Abstract
The pathogenesis of aspirin (acetylsalicylic acid, ASA)-intolerant urticaria (AIU) is still poorly understood but it has recently been suggested that it is associated with the overproduction of leukotriene (LT). This is supported by evidence that cyclooxygenase 2 inhibitor is given safely to patients with AIU. The present study was designed to investigate the role of genetic polymorphism of LT related genes in the pathogenesis of AIU via a case-control study. We screened single nucleotide polymorphisms (SNPs) in genes encoding enzymes involved in leukotriene synthesis in the Korean population with AIU (n=101), ASA-intolerant asthma (AIA, n=95) and normal healthy controls (n=123). Genotype was determined by primer extension reactions using the SNapShot ddNTP primer extension kit. Among 8 SNPs of four LT related genes, the polymorphism of ALOX5 at positions of -1708 G>A showed significant difference in genotype frequency between AIU and AIA (p=0.01). Furthermore, there were significant differences observed in the frequencies of two ALOX5 haplotypes between the AIU group and AIA group (p<0.05). However, there were no differences in allele, genotype, or haplotype frequencies of ALOX5 between the AIU group and the normal control group. These results suggested that ALOX5 has a differing contribution in two major clinical pathogenesis related to ASA-sensitivity.
Keywords: Aspirin, Hypersensitivity; Urticaria; Polymorphism, Genetic; Leukotrienes
INTRODUCTION
Aspirin (acetylsalicylic acid, ASA) sensitivity results in distinct clinical syndromes. Patients sensitive to ASA usually develop either a respiratory syndrome (ASA-intolerant asthma, AIA) or a cutaneous syndrome (ASA-intolerant urticaria/angioedema, AIU) (1, 2). ASA is also an occasional cause of anaphylaxis (3).
The pathogenesis of ASA sensitivity most likely involves inhibition of cyclooxygenase by ASA or other nonsteroidal anti-inflammatory drugs (NSAIDs) resulting in an enhanced production of leukotrienes (LTs), potent inflammatory mediators synthesized from arachidonic acid through a sequential pathway. It is widely recognized that LT biosynthesis is associated with the development and progression of AIA. Urinary LT E4 excretion is higher in patients sensitive to ASA than in patients insensitive to ASA (4, 5). Drugs that inhibit the synthesis of LT have clinical efficacy in asthma (6, 7).
In contrast to AIA, the study of the cutaneous reaction resulting from ASA sensitivity has not yet led to understanding of the mechanism underlying this reaction. Many patients with chronic urticaria exhibit increased wheal and swelling after intake of ASA (2, 8). The proportion of patients with chronic urticaria who develop exacerbation after ASA administration ranges from 20% to 30% (1), whereas ASA administration causes urticaria and/or angioedema in a percentage ranging from 1% to 6% of normal subjects (9). The mechanisms of such hypersensitivity to ASA are still poorly understood. It has been postulated that ASA sensitivity may be caused by a mechanism with increased production of cysteinyl-LT (10) as the pathogenesis of AIA. This suggestion is strengthened by several reports that antihistamine, histamine receptor 1 antagonist, may not be sufficient as a monotherapy in urticaria sensitive to ASA and LT receptor antagonist (LTRA) may be effective in chronic urticaria (11-14). It has been reported that cyclooxygenase-2 (COX2) inhibitor may be given safely to patients with AIU (15, 16). In patients with chronic urticaria, baseline urinary LT E4 excretion is higher in patients sensitive to ASA than in patients insensitive to ASA (1). These studies support the hypothesis that overproduction of LT may be important in chronic urticaria.
Although there is no clear evidence of the involvement of LT in AIU and/or angioedema, it has been proposed that LTs may participate in the pathogenesis of urticaria and angioedema in a similar mechanism to that of AIA. We (17) and others (18, 19) have previously shown that polymorphism in LT related genes is associated with AIA. Therefore, there is a rationale to investigate the genetic polymorphisms of candidate genes related with LT production in AIU and/or angioedema.
In this study, we investigated the genetic polymorphism in 8 candidate single nucleotide polymorphisms (SNPs) of four LT related genes, ALOX5 (5-lipoxygenase), ALOX5AP (5-lipoxygenase activating protein), PTGS2 (cyclooxygenase 2) and LTC4S (LTC4 synthase), in patients with AIU compared to AIA and a normal healthy control group recruited from a Korean population.
