Abstract
The central nervous system (CNS) effects of mental stress in patients with coronary artery disease (CAD) are unexplored. The present study used positron emission tomography (PET) to measure brain correlates of mental stress induced by an arithmetic serial subtraction task in CAD and healthy subjects. Mental stress resulted in hyperactivation in CAD patients compared with healthy subjects in several brain areas including the left parietal cortex [angular gyrus/parallel sulcus (area 39)], left anterior cingulate (area 32), right visual association cortex (area 18), left fusiform gyrus, and cerebellum. These same regions were activated within the CAD patient group during mental stress versus control conditions. In the group of healthy subjects, activation was significant only in the left inferior frontal gyrus during mental stress compared with counting control. Decreases in blood flow also were produced by mental stress in CAD versus healthy subjects in right thalamus (lateral dorsal, lateral posterior), right superior frontal gyrus (areas 32, 24, and 10), and right middle temporal gyrus (area 21) (in the region of the auditory association cortex). Of particular interest, a subgroup of CAD patients that developed painless myocardial ischemia during mental stress had hyperactivation in the left hippocampus and inferior parietal lobule (area 40), left middle (area 10) and superior frontal gyrus (area 8), temporal pole, and visual association cortex (area 18), and a concomitant decrease in activation observed in the anterior cingulate bilaterally, right middle and superior frontal gyri, and right visual association cortex (area 18) compared with CAD patients without myocardial ischemia. These findings demonstrate an exaggerated cerebral cortical response and exaggerated asymmetry to mental stress in individuals with CAD.
Coronary artery disease (CAD) is a major cause of death and disability. The traditional risk factors (e.g., smoking, cholesterol, hypertension), however, account for only approximately 50% of new cases (1). The influence of psychosocial stressors in the clinical presentation of CAD is widely recognized (2). Studies have found a relationship between increasing levels of self-reported stress and the incidence of myocardial infarction and sudden death (3). In populations with established CAD, high levels of stress (4) are associated with recurrent coronary events and cardiac death. Our group and others have used a reproducible laboratory model of stress (mental arithmetic) to explore the influence of stress in CAD. We found that a specific psychological profile, characterized by emotional reactivity, anger, and hostility, is associated with mental stress-induced myocardial ischemia (5) and a poor prognosis (6). The mechanism of how psychosocial stressors influence the presentation of CAD, however, is unknown.
Studies concerning the influence of mental stress in heart disease have focused on vascular physiology and psychological variables (5, 7) whereas the central nervous system effects of stress in CAD patients have not been explored. Experimental studies in animals (8–10) have identified cortical and subcortical brain areas that have direct and indirect inputs to peripheral autonomic and hormonal systems that could have an important influence on myocardial function. Increased stress-induced activation in these brain areas could lead to myocardial ischemia, either through increased sympathetic or hormonal activation, or through direct inputs to the heart.
We have designed a positron emission tomography (PET) study to assess brain blood flow during mental stress in patients with CAD. We compared patients with CAD and healthy subjects both with and without mental stress. We hypothesized that patients with CAD would develop increased activation in cortical and subcortical areas involved in memory, emotion, and the stress response.
MATERIALS AND METHODS
Population.
Subjects were 10 right-handed male patients with established CAD and 6 age-matched, right-handed normal male volunteers. All subjects were without history of psychiatric illness based on interviews by a psychiatrist using Diagnostic and Statistical Manual IV criteria. The diagnosis of CAD was established by standard clinical myocardial perfusion testing, which demonstrated myocardial ischemia. The identification of normal subjects was based on screening history, physical examination, and exercise treadmill testing to exclude CAD in volunteers over age 40. Exclusion criteria for the subjects studied included myocardial infarction within 3 months of the study, coronary artery bypass surgery or angioplasty within 3 months of the study, major cardiac rhythm disturbances, congestive heart failure, diabetes, incapacitating or life-threatening illness, major psychiatric illness or substance abuse, history of cerebral vascular disease, neurological disorder, and administration of psychotropic medication.
Research Design and Imaging Protocol (Fig. 1).
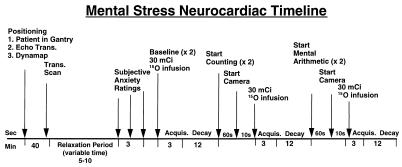
Figure 1.
Timeline of PET mental stress neurocardiac study. Subjects underwent a total of six scans: two baseline, two counting control, and two mental arithmetic. O-15 (30 mCi) was administered during each scan.
