Abstract
Respiratory isolates of Klebsiella pneumoniae in Korea during 2002-2003 were studied to determine the prevalence and types of extended-spectrum β-lactamases (ESBLs) and plasmid-mediated AmpC β-lactamases (PABLs). ESBL-production was tested by double-disk synergy, and genotypes of β-lactamases were determined by PCR and sequencing. ESBLs were detected in 28.4% of 373 isolates, and the most prevalent types were SHV-12 (63 isolates) and CTX-M-14 (9 isolates). Forty of 75 ESBL-producers (53.5%) also had PABLs: 21 isolates with CMY-2-like, 17 with DHA-1-like. Pulsed-field gel electrophoresis showed 19 types and 25 of 74 isolates had an identical pattern, indicating nosocomial spread. Dissemination of ESBL- and PABL-producing K. pneumoniae strains in Korea is a particular concern, as it limits the choice of antimicrobial agents for treatment of infections.
Keywords: beta-lactamase CTX-M-14, beta-lactamase CMY-2, beta-lactamase DHA-1, AmpC beta-lactamase, Klebsiella pneumoniae
INTRODUCTION
Extended-spectrum β-lactamases (ESBLs) produced by Gram-negative bacilli have been a serious problem in hospitalized patients worldwide (1). These enzymes are most commonly produced by strains of Klebsiella pneumoniae, one of the important nosocomial respiratory pathogens. ESBLs have been very prevalent in Korea most being of the types, SHV-12, SHV-2a, and TEM-52, however, CTX-M types have emerged recently as well (2, 3). ESBLs do not hydrolyze cephamycins, however, the cephamycin-hydrolyzing plasmid-mediated AmpC β-lactamase (PABL), CMY-1, emerged in Korea in 1988 (4). As well, various types of PABLs have been increasingly detected in intrinsically cephamycin-susceptible Gramnegative bacilli in other parts of the world (5, 6). In 2003, 36% and 37% of K. pneumoniae isolates at a Korean tertiarycare hospital showed resistance to ceftazidime and cefoxitin, respectively (unpublished data). These results suggest a high prevalence of ESBL and PABL-producing isolates. Recent increases of K. pneumoniae isolates concomitantly producing PABL and ESBL pose a serious therapeutic problem as carbapenems remain the only active β-lactams against these organisms (7).
This study was conducted to determine the prevalence and the types of ESBLs and PABLs among K. pneumoniae isolates from respiratory specimens. This information is crucial for controlling of the spread of resistance and for the decision of empirical selection of antimicrobial agents.
MATERIALS AND METHODS
Clinical isolates and antimicrobial susceptibility testing
A total of 373 non-duplicate isolates of K. pneumoniae, excluding scant growth, were isolated from the lower respiratory specimens of patients in a Korean tertiary-care hospital in Seoul over a one year period starting in September 2002. The isolates were identified by the conventional methods (8) or by using the Vitek GNI card (bioMérieux, Marcy I'Etoile, France).
Antimicrobial susceptibility was tested by the National Committee for Clinical Laboratory Standards (NCCLS) disk diffusion method (9). ESBL production was detected with the double-disk synergy test with cefotaxime, ceftazidime, aztreonam, cefepime, and amoxicillin/clavulanic acid disks, or phenotypic confirmatory tests as recommended by the NCCLS (9).
MICs of β-lactams were determined by the NCCLS agar dilution method (9). Antimicrobial agents used were cephalothin and cefepime (Sigma, St. Louis, MO, U.S.A.), ceftazidime and clavulanic acid (GlaxoSmithKline, Greenford, U.K.), cefotaxime (Aventis, Frankfurt, Germany), cefoxitin and imipenem (Merck/Sharp & Dohme, Rahway, NJ, U.S.A.), and aztreonam (Bristol-Myers Squibb, Princeton, NJ, U.S.A.).