MATERIALS AND METHODS
Study subjects
One hundred one patients with urticaria sensitive to both ASA and NSAIDs (46 male subjects; mean age: 34.2 yr; 31 patients had underlying chronic urticaria with more than 6 weeks duration), 95 patients with ASA-intolerant asthma (35 male subjects, mean age: 42.3 yr), and 123 normal healthy controls (NC) enrolled from the Department of Allergy and Rheumatology, Ajou University Hospital, Suwon, Korea were enrolled in the study. In this study, ASA-intolerant urticaria group was defined as patients having a certain history of urticaria/angioedema development after the ingestion of more than two kinds of NSAIDs and positive responders on oral ASA challenge test (classified as cross reacting group by Sanchez-Borges et al. (20)). Also NSAIDs sensitivity could be confirmed because the patients visited our Allergy Clinic or emergency room presenting current urticaria/angioedema after taking NSAIDs. In order to exclude a single ASA-intolerant urticaria, we performed skin prick test with 10 mg/mL of lysine-ASA (L-ASA) and none of them had positive skin prick test. ASA-intolerant asthma was diagnosed by a positive result to L-ASA bronchoprovocation testing and they had no history of drug allergies presenting as skin manifestations. Patients having both AIA and AIU were excluded in this study. 123 normal controls, who had non-atopy, no personal and family history of allergic diseases, and no past history of ASA and other drug hypersensitivity, were recruited from the general population. Seventy (77.8%) patients among the ASA-intolerant urticaria group and 35 (43.8%) in ASA-intolerant asthma patients were atopic. All subjects provided informed consent and the protocol used were approved by the ethics committee of Ajou University Hospital, Suwon, Korea. Skin prick tests were performed with 12 common aeroallergens (Bencard Co., U.K.) including Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog, cockroach, tree pollen mixture, grass pollen mixture, mugwort, ragweed, Hop Japanese, Aspergillus, Alternaria, with histamine and saline controls.
Oral ASA challenge and L-ASA bronchoprovocation tests
All medications, including theophyllines, beta-2 agonists, LT receptor antagonist, anti-histamine and steroids were stopped 72 hr before the procedure. After the placebo challenge, 500 mg of ASA in a tablet was orally administered, and patients were observed for 4 hr with monitoring of urticaria and changes of lung functions every 30 min. Appearance of urticaria within 4 hr without any changes of FEV1 was considered as a positive result. L-ASA bronchoprovocation test was performed according to the modified method as previously described (17, 21). The test solutions were delivered by a DeVilbiss 646 nebulizer (DeVilbiss Co., Somerset, Penn., U.S.A.) and connected to a compressed air source (5 L/min). L-ASA powder (Althargyl®, Arthromedica, Swiss) that contained 900 mg L-ASA with 100 mg aminoacetic acid was diluted in normal saline to produce a stock solution of 300 mg/mL. A placebo solution of normal saline was used as a control. Subjects inhaled the aerosol via a mouthpiece during normal tidal breathing. The L-ASA solution was inhaled every 30 min. The FEV1 and maximum mid expiratory flow (MMEF) are measured at 10 and 30 min after each dose. The provocation was stopped when the FEV1 has decreased 20% or more from the baseline. The FEV1 and MMEF after the last dose were measured at 10, 30, 60 min, and then hourly for 7 hr.
Genomic DNA preparation and SNP genotyping
Peripheral blood samples (10 mL) were collected from study subjects. Genomic DNA was extracted using a G-DEX™ for Blood Genomic DNA Extraction Kit (iNtRON Biotechnology, Korea) according to the manufacturer's instructions. For each of the 8 SNPs in the four genes, ALOX5, ALOX5AP, PTGS2 and LTC4S, analyzed, pairs of forward and reverse primers were designed to amplify the genomic region surrounding the SNP of interest (Table 1). The polymerase chain reaction (PCR) was performed with 20 ng genomic DNA, 50 mM KCl, 10 mM Tris, 2 mM MgCl2, 1.25 pmol of each primer, 200 µM dNTPs, and 0.15 U Taq DNA polymerase (Perkin Elmer, Emeryville, CA, U.S.A.) in standard buffer provided by the manufacturer. After initial denaturation for 5 min at 95℃, a touch-down PCR (22) was undertaken with 10 cycles consisting of 1 min denaturation at 94℃, 1 min annealing at 54℃ and 2 min elongation at 72℃ followed by 35 cycles of 1 min at 94℃, 1 min at 45℃ and 2 min at 72℃. A final elongation step at 72℃ for 10 min terminated the program. Primer extension reactions were performed with the SNaPSHOT ddNTP primer extension kit (Applied Biosystems) as recommend by the manufacturer using extension probes as previously described (17).