Subjects reported to the imaging center and signed an informed-consent form approved by the institutional human investigation committee. An intravenous line was established and electrocardiographic leads to monitor heart rhythm were placed. The subject was placed in the camera gantry (Posicam; Positron Corp., Houston), and the head was positioned along the canthomeatal line. After positioning, a transmission scan using a 68Ga/68Ge-rotating-rod source was used for attenuation correction. An echocardiographic probe to evaluate cardiac function was placed on the chest, and an acoustic window was established for measuring indexes of cardiac performance. Cardiac performance was measured by the presence or absence of wall motion abnormalities during the three experimental conditions throughout the procedure. A dynamap device for automated monitoring of heart rate and blood pressure was placed on the subject’s dominant arm. The subject was instructed to rest and a baseline echocardiogram was acquired. At the initiation of the protocol, subjects were instructed to immediately report symptoms of chest pain.
The subjects were asked to indicate their emotional state (calm/relaxed, anxious/tense, angry/irritated) on a five-point Likert scale. Likert scales of emotional state were collected every 5 min until there were two consecutive, unchanged ratings indicating that a stable baseline was achieved. Scans were conducted in a dimly lit room with individuals having their eyes open. O-15 water was prepared with an onsite cyclotron (-11MEV, CTI, Knoxville, TN). Study subjects received 30 mCi (1 Ci = 37 GBq) intravenous bolus O-15 water for each of the six scans.
Subjects underwent six PET scans. Two scans were conducted at baseline, two scans were conducted during a counting control (subject counts backward aloud from 500 to control for activation of verbal and auditory centers peripheral to the mental stress condition), and two scans were conducted during mental stress (serial arithmetic subtraction from a four-digit number performed aloud). The order of task presentation was consistent for all subjects (Fig. 1). A fixed order was selected to eliminate contamination of the control condition by increased emotional reactivity elicited by mental stress. Each scan lasted 2 min, with a PET scan acquisition beginning 1 min into the condition and lasting until the end of the condition. The timing of isotope injection was based on rate of isotope decay and our data, which indicate that myocardial ischemia induced by mental stress occurs within 1 min of initiation of testing in susceptible patients (5). The cognitive tasks, including mental arithmetic, were administrated by a research psychologist.
Mental Stress Testing.
The mental stress protocol has been described previously (5) and consists of an experimental task period, preceded by appropriate control periods. During these periods, the PET activation studies are accomplished. The specific laboratory task was mental arithmetic (e.g., serial subtraction and/or addition).
The arithmetic task consists of serial subtraction from a number spoken by the experimenter. The subjects were instructed to provide their response verbally. To control for task difficulty, each subject’s level of performance was adjusted to the same criterion (90% correct in 10 trials) in the following manner. For most subjects, this involved serial subtraction by using the number 7; for those patients who were unable to perform this task, easier subtraction was provided for (e.g., by 4, 3, or 2). Throughout the task, the patient was prompted for faster performance while the base number from which they were subtracting was changed (e.g., starting the patient with the number 1,013, and changing to 436 after they have performed a number of successful serial subtractions). In addition to these methods, errors were corrected in a harsh tone, thereby providing an element of harassment and increasing the pressure on task performance. The frequency of prompting and changing of base number was contingent on the patient’s performance, with an error rate of 1 error in 10 subtractions as the goal. With these methods, individual differences in mathematical ability were corrected for and the stressfulness of the task across patients was maintained.
Cardiac Echocardiography.
Two-dimensional echocardiography was performed during PET with the patient lying supine within the camera gantry. A phased-array sector scanner (Hewlett–Packard SONOS 1000) was used. Left ventricular images were obtained during each testing condition and recorded continuously on videotape. A suitable echocardiographic window in the lower parasternal area for parasternal long and short axis views was obtained. Similarly, a suitable echocardiographic window in the region of electrocardiographic leads V4 or V5, to record standard apical two- and four-chamber views, was performed. Electrodes for echocardiographic ECG tracings were placed carefully before the study was initiated to obtain a high-quality ECG tracing with a prominent R or S wave as a time marker for subsequent digitization and image storage. All recordings were videotaped continuously throughout the stress protocol, with periodic switching between recording sites as diagnostically adequate images were recorded at each site. When stress-induced wall motion abnormalities occurred, they were apparent within 30 sec of the onset of the mental stress condition. All studies were analyzed for wall motion abnormalities.
DATA ANALYSIS
PET.