Isoelectric focusing and resistance transfer
Isoelectric focusing was carried out following the manufacturer's instructions using pre-cast gels with pH gradient 3-10, and a ThermoFlow Electrophoresis Temperature Control System (Novex Experimental Technologies, San Diego, CA, U.S.A.). Resistance transfer was tested by an agar mating method with nalidixic acid-resistant recipient E. coli RG 176, rifampicin-resistant E. coli RG 488, or azide-resistant E. coli J53. Mueller-Hinton agar containing 100 mg/L of nalidixic acid, rifampicin or azide, and 2 mg/L of ceftriaxone or aztreonam was used to select transconjugants.
Molecular methods
PCR for the detection of blaTEM and blaSHV in the transconjugants was carried out as described previously (10). PCR for the detection of blaCTX-M in ESBL-producing clinical isolates was performed with primers CTXUNI-F (5'-CVATGTGCA GYACCAGTAA-3') and CTXUNI-R (5'-ARGTSACCAG AAYMAGCGG-3'). A thermocycler (Eppendorf, Hamburg, Germany) was used under the following conditions: 94℃ for 5 min, 35 cycles of 94℃ for 30 sec, 55℃ for 30 sec, and 72℃ for 45 sec, followed by 72℃ for 7 min. PCR for detection of family-specific PABL gene alleles in cefoxitin-intermediate or -resistant isolates was performed by the method of Perez-Perez and Hanson with slight modification (11). PCR for detection of blaDHA in DHA PCR positive isolates was performed using the forward primer DHA1-F (5'-TCTGCCGCTGAT AATG TCG-3') for detection of blaDHA-1 and blaDHA-2; reverse primers were DHA1-R (5'-GCCGCCGGATCATTCAGCGC-3') and DHA2-R (5'-TCTGCCGGGTCATTCAACAT-3'), respectively.
Sequencing was performed using the following primers: for blaCTX-M, CTXM-F (5'-AAAAATGATTGAAAGGTGGTTGT-3') and CTXM-R (5'-TTACAGCCCTTCGGCGATGA-3'); for blaCMY-1, CMY1E-F (5'-TATTAGAGCGGTTTAGGCTG-3') and CMY1E-R (5'-AATGTACCGCCCTCTTTC-3'); for blaCMY-2, CITS-F (5'-AACACACTGATTGCGTCTGA-3') and CITS-R (5'-TCCTGGGCCTCATCGTCAGTTAT-3'). Sequencing of blaTEM, blaSHV, and blaDHA was performed with the primers as described previously (10, 11). DNAs in the PCR products were extracted with a gel extraction kit (Qiagen, Hilden, Germany) and used for direct sequencing of ESBL and PABL genes. Both strands were analyzed by the dideoxy-chain termination method with an ABI 3700 Autosequencer (Perkin-Elmer, Foster City, CA, U.S.A.).
Genomic DNA from K. pneumoniae isolates was digested using XbaI (Takara, Japan) and separated using a CHEF-DRII system according to the manufacturer's instruction (Bio-Rad, Hercules, CA, U.S.A.). The pulsed-field gel electrophoresis (PFGE) band patterns were analyzed with the computer software UVIBand Map V.99 (UVItec Ltd., Cambridge, U.K.) by the un-weighted pair group method using arithmetic averages (UPGMA).
RESULTS
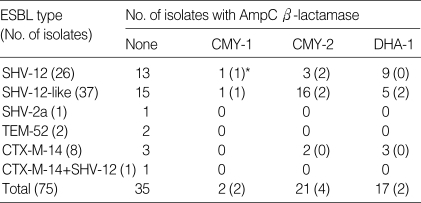
Among the 373 K. pneumoniae isolates, 106 (28.4%) tested positive using either the double-disk synergy test or NCCLS confirmatory test, indicating ESBL production. Resistance was transferable in 75 (70.8%) of the isolates. Seventy-six ESBL genes were detected by PCR in 75 transconjugants (Table 1).
Table 1.
Plasmid-mediated AmpC-β-lactamases detected from extended-spectrum β-lactamase-producing K. pneumoniae isolates
*The number of isolates with their AmpC genes sequenced is shown in parenthesis.