Table 1.
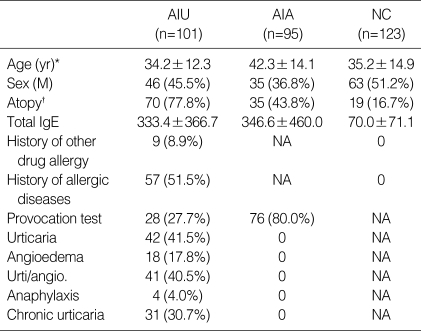
Clinical characteristics of the study subjects
AIU, ASA-intolerant urticaria; AIA, ASA-intolerant asthma; NC, normal controls; NA, not applicable.
*p<0.01 (AIU vs. AIA). †p<0.01 (AIU vs. AIA and AIU vs. NC).
Data analysis
Statistical analyses were completed using SPSS version 10 (SPSS Inc., Chicago, IL, U.S.A.) and a p-value <0.05 was considered to be significant. Allele frequencies were calculated for each polymorphic site by the allele counting method, and significant departures of genotype frequency from the Hardy-Weinberg equilibrium (HWE) at each polymorphic site were tested by chi-square analysis. Differences in genotype frequency between patients and controls were tested by Fisher's exact test. Differences in the mean value of the phenotypic characteristics within patient groups were compared using ANOVA and t-test. Haplotypes of ALOX5 were analyzed by using the Haploview version 2.05 (23).
RESULTS
The characteristics of the participants were shown in Table 1. The three groups significantly differed with respect to age (p<0.01 for AIU vs. AIA) and prevalence of atopy (p<0.01 for AIU vs. NC and AIU vs. AIA) between AIU and other control groups. Seventy (77.8%) patients with AIU and 35 (43.8%) AIA patients were atopic. Patients with AIU were composed of 31 (30.7%) chronic urticaria exacerbated by ASA, 18 (17.8%) combined with angioedema and 4 (4.0%) combined with anaphylaxis. Patients with both AIA and AIU were excluded in this study.
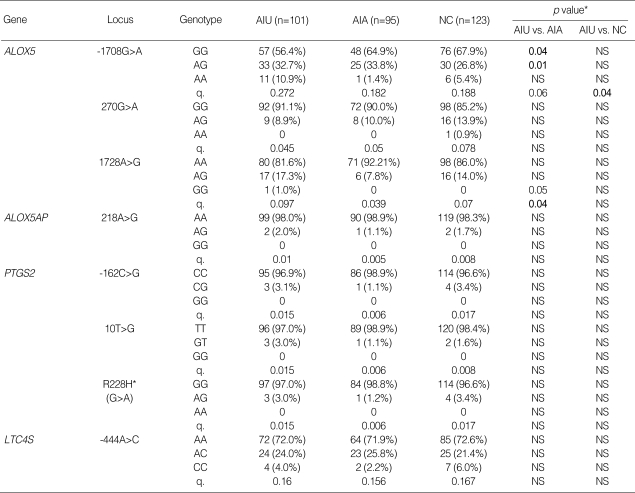
We investigated 8 SNPs of ALOX5, ALOX5AP, PTGS2 and LTC4S in AIU compared to other control groups, AIA and NC. Genotype distributions of all loci were in Hardy-Weinberg equilibrium (p>0.05). Allele and genotype frequencies of each SNP in four genes are shown in Table 2. Of all SNPs tested, the polymorphism of ALOX5 at positions of -1708 G>A showed significant difference in genotype frequency between AIU and AIA; the frequency of minor genotype of ALOX5-1708G>A was significantly higher in AIU group compared to AIA group (p=0.01 in dominant model, OR=8.9, 95% CI=1.13-10.73). The p value remained significant after correction for multiple comparisons (Pc=0.045). For all other SNPs tested, there were no significant differences in allele and genotype frequencies among the three groups.
Table 2.