Images were reconstructed and analyzed on a SunSparc Workstation (Sun Computer, Mountain View, CA). Images were analyzed by using statistical parametric mapping (spm95) to compare areas of increased and decreased blood flow between control and mental stress conditions. A voxel-by-voxel analysis in CAD patients and healthy subjects by using multiple linear regression in accordance with the general linear model was performed. Analyses were performed by using analysis of covariance, with global blood flow considered as a confounding covariant. In the current study, there was an a priori hypothesis on increased activation in cortical and subcortical areas involved in memory, cognition, and emotion in CAD patients relative to controls. Areas of increased blood flow with mental stress versus the counting control conditions in hypothesized areas at a threshold of P < 0.01 (z score > 2.33) were noted within CAD and healthy subject groups. In addition, a between-groups comparison was performed by using the general linear model taking into account group mean values and variance at each voxel. Results of analyses (areas of increased and decreased blood flow) with mental stress versus counting control in voxels corresponds to the value of the t statistic for that voxel (11). Location of areas of activation were identified as the distance from the anterior commissure in mm, with x, y, and z coordinates, using the standard Talairach coordinate space (12). The correlation between increase in rate pressure product (heart rate × blood pressure) during mental stress and cerebral blood flow response to mental stress also was examined by using spm95. Images for each patient were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration of each slice and adjusted to the nominal value of 50 ml/min per 100 g. The data were then rescaled and smoothed with a three-dimensional Gaussian filter to 16 mm full-sixth half-maximum. Images from the two scans conducted within each condition (two baseline, two counting control, two arithmetic stress) were pooled for subjects, and images of mean difference with values in z score units were calculated by subtracting pooled scans for the counting control condition from the mental stress condition. Areas of increased and decreased blood flow with the mental stress condition (compared with the counting control condition) then were compared between groups, and areas of activation were expressed in terms of standard three-dimensional anatomical coordinates (x, y, and z axes) (12).
Cardiac Echocardiography.
For the echocardiographic analysis, representative cycles of baseline, counting control, and mental stress were positioned side by side on a quad-screen format. The images were corrected for differences in heart rate. The left ventricle was divided into eight short-axis segments (two anterior, two septal, two inferior, two lateral). Wall motion was scored as follows: normal (score = 1), hypokinetic (score = 2), akinetic (score = 3), or dyskinetic (score = 4) as determined by two independent observers blinded to the condition and clinical status (CAD patient vs. healthy volunteer) of the subjects (I.C. and R.S.). Wall motion during the baseline was compared with wall motion during counting control and mental stress. Mental stress-induced myocardial ischemia was determined from echocardiographic data. The criterion included an increase in scored wall motion abnormality from baseline to mental stress condition (score mental stress − score baseline).
Hemodynamics.
The heart rate and blood pressure changes from counting control to the mental stress condition were recorded. The product of heart rate and blood pressure (rate pressure product) was calculated for counting and mental stress conditions. These hemodynamic measurements were compared between groups and counting and mental stress conditions by using ANOVA. Repeated measures of ANOVA was used to measure any association between brain activation and heart rate–blood pressure double product.
RESULTS
Hemodynamics.
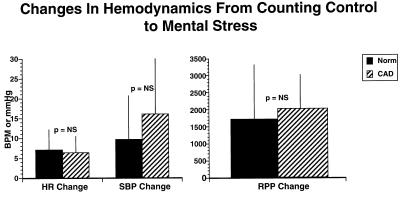
The heart rate and blood pressure changes from counting control to the mental stress condition reinforced the adequacy of the stress in normal subjects and those with heart disease (Fig. 2), and were comparable in both groups. The increase in rate pressure product revealed an appropriate hemodynamic response at peak stress that did not differ between the healthy and CAD groups. These responses are similar to other reports that have shown that hemodynamic cardiovascular response to stress is not as robust as in response to exercise (5, 13, 14).
Figure 2.
Effect of mental stress of heart rate and blood pressure in patients with coronary artery disease (CAD) and normal subjects. There is no significant difference in the increase in heart rate (HR), systolic blood pressure (SBP), and rate pressure product (RPP) from control to mental stress conditions among CAD and normal subjects.
CNS Activation Patterns.
