding symptoms, which include hesitancy and a weak stream, have been attributed to a static component caused by the mass of the enlarged gland and a dynamic component caused by the tone of the smooth muscle of the prostatic stroma. Storage symptoms such as frequent and urgent urination have been associated with bladder dysfunction caused by bladder outlet obstruction (BOO) (1).
Evidence of a link between BPH and voiding symptoms is well established, but there is no direct evidence of a link between BPH and storage symptoms. Although obstructions can be relieved by prostatectomy or administration of alpha-adrenergic blockers, up to 38% of men with BPH continue to suffer from storage symptoms (2). Obstructed bladder dysfunction caused by BPH is characterized by alterations in bladder mass, tissue composition, capacity, compliance and the response to pharmacological agents. Storage symptoms are displayed in the compensatory stage subsequent to these changes (3). Many pathophysiological mechanisms have been proposed, including changes in detrusor morphology and innervation, intercellular communication and electrical properties, detrusor receptors, ischemic/reperfusion injury, increased synthesis and deposition of connective tissue, urothelial mechanoafferent signaling, and central nervous system regulation (4, 5). Recently, many scientists have become interested in the potential of molecular biology for identifying pathophysiological mechanisms. It has been suggested that hyperplasia and apoptosis of bladder muscle following BOO are opposing cellular processes, which mediate the bladder's response to short-term obstructive stimuli, and that local synthesis of growth-promoting and growth-inhibitory factors may be responsible for initiating both of these responses (6). Moreover, following obstruction of the bladders of animal models and patients with BPH, there were acute increases in the expression of bFGF (7), the transcription factors, upstream binding factor, c-Jun, and cyclins E and C (8), nerve growth factor (9), N-ras and c-myc (10), and decreased expression of TGF-beta 1 (7). This suggests that these changes may play a role in obstruction-induced bladder hypertrophy and dysfunction. Because these changes cannot explain all the pathophysiological mechanisms of storage symptoms exhibited following BOO, an alternative tool is needed.
Recent developments in proteomic technologies have provided tools for discovering and identifying disease-associated biomarkers. As data generated using proteomic technologies reflect the impact on the cell of intrinsic genetic effects and the environment, these methods are very useful for determining biomarkers. In humans, it is difficult to investigate the cellular mechanisms that mediate obstructive bladder dysfunction.
We investigated the proteomics of this disease using the rat because many of the structural and functional changes associated with human bladder pathology can be induced in animal models of partial outlet obstruction (11). Although application of these technologies to search for potential diagnostic/prognostic biomarkers associated with BOO has been limited, this database will provide a more valuable resource for identification of biomarkers than genomic methods.
MATERIALS AND METHODS
Procedures of partial bladder outlet obstruction
Female Sprague-Dawley rats were obtained at 8 weeks of age. The rats were housed in groups of three with hardwood bedding in windowless animal rooms. Animal rooms were illuminated according to a 12 hr light/dark cycle and were maintained at a temperature of 22±1℃ and a relative humidity of 50±20%. After a quarantine period of 1 week, animals were randomized into three groups: a control group (10 rats), a sham operation group (10 rats) and a BOO group (30 rats). The BOO group was randomly sub-divided into 1-, 3-, and 5-day sub-groups of 10 rats each. Polyethylene catheters (PE200, inner diameter 1.40 mm; Clay-Adams, Parsippany, NJ, U.S.A.) were used to create partial BOO. After establishing anesthesia with ketamine (100 mg/kg body weight intramuscularly), the bladder and proximal urethra were exposed through an incision in the lower abdomen. The proximal urethra was freed from the vaginal wall by careful dissection to avoid injury to the periurethral blood vessels. The wall of a 2 mm wide transverse slice of catheter material was cut to open the ring, which was placed around the proximal urethra. The implanted catheters fitted loosely around the urethra, resulting in a constant and mild degree of BOO. The abdominal wound was closed and the animals received 150 mg/kg body weight ampicillin intramuscularly. No rats died of urinary complications. Sham operated rats underwent similar procedures but the implanted catheter was removed before closing the abdominal wound. 1, 3 or 5 days following the operation, urinary bladders were excised under anesthesia induced by intraperitoneal injection. Following this, all rats were exsanguinated after cervical dislocation. The bladders were placed in liquid nitrogen after weighing.