Allele and genotype frequencies of the SNPs in the candidate genes
AIU, ASA-intolerant urticaria; AIA, ASA-intolerant asthma; NC, normal controls; n, number of patients; q, minor allele frequency. R, arginine; H, histidine; NS, not significant. *Each p value was calculated with co-dominant, dominant and recessive models.
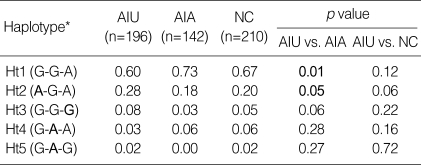
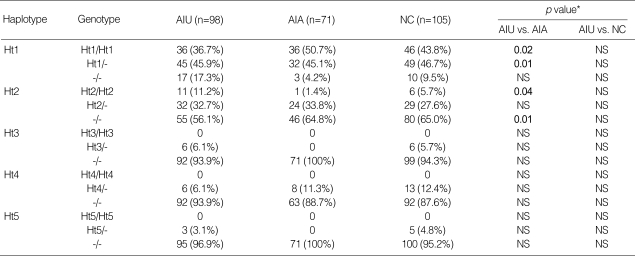
Using Haploview program, haplotypes were constructed for 3 SNPs and the frequency of each haplotype in the patient groups was assessed. The frequency of five haplotypes of ALOX5 showing ≥1% frequency in the population was shown in Table 3. There were significant differences observed in the frequency of the ALOX5 haplotypes between the AIU group and AIA group; the frequency of ALOX5 ht1 [G-G-A] carrying major alleles of 3 SNPs in the AIU group was significantly lower than that in the AIA group (p=0.01, OR=0.541, 95% CI=0.339-0.865), but the frequencies of haplotypes carrying minor allele of ALOX5-1708G>A, ht2 [A-G-A] was significantly higher in AIU group compared to AIA group (p=0.05, OR=1.697, 95% CI=1.00-2.88). Analysis of the genotype distributions of the ALOX5 haplotypes (Table 4) also revealed that frequencies of genotype containing ht1 [G-G-A] and ht2 [A-G-A] were significantly different between AIA and AIU (p=0.01 in dominant model for ht1, p=0.02 in recessive model for ht2). However, there were no differences in allele, genotype, or haplotype frequencies of ALOX5 polymorphisms between the AIU group and the normal control. Haplotypic analysis of PTGS2 polymorphism was not undertaken due to the extremely low frequencies of the variant alleles.
Table 3.
Haplotype frequencies of ALOX5 gene. The haplotype of ALOX5 is in order of -1708G>A, 270 G>A, 1728 A>G
AIU, ASA-intolerant urticaria; AIA, ASA-intolerant asthma; NC, normal controls; Ht,haplotype; N, number of chromosomes.
*Minor alleles are given in bold.
Table 4.
Genotype distributions of ALOX5 haplotypes
†AIU, ASA-intolerant urticaria; AIA, ASA-intolerant asthma; NC, normal controls; Ht, haplotype.
*Each p value was calculated with co-dominant, dominant and recessive models.
DISCUSSION
In this study, we have investigated genetic association between polymorphisms of LT related genes (ALOX5, ALOX5AP, PTGS2 and LTC4S) and AIU in a Korean population.
Among 8 SNPs of four LT related genes, the polymorphism of ALOX5 at positions of -1708 G>A showed highly significant differences in genotype frequency between AIU and AIA (p=0.01). Furthermore, there were significant differences observed in the frequency of the ALOX5 haplotypes (ht1 and ht2) between the AIU group and AIA group. However there were no differences in allele, genotype, or haplotype frequencies in ALOX5 -1708 G>A between the AIU group and the normal control. In association study of 8 SNPs in LT related genes, ALOX5, LTC4S, PTGS2 and ALOX5AP with phenotypes (sex, age, atopy, tIgE), we could not find any significant difference.