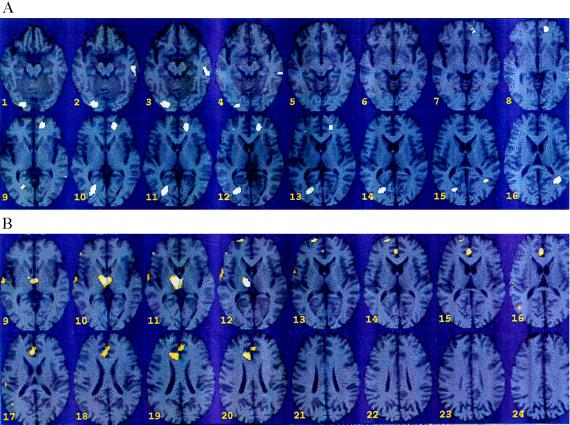
Mental stress resulted in increased activation (between mental stress and counting) in CAD patients compared with healthy subjects in several brain areas including the left parietal cortex [angular gyrus/parallel sulcus (area 39)], left anterior cingulate (area 32), right visual association cortex (area 18), left fusiform gyrus, and cerebellum (Fig. 3A; z score > 2.33; P < 0.01). These same regions were activated within the CAD patient group during mental stress versus control conditions (z score > 3.0; P < 0.001). In the group of healthy subjects, activation was significant only in the left inferior frontal gyrus (z score > 3.0; P < 0.001) during mental stress compared with counting controls. Decreases in blood flow were also produced by mental stress in CAD versus healthy subjects in right thalamus (DL, LP), right superior frontal gyrus (areas 32, 24, and 10), and right middle temporal gyrus (area 21) (in the region of the auditory association cortex) (Fig. 3B; z score > 3.00; P < 0.001).
Figure 3.
(A) Statistical parametric map overlaid on an MRI template of areas of increased blood flow with mental stress in CAD patients (n = 10) versus healthy subjects (n = 6). There were significant increases in visual association cortex (18) [(Talairach coordinates) x = −26, y = −80, z = 12, z score = 3.32, P < 0.001]; anterior cingulate (32) (x = 14, y = 46, z = 4, z score = 3.08, P < 0.001); cerebellum (x = −22, y = −90, z = −20, z score = 3.00, P < 0.001); left parietal cortex (x = 36, y = −58, z = 36, z score = 2.94, P < 0.002); left fusiform gyrus (x = 52, y = −32, z = −20, z score = 2.88, P < 0.002). (B) Statistical parametric map overlaid on an MRI template of areas of decreased blood flow with mental stress versus counting control in CAD patients (n = 10) versus healthy controls (n = 6). There were significant decreases in thalamus (x = −10, y = −18, z = 16, z score = 4.07, P < 0.001); superior frontal gyrus/cingulate gyrus (32, 24) (x = −6, y = 32, z = 32, z score = 3.29, P < 0.001); superior frontal gyrus (10) (x = −20, y = 58, z = 20, z score −3.15, P < 0.001); right middle temporal gyrus (21) (x = − 60, y = −18, z = 4, z score = 3.08, P < 0.001).
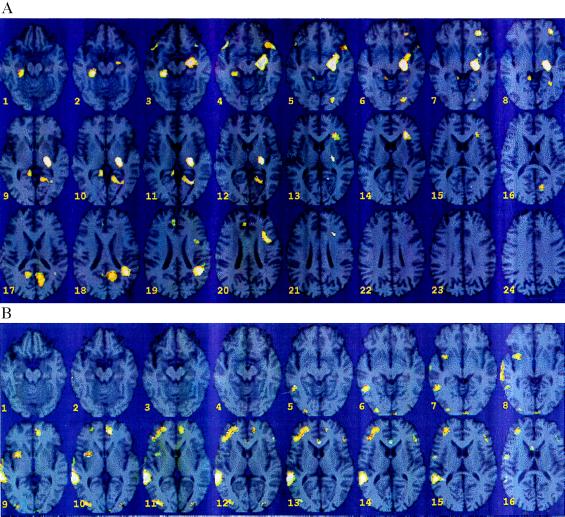
During the course of the study, 3 of 10 CAD patients (33%) demonstrated a worsening of their heart function during mental stress (two inferior, one anterior myocardial segments), which is an indicator of myocardial ischemia. The provocation of myocardial ischemia provided an opportunity to compare patients with and without mental stress-induced myocardial ischemia within the CAD group. There was significantly greater activation in left hippocampus and left parietal cortex (inferior parietal lobule) (area 40), left superior and middle frontal gyrus (areas 8 and 10), left visual association cortex (area 18), and right temporal pole (area 38) in CAD patients with mental stress-induced myocardial ischemia compared with CAD patients without myocardial ischemia (Fig. 4A; z score > 3.00; P < 0.001). Of interest, there was a concomitant decrease in activation observed in the right superior and middle temporal gyri (areas 22 and 37), right middle frontal gyrus (area 10), right visual association cortex (area 18) (Fig. 4B; z score >3.00; P < 0.001). There was bilateral deactivation of the anterior cingulate (area 24), (left: z score > 3.00, P < 0.001; right: z score = 2.89, P = 0.002).