The most common ESBL type, SHV-12, was identified in 26 of 63 blaSHV allele-positive isolates, which showed β-lactamase bands with a pI of 8.2. The second most common type was CTX-M-14, being detected in nine isolates. SHV-2a and TEM-52 were identified in only one and two isolates, respectively.
Forty of 75 (53.3%) clinical isolates with ESBL genes had PABLs. Twenty one contained blaCMY-2-like, 17 contained blaDHA-1-like, and two contained blaCMY-1 (Table 1). Antibiotic resistance by disk diffusion method of isolates without ESBL and PABL genes, those with ESBL genes, and those with both ESBL and PABL genes were: to cefoxitin 6%, 3%, and 100%; to aminoglycosides 5-9%, 40-97%, and 90-100%; and to levofloxacin 4%, 60%, and 80%, respectively.
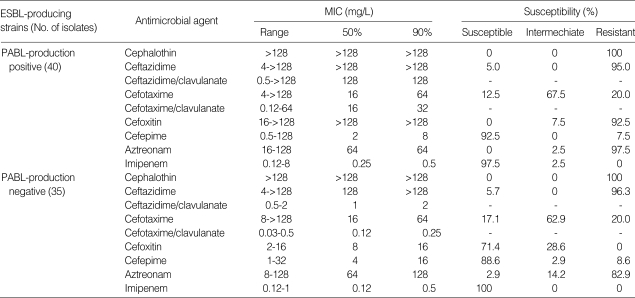
The MIC90S of β-lactams to ESBL-producers were: cefotaxime and ceftazidime for both PABL-nonproducer and -producers 64 mg/L and >128 mg/L, respectively; cefotaxime/clavulanate and ceftazidime/clavulanate for PABL-nonproducer 2 mg/L and 0.25 mg/L, respectively, and PABL-producer 32 mg/L and 128 mg/L, respectively; cefoxitin for PABL-nonproducer and -producer 16 mg/L and >128 mg/L, respectively (Table 2).
Table 2.
Antimicrobial susceptibilities of ESBL-producing K. pneumoniae isolates with and without concomitant production of PABLs
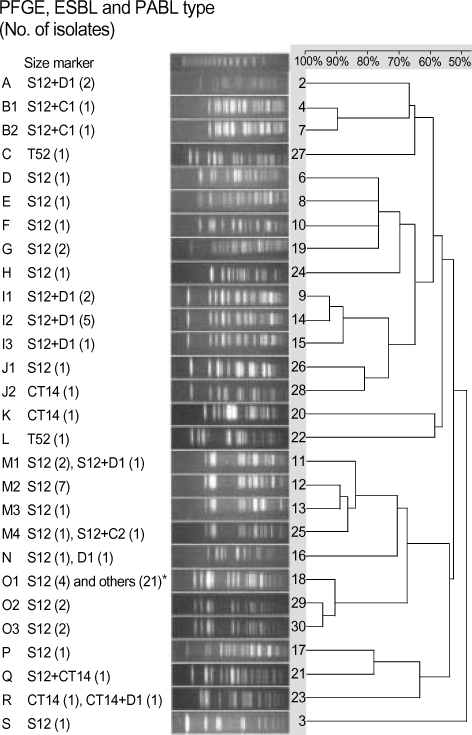
PFGE of XbaI-digested genomic DNA of 74 ESBL producers showed 19 types including nine subtypes. Twenty-five isolates gave an identical pattern, O1 (Fig. 1), which was mostly isolated from NCU patients. Among these, 18 isolates produced both SHV-12-like and CMY-2-like enzymes.
Fig. 1.
PFGE band pattern of 74 strains of K. pneumoniae were analyzed with UVIBand Map V.99 by the un-weighted pair group method using arithmetic averages (UPGMA). *The β-lactamases produced include S12+C2 (18), S12+D1 (1), and CT14+C2 (2). C1, CMY-1; C2, CMY-2; CT14, CTX-M-14; D1, DHA-1; S12, SHV-12; T52, TEM-52.