In our previous study, we found that an ALOX5-ht1 [G-C-G-A] was associated with AIA but not ASA-tolerant asthma; the frequency of the ALOX5-ht1 [G-C-G-A] carrying major alleles of these 3 SNPs in the AIA group was significantly higher than its frequency in the control group. In addition, consistent with the present study, significant differences in frequency of the ALOX5 polymorphism at positions of -1708G>A was also observed between the AIU group and the AIA group. The results of the present study however, suggested that unlike AIA, susceptibility to AIU is associated with minor genotype of ALOX5 -1708G>A. There was a significant difference in atopy frequency between the AIA and AIU groups. However this may be unlikely to influence the results as previous studies have not identified association of LT related polymorphism with atopy (24, 25). It may be possible that ALOX5 polymorphism may contribute to AIU susceptibility with odds ratio (OR) of 8.9 and 95% confidence interval (95% CI) of 1.13-70.73 relative to AIA. These results suggested that ALOX5, which catalyzes the first step in the formation of LT, has a differing contribution in two major clinical pathogenesis related to ASA-sensitivity.
This study has several potential limitations. It is possible to observe a discordant result, oral tests were postitive but the bronchial tests were negative, according to the provocation route (26). Our limited power to detect modest effects may at least partially explain our modest evidence of association to ALOX5 SNP. While the minimum p-values we observed were modest, this is consistent with our expectations regarding common, complex conditions such as AIA and AIU.
In conclusion, we have shown that ALOX5 has a differing contribution in two major clinical pathogenesis related to ASA-sensitivity. Our studies suggested that ALOX5 may be a potentially important gene in AIU susceptibility in the Korean population, and that promoter polymorphism of the ALOX5 gene might contribute to AIU susceptibility relative to AIA. Further studies will be needed to confirm functional effect of the ALOX5 -1708 G>A on the pathogenic mechanism of AIU.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D project. Ministry of Health & Welfare, Republic of Korea (01-PJ10-PG6-01GN14-0002).
References
- 1.Grattan CE. Aspirin sensitivity and urticaria. Clin Exp Dermatol. 2003;28:123–127. doi: 10.1046/j.1365-2230.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 2.Mastalerz L, Setkowicz M, Sanak M, Szczeklik A. Hypersensitivity to aspirin: Common eicosanoid alterations in urticaria and asthma. J Allergy Clin Immunol. 2004;113:771–775. doi: 10.1016/j.jaci.2003.12.323. [DOI] [PubMed] [Google Scholar]
- 3.Quiralte J, Blanco C, Castillo R, Ortega N, Carrillo T. Anaphylactoid reactions due to nonsteroidal antiinflammatory drugs: clinical and cross-reactivity studies. Ann Allergy Asthma Immunol. 1997;78:293–296. doi: 10.1016/S1081-1206(10)63184-5. [DOI] [PubMed] [Google Scholar]
- 4.Christie PE, Tagari P, Ford-Hutchinson AW, Black C, Markendorf A, Schmitz-Schumann M, Lee TH. Urinary leukotriene E4 after lysine-aspirin inhalation in asthmatic subjects. Am Rev Respir Dis. 1992;146:1531–1534. doi: 10.1164/ajrccm/146.6.1531. [DOI] [PubMed] [Google Scholar]
- 5.Smith CM, Hawksworth RJ, Thien FC, Christie PE, Lee TH. Urinary leukotriene E4 in bronchial asthma. Eur Respir J. 1992;5:693–699. [PubMed] [Google Scholar]
- 6.Holgate ST, Sampson AP. Antileukotriene therapy. Future directions. Am J Respir Crit Care Med. 2000;161:147–153. doi: 10.1164/ajrccm.161.supplement_1.ltta-29. [DOI] [PubMed] [Google Scholar]
- 7.Drazen JM, Israel E, O'Byme PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 8.Doeglas HM. Reactions to aspirin and food additives in patients with chronic urticaria, including the physical urticarias. Br J Dermatol. 1975;93:135–144. doi: 10.1111/j.1365-2133.1975.tb06732.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson DD, Simon RA. Sensitivity to aspirin and nonsteroidal anti-inflammatory drugs. In: Middleton E, Reed CE, Ellis EF, et al., editors. Allergy: Principles and Practice. 4th edn. Mosby; 1993. pp. 1747–1765. [Google Scholar]