Figure 4.
(A) Statistical parametric map overlaid on an MRI template of areas of increased blood flow with mental stress versus counting control in mental stress ischemic CAD patients (n = 3) versus mental stress nonischemic CAD patients (n = 7). There were significant increases in left hippocampus (x = 22, y = −22, z = −8, z score = 4.43, P < 0.001); left parietal cortex (inferior parietal lobule) (40) (x = 40, y = −54, z = 44, z score = 3.80, P < 0.001); left superior frontal gyrus (8) (x = 26, y = 16, z = 52, z score = 3.83, P < 0.001); left middle frontal gyrus (10) (x = 28, y = 36, z = −4, z score = 3.58, P < 0.001); posterior cingulate, corpus callosum (x = 24, y = −62, z = 12, z score = 3.65, P < 0.001); right hippocampus (x = −26, y = −36, z = −24, z score = 3.44, P < 0.001); temporal pole (38) (x = −44, y = 14, z = −16, z score = 3.45, P < 0.001); precuneus (31) (x = −14, y = −58, z = 36, z score = 3.19, P < 0.001); visual association cortex (18) (x = 22, y = −86, z = −12, z score = 3.15, P < 0.001). (B) Statistical parametric map overlaid on an MRI template of areas of decreased blood flow with mental stress versus counting control in mental stress ischemic CAD patients (n = 3) versus mental stress nonischemic CAD patients (n = 7). There were significant decreases in right superior temporal gyrus (22) (x = −58, y = −42, z = 12, z score = 4.84, P < 0.001); right-middle temporal gyrus (37) (x = −46, y = −56, z = −8, z score = 3.60, P < 0.001); left anterior cingulate (x = 8, y = 42, z = 8, z score = 3.91, P < 0.001); right anterior cingulate (24) (x = −8, y = 6, z = 36, z score = 2.89, P = 0.002); visual association cortex (18) (x = −36, y = −88, z = 12, z score = 3.73, P < 0.001); area 19 (x = 34, y = −76, z = 36, z score = 2.66, P < 0.001); right-middle frontal gyrus (10) (x = −24, y = 48, z = 16, z score = 3.61, P < 0.001); insula (x = −32, y = 0, z = 0, z score = 3.45, P < 0.001); precentral (x = −52, y = −6, z = 32, z score = 2.81, P = 0.002); lingual gyrus (x = −16, y = −70, z = 0, z score = 2.39, P = 0.008).
Myocardial ischemia often is associated and aggravated by increases in heart rate and blood pressure. It is interesting that although both CAD patients and healthy subjects had comparable increases in rate pressure product during mental stress (Fig. 2), significant correlations between rate pressure product and cortical blood flow were found only in CAD patients but not in healthy subjects. There was a positive correlation in cerebellum (x = −6, y = −76, z = −28, z score = 3.79, P < 0.001); midbrain (periaqueductal gray) (x = −4, y = −40, z = −8, z score = 3.67, P < 0.001); right inferior frontal gyrus (47) (x = −48, y = 26, z = −4, z score = 3.66, P < 0.001); middle frontal gyrus/orbitofrontal (11) (x = 26, y = 38, z = −12, z score = 3.62, P < 0.001).
DISCUSSION
Mental stress resulted in hyperactivation in CAD patients compared with healthy subjects in several brain areas including the left parietal cortex (angular gyrus), anterior cingulate, visual association cortex, left fusiform gyrus, and cerebellum (Fig. 3A). Decreased blood flow was found in the right: parietal cortex, thalamus, superior frontal gyrus (areas 32, 24, and 10), and middle temporal gyrus (area 21). The regions found to be activated in CAD patients during mental stress are well documented as being involved in visual and verbal memory and presumably are integral to performance on the mental arithmetic task. Indeed, some of these areas, e.g., the angular gyrus (15–17) and the fusiform gyrus (17–24) have been specifically implicated in mental calculations (15–17) and/or in the relevant processes of working memory (25), attention (19, 20, 26–29), visual imagery (18, 21–24, 30–33), and semantic memory/word forming (34–37). Even the cerebellum, which traditionally has been considered to be involved in coordination of movement, is also activated in declarative memory tasks (38). Many of these same regions (angular gyrus, anterior cingulate, visual association cortex) have also been specifically implicated in stress and emotion (36, 37, 39, 40).