Procedures for protein extraction
Proteins were extracted from rat urinary bladder tissue according to the German Heart Center method (http://userpage.chemie.fu-berlin.de/~pleiss/tissue.html; accesssed on December 2004). Bladder tissue was washed in buffer containing 50 mM Tris-HCl (pH 7.1), 100 mM KCl, 60 mM EDTA, 5.8 mM benzamidine and 0.2 mM PMSF and is lyophilized for 1 hr. Samples of 30 mg were crushed after immersion in liquid nitrogen and were solublized in 100 µL buffer containing 7 M urea, 2 M thiourea, 4% CHAPS, 100 mM DTT, 25 mM Tris-HCl (pH 7.1), 50 mM KCl, and 0.2% Bio-Lyte 3/10 Ampholyte. DNase was added (210 U) and the solution was then incubated at room temperature for 30 min. Insoluble material was removed by centrifugation at 14,737×g for 20 min at 10℃. Protein concentration was determined using a commercial Bradford reagent (Bio-Rad protein assay kit, Hercules, CA, U.S.A.), and the samples were stored at -70℃ until analysis.
Two-dimensional electrophoresis
The first-dimensional gel separation was carried out with 17cm pH 4-7 immobile pH gradient (IPG) strips following the manufacturer's protocol (Bio-Rad) with minor modifications. Samples containing up to 1 mg of protein were loaded and isoelectric focusing (IEF) was performed using a PROTEAN IEF cell for 145 kV hr at 20℃. After IEF, strips were equilibrated for 15 min in 6 M urea, 2% SDS, 0.05 M Tris-HCl (8.8) and 20% glycerol containing 2% DTT, and then equilibrated again for 15 min in the same buffer containing 2.5% iodoacetamide. Equilibrated IPG strips were transferred onto 15% uniform polyacrylamide gels and run in a PROTEAN II Xi cell tank at 30 mA per gel. The gels were visualized using Coomassie Brilliant Blue R250 staining. After staining, 2D gels were imaged using Powerlook 1100 (UMAX, Fremont, CA, U.S.A.). The images were analyzed using Melanie III (Swiss Institute of Bioinformatics, Geneva, Switzerland). All samples were processed at least three times to confirm reproducibility.
Protein identification
Proteins that are differentially expressed in the bladder tissue are identified by matrix assisted laser desorption/ionization-the time of flight mass spectrometry (MALDI-TOF MS) analysis (Yonsei Proteome Research Center, Seoul, Korea) with the search programs called ProFound and MS-FIT.
RESULTS
The mean bladder masses of the control and sham-operated rats were 122±15 mg and 131±19 mg, respectively. Mean bladder mass of the obstructed rats was 275±68 mg at 24 hr and increased to 467±212 mg by 120 hr. The control group did not exhibit any pathological lesions of the urinary bladder. The urinary bladders of the obstructed rats were generally thickened.
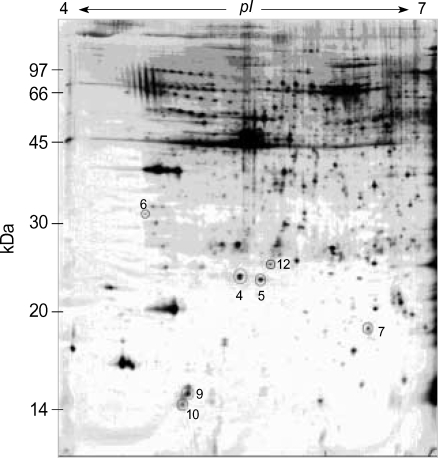
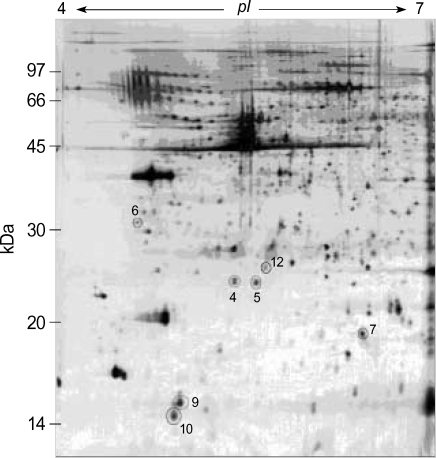
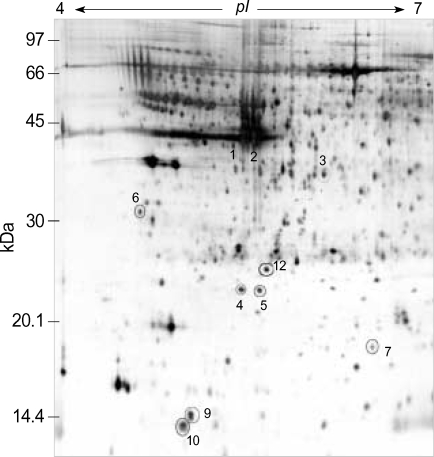
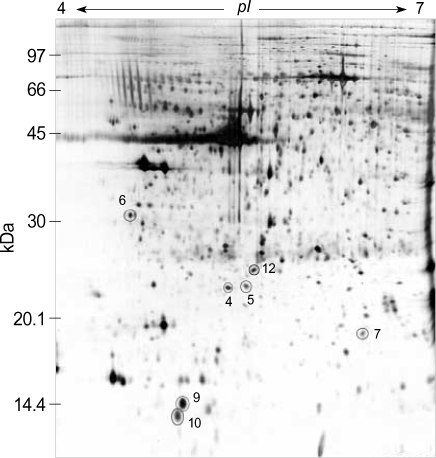