DISCUSSION
The prevalence of ESBLs among clinical isolates varies depending on countries and institutions. In the United States occurrence of ESBL production in Enterobacteriaceae ranges from 0 to 25%, with the national average being around 3% (1). ESBL production among K. pneumoniae isolates was comparatively higher in Taiwan and Hong Kong, 8.5% and 13%, respectively, but very low in Japan, less than 1% (1). The prevalence in our study during 2002-2003 was 28.4%, which is significantly higher than the United States, Taiwan, or Hong Kong, but still similar to that of other Korean study (10).
SHV-12, TEM-52, and SHV-2a were common in the late 1990s in Korea when compared to other countries (2). In this study, SHV-12-like enzyme remained prevalent (64 of 75), however SHV-2a and TEM-52 were found in only one and two isolates, respectively.
Recently, strains with CTX-M type ESBLs have been increasingly isolated from many parts of the world (1). In Korea, a few strains harboring CTX-M-14 β-lactamase have been reported since 2000 (3, 10). In this study, nine of 106 isolates harbored CTX-M-14 type ESBLs, indicating a gradual increase of this type.
Since 1989 over 20 types of PABLs have been reported worldwide (6). In 1995, CMY-1b was reported in Korea with a one amino acid change, Asn346Ile, compared to CMY-1 (5). It was later renamed CMY-10 (12). This type was however not detected in this study; neither was CMY-11 which was first identified in E. coli in 2002 in Korea (13).
Korean Antimicrobial Surveillances showed that the cefoxitin-resistance rate of K. pneumoniae increased from 14% in 1998 to 20% in 2001 (14), suggesting dissemination of PABL-producing isolates. In this study, 41 of 75 (54.7%) isolates were cefoxitin resistance, and 40 isolates had PABL genes. Isolates with blaCMY-2-like and blaDHA-1-like were detected in 21 and 17 isolates respectively, while blaCMY-1 was found in only two isolates.
CMY-2 (BIL-1, LAT-2) was first identified in K. pneumoniae isolates from Greece in 1990 (15). CMY-2 was the most prevalent and most geographically distributed PABL, with reports from Africa, Europe, India, Taiwan, and the United States (6). It is a concern that as is ACT-1 enzyme, CMY-2 together with reduced permeability can render the isolate resistant even to imipenem (16, 17).
DHA-1, an inducible PABL, which was first identified in Salmonella enteritidis isolated in Saudi Arabia in 1992, has been reported in several other countries including Korea (18, 19). DHA-1-producing K. pneumoniae isolates were reported to be especially prevalent in a Korean university hospital (20).
ESBL or PABL-producing strains were often resistant to aminoglycosides and fluoroquinolones, too. In our study, isolates with both ESBLs and PABLs, were more often resistant to cefoxitin, aminoglycosides, and levofloxacin.
The MIC90S of cefotaxime and ceftazidime for ESBL-producing strains were similar to those for strains producing both ESBLs and PABLs. Addition of clavulanic acid cefotaxime and ceftazidime reduced the MIC90S for ESBL producers, but not for strains producing both ESBLs and PABLs.
In this study, PFGE of XbaI-digested genomic DNA showed that many isolates had the patterns O (29 isolates) and M (13 isolates). Some isolates with different ESBLs, or both ESBLs and PABLs, showed a few identical patterns, implying that both clonal and horizontal spread, and the propensity of K. pneumoniae to acquire resistance genes, has a role for their high prevalence in this study.
In conclusion, SHV-12 type ESBL-producing K. pneumoniae are increasing among lower respiratory isolates. As well, it seems that CTX-M-14-producing isolates are emerging in Korea. It also seems that the coexistence of PABLs such as CMY-2 and DHA-1 is common. Spreading of ESBL-producing strains with PABL genes is a concern, as it causes limitations in the selection of antimicrobial agents for optimal treatment of patients.
Footnotes
This study was supported by a grant of the Korean Health 21 Research and Development Project, Ministry of Health and Welfare of Korea (01-PJ10-PG6-010GM03-002).