The greater increases in blood flow observed during mental stress in CAD patients, compared with normals, may be interpreted in several ways. The greater activation of a network of cortical areas involved in visual imagery and mental activity may indicate that for these CAD patients the task of mental calculation, which engages these areas, required more effort. An alternative explanation is that these findings demonstrate an activation pattern driven by emotional variables. We found that the added component of stress and emotionality increased activation of those cortical regions that are known or presumed to be engaged by the specific mental task performance required in the present study. The considerable overlap between brain regions activated during stress/emotion and cognition/memory (41) may indicate that these processes are interdependent, intermingled, or largely inseparable at the cortical level.
During the course of the study, 3 of 10 CAD patients (33%) demonstrated a worsening of their heart function during mental stress, which is an indicator of myocardial ischemia. These patients exhibited significantly greater stress-induced activation in the left hippocampus and left inferior parietal lobule, left superior and middle frontal gyrus, left temporal pole, and left visual association cortex than CAD patients without myocardial ischemia (Fig. 4A). This hyperresponsiveness is interpretable in terms of task demand, because many of these areas also have been related to mnemonic functions (25–27, 42–55), mental calculations (56), verbal memory encoding (57–60), and retrieval (38, 60, 61) as well as in the stress response (46, 62, 63). Although task performance was phenomenologically equated between groups (as evidenced by similar peripheral hemodynamic response), there were different cortical responses to mental stress. Thus, our findings demonstrate that equating performance in cognitive tasks does not necessarily equate for mental effort.
The hemispheric patterns of activation and deactivation (activation in visually related areas in the left hemisphere; deactivation inclusive of other areas in the right hemisphere) are similar to those found in PET studies of memory and cognition in normal subjects (38, 43, 44, 57). Thus, the pattern of hemispheric asymmetry during mental calculation suggests that mental stress exacerbates a normal hemispheric asymmetry during mentation, generally, and especially mental calculation. Specifically, depression of right hemisphere areas may mean that the strategies of the right hemisphere are underutilized particularly by CAD patients vulnerable to mental stress-induced myocardial ischemia.
Increases in heart rate and blood pressure augment myocardial demand; thus these hemodynamic variables are major determinants of myocardial ischemia. Despite comparable increases in rate pressure product during mental stress between CAD and healthy subjects, significant correlations between rate pressure product and cortical blood flow were found only in CAD patients and not in healthy volunteers. These correlations, which occurred in the right middle and inferior frontal gyri, as well as in the cerebellum and midbrain, may implicate these areas in the regulation of peripheral autonomic and hormonal response to stress or, as likely, again reflect the role of cognitive demand in provoking cardiac ischemia. In any event, the present findings demonstrate that mental stress has distinct cortical correlates that may serve to augment sympathetic stimulation in part through central sympathetic and neuroendocrine-mediated pathways.
Ischemic responses to mental stress occurring in daily life often are not associated with typical anginal pain. Therefore, it is of interest that there was bilateral deactivation in the anterior cingulate with the onset of ischemic-induced wall motion abnormalities in the absence of pain (angina pectoris). The anterior cingulate is involved in pain perception (64). Other areas with decreased blood flow were observed in the right hemisphere: superior and middle temporal lobe (auditory cortex), and the visual association cortex (Fig. 4B) (32, 33).
The identification of brain regions that mediate the physiological effects of stress on CAD may have important treatment implications. For instance, PET studies in patients with psychiatric disorders related to abnormalities of stress and emotion have shown alterations in similar brain regions as those in the current study, including hippocampus, prefrontal, parietal and temporal cortex, and cingulate (65–69). Treatment resulted in a reversal of deficits in some studies (57). One could envision specific cognitive–behavioral and/or pharmacologic treatment strategies that could reverse alterations in brain function in specific brain regions in those CAD patients where mental stress plays an important role. The current study provides the basis for identifiable physiologic markers of the mental stress response in CAD patients, which may be used to develop novel therapeutic strategies and evaluate the effectiveness of treatment.
Acknowledgments
This work was supported by a Merit Review Grant, Department of Veteran Affairs, to R.S. and M.B.