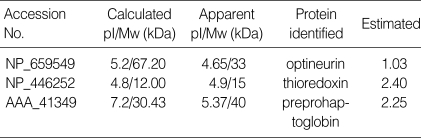
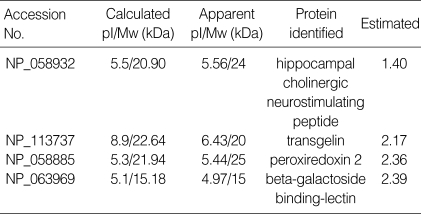
Two-dimensional electrophoresis of bladder tissue produced about 1,000 spots per gel. Electrophoretic images of samples taken from normal and sham-operated bladder tissue were depicted in Fig. 1, 2, which show that there were no significant differences between these groups. The proteins shown in these figures were not all identified because, for many, the expression in obstructed bladder tissue was similar to that in normal bladder tissue. Electrophoretic images of up-regulated and down-regulated proteins are shown in Fig. 3, 4. A comparison of images of proteins from bladders of obstructed rats with those of the controls showed that three proteins, optineurin (OPTN), thioredoxin and preprohaptoglobin, were over-expressed in the bladders of obstructed rats. Of these, thioredoxin and preprohaptoglobin increased transiently at 24 hr. Four proteins (peroxiredoxin 2, transgelin [SM22 alpha], hippocampal cholinergic neurostimulating peptide [HCNP] and beta-galactoside-binding lectin) were under-expressed in the obstructed bladder. Beta-galactoside-binding lectin was shown to be decreased transiently at 24 hr. Of the changes in protein expression detected in this proteomic analysis, the over-expression of OPTN and the under-expression of transgelin and HCNP were most noteworthy (Fig. 5) (Table 1, 2).
Fig. 1.
Two-dimension electrophoresis pattern of rat normal bladder tissue. Expressed number indicated proteins to be up-regulated or down-regulated at post-operative study (4; peroxiredoxin, 5; hippocampal cholinergic neurostimulating peptide, 6; optineurin, 7; transgelin, 9; beta-galactoside-binding lectin, 10; thioredoxoin, 12; albumin).
Fig. 2.
Two-dimension electrophoresis pattern of rat bladder tissue of sham-operated group. There is no difference from control group (4; peroxiredoxin, 5; hippocampal cholinergic neurostimulating peptide, 6; optineurin, 7; transgelin, 9; beta-galactoside-binding lectin, 10; thioredoxoin, 12; albumin).
Fig. 3.
Two-dimension electrophoresis pattern of rat bladder tissue of obstructed group (post-operative 1 day). Electrophoretic images of up-regulated and down-regulated proteins were shown. Number 1, 2, and 3 were prehaptoglobins and increased transiently at 24 hr. (4; peroxiredoxin, 5; hippocampal cholinergic neurostimulating peptide, 6; optineurin, 7; transgelin, 9; beta-galactoside-binding lectin, 10; thioredoxoin, 12; albumin).
Fig. 4.
Two-dimension electrophoresis pattern of rat bladder tissue of obstructed group (post-operative 5 day). Electrophoretic images of up-regulated and down-regulated proteins were shown. (4; peroxiredoxin, 5; hippocampal cholinergic neurostimulating peptide, 6; optineurin, 7; transgelin, 9; beta-galactoside-binding lectin, 10; thioredoxoin, 12; albumin).