- 10.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 11.Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: a single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110:484–488. doi: 10.1067/mai.2002.126676. [DOI] [PubMed] [Google Scholar]
- 12.Pacor ML, Di Lorenzo G, Corrocher R. Efficacy of leukotriene receptor antagonist in chronic urticaria. A double-blind, placebo-controlled comparison of treatment with montelukast and cetirizine in patients with chronic urticaria with intolerance to food additive and/or acetylsalicylic acid. Clin Exp Allergy. 2001;31:1607–1614. doi: 10.1046/j.1365-2222.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellis MH. Successful treatment of chronic urticaria with leukotriene antagonists. J Allergy Clin Immunol. 1998;102:876–877. doi: 10.1016/s0091-6749(98)70032-6. [DOI] [PubMed] [Google Scholar]
- 14.Perez C, Sanchez-Borges M, Capriles E. Pretreatment with montelukast blocks NSAID-induced urticaria and angioedema. J Allergy Clin Immunol. 2001;108:1060–1061. doi: 10.1067/mai.2001.120275. [DOI] [PubMed] [Google Scholar]
- 15.Zembowicz A, Mastalerz L, Setkowicz M, Radziszewski W, Szczeklik A. Safety of cyclooxygenase 2 inhibitors and increased leukotriene synthesis in chronic idiopathic urticaria with sensitivity to nonsteroidal anti-inflammatory drugs. Arch Dermatol. 2003;139:1577–1582. doi: 10.1001/archderm.139.12.1577. [DOI] [PubMed] [Google Scholar]
- 16.Quiralte J, Saenz de San Pedro B, Florido JJ. Safety of selective cyclooxygenase-2 inhibitor rofecoxib in patients with NSAID-induced cutaneous reactions. Ann Allergy Asthma Immunol. 2002;89:63–66. doi: 10.1016/s1081-1206(10)61912-6. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Park HS, Oh HB, Lee JH, Suh YJ, Park CS, Shin HD. Leukotriene-related gene polymorphisms in ASA-intolerant asthma: an association with a haplotype of 5-lipoxygenase. Hum Genet. 2004;114:337–344. doi: 10.1007/s00439-004-1082-1. [DOI] [PubMed] [Google Scholar]
- 18.Sanak M, Simon HU, Szczeklik A. Leukotriene C4 synthase promoter polymorphism and risk of aspirin-induced asthma. Lancet. 1997;350:1599–1600. doi: 10.1016/s0140-6736(05)64015-9. [DOI] [PubMed] [Google Scholar]
- 19.Sanak M, Pierzchalska M, Bazan-Socha S, Szczeklik A. Enhanced expression of the leukotriene C(4) synthase due to overactive transcription of an allelic variant associated with aspirin-intolerant asthma. Am J Respir Cell Mol Biol. 2000;23:290–296. doi: 10.1165/ajrcmb.23.3.4051. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. Cutaneous reactions to aspirin and nonsteroidal anti-inflammatory drugs. Clin Rev Allergy Immunol. 2003;24:125–136. doi: 10.1385/CRIAI:24:2:125. [DOI] [PubMed] [Google Scholar]
- 21.Park HS. Early and late onset asthmatic responses following lysineaspirin inhalation in aspirin-sensitive asthmatic patients. Clin Exp Allergy. 1995;25:38–40. doi: 10.1111/j.1365-2222.1995.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 22.Schunck B, Kraft W, Truyen U. A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J Virol Methods. 1995;55:427–433. doi: 10.1016/0166-0934(95)00069-3. [DOI] [PubMed] [Google Scholar]
- 23.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 24.Sayers I, Barton S, Rorke S, Sawyer J, Peng Q, Beghe B, Ye S, Keith T, Clough JB, Holloway JW, Sampson AP, Holgate ST. Promoter polymorphism in the 5-lipoxygenase (ALOX5) and 5-lipoxygenase-activating protein (ALOX5AP) genes and asthma susceptibility in a Caucasian population. Clin Exp Allergy. 2003;33:1103–1110. doi: 10.1046/j.1365-2222.2003.01733.x. [DOI] [PubMed] [Google Scholar]
- 25.Sayers I, Barton S, Rorke S, Beghe B, Hayward B, Van Eerdewegh P, Keith T, Clough JB, Ye S, Holloway JW, Sampson AP, Holgate ST. Allelic association and functional studies of promoter polymorphism in the Leukotriene C4 synthase gene (LTC4S) in asthma. Thorax. 2003;58:417–424. doi: 10.1136/thorax.58.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nizankowska E, Bestynska-Krypel A, Cmiel A, Szczeklik A. Oral and bronchial provocation tests with aspirin for diagnosis of aspirininduced asthma. Eur Respir J. 2000;15:863–869. doi: 10.1034/j.1399-3003.2000.15e09.x. [DOI] [PubMed] [Google Scholar]