ABBREVIATIONS
- PET
positron emission tomography
- CAD
coronary artery disease
References
- 1.Anderson K M, Wilson P W F, Odell P M, Kannel W B. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 2.Pepine C D J. Ann Int Med. 1996;124:1006–1007. doi: 10.7326/0003-4819-124-11-199606010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Tibblin G, Wilhelmsen L. Am J Cradiol. 1991;68:1171–1175. doi: 10.1016/0002-9149(91)90189-r. [DOI] [PubMed] [Google Scholar]
- 4.Ruberman W, Weinblatt E, Goldberg J D, Chaudrey B S. N Engl J Med. 1984;311:552–559. doi: 10.1056/NEJM198408303110902. [DOI] [PubMed] [Google Scholar]
- 5.Burg M M, Jain D, Soufer R, Kerns R D, Zaret B L. J Am Coll Cardiol. 1993;22:440–448. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Burg M M, Soufer R, Zaret B L. Am J Cardiol. 1995;76:31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 7.Yeung A C, Vekshtein V I, Krantz D S. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 8.Fulton J F. Physiology of the Nervous System. New York: Oxford Univ. Press; 1949. p. 12. , 202–235. [Google Scholar]
- 9.Skinner J E. Electroencephalogr Clin Neurophysiol Suppl. 1991;42:270–283. [PubMed] [Google Scholar]
- 10.LeDoux J L. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- 11.Friston K J, Frith C D, Liddle P F, Dolan R J, Lammertsma A A, Frackowiak R S. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- 12.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 13.Becker L C, Pepine C J, Bonsall R, Cohen J D, Goldberg A D, Coghlan C, Stone P H, Forman S, Knatteurud G, Sheps D S, Kaufmann P G. Circulation. 1996;94:2768–2777. doi: 10.1161/01.cir.94.11.2768. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener J S, Krantz D S, Howell R H, Hecht G M, Klein J, Falconer J J, Rozanski A. J Am Coll Cardiol. 1994;24:1645–1651. doi: 10.1016/0735-1097(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 15.Gold M, Adair J C, Daniel H, Heilman J, Heilman K. Cortex. 1995;31:267–283. doi: 10.1016/s0010-9452(13)80362-0. [DOI] [PubMed] [Google Scholar]
- 16.Roland P E, Friberg L. J Neurophysiol. 1985;31:1219–1243. doi: 10.1152/jn.1985.53.5.1219. [DOI] [PubMed] [Google Scholar]
- 17.Dehaene S, Tzourio N, Frak V, Raynaud L, Cohen L, Mehler J, Mazoyer B. Neuropsychologia. 1996;34:1097–1106. doi: 10.1016/0028-3932(96)00027-9. [DOI] [PubMed] [Google Scholar]
- 18.Nobre A, Allison T, McCarthy G. Nature (London) 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- 19.Heinze H J, Mangun G R, Burchert W, Hinrichs H, Scholz M, Munte T F, Gos A, Scherg M, Johannes S, Hundeshagen H, et al. Nature (London) 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 20.Gulyas B, Roland P E, Heywood C A, Popplewell D A, Cowey A. NeuroReport. 1994;5:2367–2371. doi: 10.1097/00001756-199411000-00039. [DOI] [PubMed] [Google Scholar]
- 21.Allison T, McCarthy G, Nobre A, Puce A, Belger A. Cerebral Cortex. 1994;5:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- 22.Puce A, Allison T, Gore J C, McCarthy G. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- 23.Puce A, Allison T, Asgari M, Gore J, McCarthy G. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanwisher N, McDermott J, Chun M M. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swartz B E, Halgren E, Simpkins F, Mandelkern M. Life Sci. 1996;58:2057–2064. doi: 10.1016/0024-3205(96)00198-1. [DOI] [PubMed] [Google Scholar]
- 26.Cohen R M, Semple W E, Gross M, King A C, Nordahl T E. Exp Brain Res. 1992;92:165–172. doi: 10.1007/BF00230392. [DOI] [PubMed] [Google Scholar]
- 27.Coull J T, Frith C D, Frackowiak R S J, Graspy P M. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 28.Devinsky O, Morrell M J, Vogt B A. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 29.Pardo J V, Pardo P J, Janer K W, Raichle M E. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawashima R, Roland P E, O’Sullivan B T. Cereb Cortex. 1995;2:111–122. doi: 10.1093/cercor/5.2.111. [DOI] [PubMed] [Google Scholar]
- 31.Roland P E, Gulyas B. Cereb Cortex. 1995;1:79–93. doi: 10.1093/cercor/5.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Moscovitch M, Kapur S, Kohler S, Houle S. Proc Natl Acad Sci USA. 1995;92:3721–3725. doi: 10.1073/pnas.92.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haxby J V, Horwitz B, Ungerleider L G, Maisog J M, Pietrini P, Grady C L. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demonet J F, Price C, Wise R, Frackowiak R S J. Neurosci Lett. 1994;182:25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 35.Penniello M J, Lambert J, Eustache F, Petit-Taboue M C, Barre L, Viader F, Morin P, Lechevalier B, Baron J C. Brain. 1995;118:697–706. doi: 10.1093/brain/118.3.697. [DOI] [PubMed] [Google Scholar]