Fig. 5.
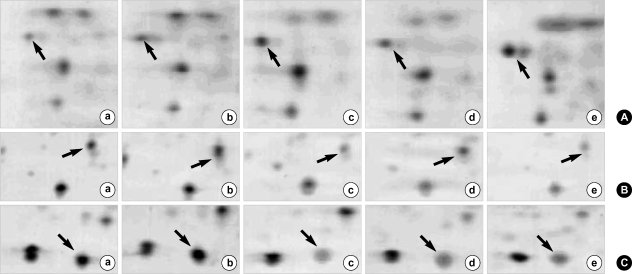
2-DE patterns showing the up- or down-regulation of important proteins in obstructed bladder tissues as compared to normal tissues. a: normal, b: sham-operative group, c: post-operative 1 day, d: post-operative 3 day, e: post-operative 5 day. (A) 2-DE patterns showing the up-regulation of optineurin (arrow) in bladder hyperplastic tissues as compared to normal tissues. (B) 2-DE patterns showing the down-regulation of transgelin (arrow) in bladder hyperplastic tissues. (C) 2-DE patterns showing the down-regulation of hippocampal cholinergic neurostinulatind peptide (arrow) in bladder hyperplastic tissues.
Table 1.
Up-regulated proteins in hyperplastic bladder tissue
*The "Z score" is estimated when the search result is compared against an estimated random match population. For instance, a Z score of 1.65 for a search means that the search is in the 95th percentile. Conceptually, this "95th percentile" is different from "95% confidence" that the search is a correct identification. (http://129.85.19.192/profound/help.html#ZSCORE)
Table 2.
Down-regulated proteins in hyperplastic bladder tissue
*The "Z score" is estimated when the search result is compared against an estimated random match population. For instance, a Z score of 1.65 for a search means that the search is in the 95th percentile. Conceptually, this "95th percentile" is different from "95% confidence" that the search is a correct identification. (http://129.85.19.192/profound/help.html#ZSCORE)
DISCUSSION
LUTS such as frequent or urgent urination may occur in BPH patients with bladder outlet obstruction. The exact causes and mechanisms of LUTS are unknown, although many theories have been proposed. We investigated a possible mechanism of LUTS in BOO using a proteomic approach. When the human genome project is completed, the practice of medicine will be altered fundamentally. Proteomic methods detect the functional units of expressed genes using protein fingerprinting (12, 13). At present, it is commonly held that proteins are the main functional outputs of genes and that proteomics will lead biology and medicine beyond genomics. The identification, quantification, classification, and functional assignment of proteins will be essential for a full understanding of these molecular events. Such information will likely prove to be crucial for amelioration of bladder changes incurred following partial BOO (14, 15).
In this study, we detected the over-expression of three proteins (OPTN, thioredoxin and preprohaptoglobin), and the under-expression of four proteins (peroxiredoxin 2, transgelin [SM22 alpha], HCNP, and beta-galactoside-binding lectin). Of these HCNP, OPTN and transgelin were of particular interest. HCNP was originally purified from the hippocampus of the young rat, and is associated with memory and learning (16). HCNP or its precursor, or both, may have a function in the peripheral as well as in the central nervous system (16). HCNP has been detected in variety of tissues and organs, such as the salivary gland, bronchiole, adrenal gland, small intestine and testis. It has been shown that HCNP-immunopositive nerve cell bodies and nerve fibers are present in the submucosal and myenteric plexuses of the small intestine of the rat (17). Human and rat HCNP are 54.5% homologous, and both stimulate acetylcholine synthesis (18). It has been shown that mutations in the OPTN gene are the principal cause of adult-onset primary open angle glaucoma. The existing evidence suggests that the direct interaction of OPTN with the E3-14.7K protein probably uses TNF-alpha or Fas-ligand pathways to mediate apoptosis, inflammation, or vasoconstriction (19). OPTN may play a neuroprotective role in the eye and optic nerve (20). Transgelin is an actin cross-linking/gelling protein found in fibroblasts and smooth muscle and is sensitive to transformation and shape-changes (21). Its expression is down-regulated in many cell lines, and this property may be an early and sensitive marker for the onset of transformation (22). Ablation of transgelin decreases the contractility and actin content of mouse vascular smooth muscle. Thus, transgelin plays a role in contractility, possibly by affecting actin filament organization (23). Beside these proteins, other proteins are related to oxidative stress or have a nonspecific function. Thioredoxin (Trx) is small globular proteins that proved to be excellent model for investigating the relationship between the structure of protein and their physico-chemical and functional properties (24) and has a role as an antioxidant (25). Plasma/serum levels of Trx 1 are good markers for oxidative stress. Exogenous Trx 1 shows cytoprotective and anti-inflammatory effects and has a good potential for clinical application (25). Preprohaptoglobin has a function protecting the tissues from oxidative damage (26). In this study thioredoxin and preprohaptoglobin increased transiently at 24 hr. This suggests that BOO induced hypoxia/reperfusion injury, but this finding needs to more experiments and information about that. Peroxiredoxins are nuclear encoded thiol-proteins with molecular masses of 17 to 24 kDa. They are ubiquitious peroxidases reducing a broad spectrum of peroxides like H2O2, lipid hydroperoxide and peroxinitrite, and related to oxidative stress (27). Beta-galactoside-binding lectin (galectin) has recently been designated as galectins and has related to variable pathogenesis; immunity, carcinogenesis, neural pain, etc (28-30). In this study the function of galectin remains unknown to the development of BOO symptoms.