References
- 1.Bradford PA. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai H. The characteristics of extended-spectrum β-lactamases in Korean isolates of Enterobacteriaceae. Yonsei Med J. 1998;39:514–519. doi: 10.3349/ymj.1998.39.6.514. [DOI] [PubMed] [Google Scholar]
- 3.Pai H, Choi EH, Lee HJ, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol. 2001;39:3747–3749. doi: 10.1128/JCM.39.10.3747-3749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Chong Y, Schweighart S. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989;17:316–321. doi: 10.1007/BF01650718. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Chong Y, Lee K. Plasmid-encoded AmpC β-lactamases: How far have we gone 10 years after the discovery? Yonsei Med J. 1998;39:520–525. doi: 10.3349/ymj.1998.39.6.520. [DOI] [PubMed] [Google Scholar]
- 6.Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother. 2002;46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan JJ, Ko WC, Wu HM, Tsai SH, Chuang CL, Wu JJ. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a Teaching Hospital in Taiwan. J Clin Microbiol. 2004;42:5337–5340. doi: 10.1128/JCM.42.11.5337-5340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer JJ., III . Enterobacteriaceae: introduction and identification. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 7th ed. Washington, DC: ASM; 1999. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; Twelfth informational supplement. Wayne, PA: NCCLS; 2002. M100-S12. [Google Scholar]
- 10.Hong SG, Kim S, Jeong SH, Chang CL, Cho SR, Ahn JY, Shin JH, Lee HS, Song WK, Uh Y, Yum JH, Yong D, Lee K, Chong Y. Prevalence and diversity of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates in Korea. Korean J Clin Microbiol. 2003;6:149–155. [Google Scholar]
- 11.Perez-Perez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Jeong SH, Park YM. Characterization of blaCMY-10 a novel, plasmid-encoded AmpC-type β-lactamase gene in a clinical isolate of Enterobacter aerogenes. J Appl Microbiol. 2003;95:744–752. doi: 10.1046/j.1365-2672.2003.02040.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Kim JY, Lee GS, Cheon SH, An YJ, Jeong SH, Lee KJ. Characterization of blaCMY-11, an AmpC-type plasmid-mediated β-lactamase gene in a Korean clinical isolate of Escherichia coli. J Antimicrob Chemother. 2002;49:269–273. doi: 10.1093/jac/49.2.269. [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Jang SJ, Lee HJ, Ryoo N, Kim M, Hong SG, Chong Y. Increasing prevalence of vancomycin-resistant Enterococcus faecium, expanded-spectrum cephalosporin-resistant Klebsiella pneumoniae, and imipenem-resistant Pseudomonas aeruginosa in Korea: KONSAR study in 2001. J Korean Med Sci. 2004;19:8–14. doi: 10.3346/jkms.2004.19.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmid β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother. 1996;40:221–224. doi: 10.1128/aac.40.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poirel L, Heritier C, Spicq C, Nordmann P. In vivo acquisition of high-level resistance to imipenem in Escherichia coli. J Clin Microbiol. 2004;42:3831–3833. doi: 10.1128/JCM.42.8.3831-3833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford PA, Urban C, Mariano N, Projan SJ, Rahal JJ, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaillot O, Clement C, Simonet M, Philippon A. Novel transferable β-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J Antimicrob Chemother. 1997;39:85–87. doi: 10.1093/jac/39.1.85. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Park YJ, Lee SO, Song W, Jeong SH, Yoo YA, Lee KY. Case report: Bacteremia due to Salmonella enterica serotype Montevideo producing plasmid-mediated AmpC beta-lactamase (DHA-1) Ann Clin Lab Sci. 2004;34:214–217. [PubMed] [Google Scholar]
- 20.Pai H, Kang CI, Byeon JH, Lee KD, Park WB, Kim HB, Kim EC, Oh MD, Choe KW. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:3720–3728. doi: 10.1128/AAC.48.10.3720-3728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]