- 36.Kosslyn S M, Shin L M, Thompson W L, McNally R J, Rauch S L, Pitman R K, Alpert N M. NeuroReport. 1996;7:1569–1576. doi: 10.1097/00001756-199607080-00007. [DOI] [PubMed] [Google Scholar]
- 37.Binder J R, Frost J A, Hammeke T A, Rao S M, Cox R W. Brain. 1996;119:1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen N C, O’Leary D S, Arndt S, Cizadlo T, Hurtig R, Rezai K, Leonard W G, Boles Ponto L L, Hichwa R D. Proc Natl Acad Sci USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bench C J, Frith C D, Grasby P M, Friston K J, Paulesu E, Frackowiak R S J, Dolan R J. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 40.George M S, Ketter T A, Gill D S, Haxby J V, Ungerleider L G, Herscovitch P, Post R. J Neuropsychiatry Clin Neurosci. 1993;5:384–394. doi: 10.1176/jnp.5.4.384. [DOI] [PubMed] [Google Scholar]
- 41.Leiner H C. Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- 42.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintum M A. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 43.Tulving E, Kapur S, Markowitsch H J, Craik F I M, Habib R, Houle S. Proc Natl Acad Sci USA. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grasby P M, Frith C D, Friston K, Frackowiak R S J, Dolan R J. Neurosci Lett. 1993;163:185–188. doi: 10.1016/0304-3940(93)90378-x. [DOI] [PubMed] [Google Scholar]
- 45.Grasby P M, Frith C D, Friston K J, Bench C, Frackowiak R S J, Dolan R J. Brain. 1993;116:1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Akshoomoff N A, Courchesne E. Behav Neurosci. 1992;106:731–738. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- 47.Squire L R, Szola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 48.Sapolsky R M, Uno H, Rebert C S, Finch C E. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldman-Rakic P S. In: Handbook of Physiology. Plum F, editor. Washington, DC: Am. Physiol. Soc.; 1987. , Section I, Vol. V, Part 1, Chapter 9, pp. 373–417. [Google Scholar]
- 50.Baddeley A. Science. 1995;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy G, Blamire A M, Puce A, Nobre A C, Bloch G, Hyder F, Goldman-Rakic P, Shulman R G. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy G, Charney D S, Innis R B. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen SE, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 54.Pardo J V, Fox P T, Raichle M E. Nature (London) 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 55.Goldman-Rakic P S. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 56.Burbaud P, Degreze P, Lafon P, Franconi J M, Bouligand B, Bioulac B, Caille J M, Allard M. J Neurophysiol. 1995;74:2194–2200. doi: 10.1152/jn.1995.74.5.2194. [DOI] [PubMed] [Google Scholar]
- 57.Fletcher P C, Frith C D, Grasby P M, Shallice T, Frackowiak R S J, Dolan R J. Brain. 1995;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 58.Kapur S, Craik F I M, Tulving E, Wilson A A, Houle S, Brown G M. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shallice T, Fletcher P, Frith C D, Grasby P, Frackowiak R S J, Dolan R J. Nature (London) 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 60.Tulving E, Kapur S, Craik F I M, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nyberg L, McIntosh A R, Houle S, Nilsson L G, Tulving E. Nature (London) 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- 62.Gray J A. The Neuropsychology of Anxiety. New York: Oxford Univ. Press; 1982. [Google Scholar]
- 63.McEwen B S, Gould E A, Sakai R R. Br J Psychiatry. 1992;160:18–24. [PubMed] [Google Scholar]
- 64.Talbot J D, Marrett S, Evans A C, Meyer E, Bushnell M C, Duncan G H. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 65.Bremner J D, Innis R B, Salomon R M, Staib L, Ng C K, Miller H L, Bronen R A, Duncan J, Krystal J H, Rich D, et al. Arch Gen Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 66.Bremner J D, Innis R B, Ng C K, Staib L, Duncan J, Bronen R, Zubal G, Rick D, Krystal J H, Dey H, et al. Arch Gen Psychiatry. 1997;54:146–156. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 67.Bremner J D, Randal P R, Scott T M, Bronen R A, Delaney R C, Seibyl J P, Southwick S M, McCarthy G, Charney D S, Innis R B. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bremner J D, Randall P, Vermetten E, Staib L, Bronen R A, Mazure C M, Capelli S, McCarthy G, Innis R B, Charney D S. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.George M S, Ketter T A, Post R M. Depression. 1994;2:59–72. [Google Scholar]