In conclusion, bladder outlet obstruction resulted in an increase in the expression of three proteins and a decrease in the expression of four proteins in our rat model system. Of these proteins, HCNP, OPTN, and transgelin are of particular interest. Up-regulation of OPTN may protect against nerve injury. Down-regulation of HCNP may make detrusor muscle supersensitive to acetylcholine and down-regulation of transgelin may decrease its contractility. Beside these proteins, other proteins are related to oxidative stress or have a nonspecific function in this study. So proteins of urinary bladder changed by BOO seem to be responsible to the damage for bladder function in this animal study. However, more information on human bladder tissue and other urodynamic study are needed before clinical application of these findings is warranted.
Footnotes
This study was supported by the grant of Bio-medical Research Center of Medical College of Dankook University in 2003.
References
- 1.Caine M. The present role of alpha-adrenergic blockers in the treatment of benign prostatic hypertrophy. J Urol. 1986;136:1–4. doi: 10.1016/s0022-5347(17)44709-4. [DOI] [PubMed] [Google Scholar]
- 2.Abrams PH, Farrar DJ, Turner-Warwick RT, Whiteside CG, Feneley RC. The results of prostatectomy: a symptomatic and urodynamic analysis of 152 patients. J Urol. 1979;121:640–642. doi: 10.1016/s0022-5347(17)56918-9. [DOI] [PubMed] [Google Scholar]
- 3.Barry MJ, Meigs JB. The natural history of benign prostatic hyperplasia. In: Lepor H, editor. Prostatic disease. Philadelphia: Saunders; 2000. pp. 106–115. [Google Scholar]
- 4.Anderson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology. 2003;62:3–10. doi: 10.1016/j.urology.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Levin RM, Chichester P, Hass MA, Goslin JA, Buttyan R. Obstructive bladder dysfunction: morphological, biochemical and molecular changes. Eur Urol suppl. 2002;1:14–20. [Google Scholar]
- 6.Santarosa R, Colombel MC, Kaplan S, Monson F, Levin RM, Buttyan R. Hyperplasia and apoptosis. Opposing cellular processes that regulate the response of the rabbit bladder to transient outlet obstruction. Lab Invest. 1994;70:503–510. [PubMed] [Google Scholar]
- 7.Chen MW, Krasnapolsky L, Levin RM, Buttyan R. An early molecular response induced by acute overdistension of the rabbit urinary bladder. Mol Cell Biochem. 1994;132:39–44. doi: 10.1007/BF00925673. [DOI] [PubMed] [Google Scholar]
- 8.Flynn BJ, Mian HS, Cera PJ, Kabler RL, Mowad JJ, Cavanaugh AH, Rothblum LI. Early molecular changes in bladder hypertrophy due to bladder outlet obstruction. Urology. 2002;59:978–982. doi: 10.1016/s0090-4295(02)01619-9. [DOI] [PubMed] [Google Scholar]
- 9.Steers WD. Overactive bladder (OAB): What we thought we knew and what we know today. Eur Urol suppl. 2002;1:3–10. [Google Scholar]
- 10.Levin RM, Wein AJ, Buttyan R, Monson FC, Longhurst PA. Update on bladder smooth-muscle physiology. World J Urol. 1994;12:226–232. doi: 10.1007/BF00191201. [DOI] [PubMed] [Google Scholar]
- 11.Levin RM, Brading AF, Mills IW, Longhust PA. Experimental models of bladder obstruction. In: Lepor H, editor. Prostatic disease. Philadelphia: Saunders; 2000. pp. 169–196. [Google Scholar]
- 12.Wilkins MR, Sanchez JC, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF, Williams KL. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 13.Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- 14.Adam GC, Cravatt BF, Sorensen EJ. Profiling the specific reactivity of the proteome with non-directed activity-based probes. Chem Biol. 2001;8:81–95. doi: 10.1016/s1074-5521(00)90060-7. [DOI] [PubMed] [Google Scholar]
- 15.Chambers G, Lawrie L, Cash P, Murray GI. Proteomics: a new approach to the study of disease. J Pathol. 2000;192:280–288. doi: 10.1002/1096-9896(200011)192:3<280::AID-PATH748>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Katada E, Mitake S, Matsukawa N, Otsuka Y, Tsugu Y, Fujimori O, Ojika K. Distribution of hippocampal cholinergic neurostimulating peptide (HCNP)-like immunoreactivity in organs and tissues of young Wistar rats. Histochem Cell Biol. 1996;105:43–51. doi: 10.1007/BF01450877. [DOI] [PubMed] [Google Scholar]
- 17.Katada E, Ojika K, Mitake S, Ueda R. Neuronal distribution and subcellular localization of HCNP-like immunoreactivity in rat small intestine. J Neurocytol. 2000;29:199–207. doi: 10.1023/a:1026555107647. [DOI] [PubMed] [Google Scholar]
- 18.Ojika K, Ueki Y, Mitake S, Tsugu Y, Otsuka Y, Katada E. Demonstration of the biological activity of peptide fragments related to human and rat hippocampal cholinergic neurostimulating peptide (HCNP) Neurosci Lett. 1996;215:127–130. [PubMed] [Google Scholar]
- 19.Sarfarazi M, Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 2003;16:529–541. doi: 10.1016/s0896-1549(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 20.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 21.Shapland C, Hsuan JJ, Totty NF, Lawson D. Purification and properties of transgelin: a transformation and shape change sensitive actin-gelling protein. J Cell Biol. 1993;121:1065–1073. doi: 10.1083/jcb.121.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson D, Harrison M, Shapland C. Fibroblast transgelin and smooth muscle SM22 alpha are the same protein, the expression of which is down-regulated in many cell lines. Cell Motil Cytoskeleton. 1997;38:250–257. doi: 10.1002/(SICI)1097-0169(1997)38:3<250::AID-CM3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Zeidan A, Sward K, Nordstrom I, Ekblad E, Zhang JC, Parmacek MS, Hellstrand P. Ablation of SM22alpha decreases contractility and actin contents of mouse vascular smooth muscle. FEBS Lett. 2004;562:141–146. doi: 10.1016/S0014-5793(04)00220-0. [DOI] [PubMed] [Google Scholar]
- 24.Stefankova P, Kollarova M, Barak I. Thioredoxin-structural and functional complexity. Gen Physiol Biophys. 2005;24:3–11. [PubMed] [Google Scholar]
- 25.Nakamura H. Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal. 2005;7:823–828. doi: 10.1089/ars.2005.7.823. [DOI] [PubMed] [Google Scholar]
- 26.Lim YK, Jenner A, Ali AB, Wang Y, Hsu SI, Chong SM, Baumman H, Halliwell B, Lim SK. Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int. 2000;58:1033–1044. doi: 10.1046/j.1523-1755.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 27.Dietz KJ, Horling F, Konig J, Baier M. The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot. 2002;53:1321–1329. [PubMed] [Google Scholar]
- 28.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1991;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 29.Imbe H, Okamoto K, Kadoya T, Horie H, Senba E. Galectin-1 is involved in the potentiation of neuropathic pain in the dorsal horn. Brain Res. 2003;993:72–83. doi: 10.1016/j.brainres.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 30.Stillman BN, Mischel PS, Baum LG. New roles for galectins in brain tumors--from prognostic markers to therapeutic targets. Brain Pathol. 2005;15:124–132. doi: 10.1111/j.1750-3639.2005.tb00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]