Abstract
CD26 is a T-cell costimulatory molecule with dipeptidyl peptidase IV (DPPIV) activity in its extracellular region. We previously reported that recombinant soluble CD26 enhances peripheral blood T-cell proliferation induced by the recall antigen tetanus toxoid (TT). Recently, we demonstrated that CD26 binds caveolin-1 on antigen-presenting cell (APC), and that residues 201–211 of CD26 along with the serine catalytic site at residue 630, which constitute a pocket structure of CD26/DPPIV, contribute to binding to caveolin-1 scaffolding domain. In addition, following CD26–caveolin-1 interaction on TT-loaded monocytes, caveolin-1 is phosphorylated, with linkage to NF-κB activation, followed by upregulation of CD86. Finally, reduced caveolin-1 expression on APC inhibits CD26-mediated CD86 upregulation and abrogates CD26 effect on TT-induced T-cell proliferation, and immunohistochemical studies revealed an infiltration of CD26+ T cells in the sublining region of rheumatoid synovium and high expression of caveolin-1 in the increased vasculature and synoviocytes of the rheumatoid synovium. Taken together, these results strongly suggest that CD26–cavolin-1 interaction plays a role in the upregulation of CD86 on TT-loaded APC and subsequent engagement with CD28 on T cells, leading to antigen-specific T-cell activation such as the T-cell-mediated antigen-specific response in rheumatoid arthritis.
Key words: Caveolin-1, CD26, Memory T cell, Rheumatoid arthritis (RA), Synovial cell
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the progressive destruction of cartilage and bone in the synovial joints, which is associated with proliferation of synovial cells and infiltration of activated memory T cells, antigen-presenting cells (APCs) and plasma cells.1 Proposed etiologies for RA include genetic predisposition, dysregulation of self-tolerance, immune dysregulation triggered by environmental agents, and subsequent transformation of synovial cells.1–3 Macrophages and/or T cells are important mediators of RA pathogenesis, with cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) being proven therapeutic targets. In fact, antagonists against such cytokines have been used recently as effective RA therapy, decreasing joint damage and slowing radiographic progression of disease in patients of RA with inadequate response to methotrexate.4–7 However, as many patients do not experience effective relief even with the use of these newer biological agents, additional novel therapeutic approaches are still needed.8–10
Major-histocompatibility-complex (MHC) class II phenotype such as HLA-DR1, DR-4 and DR-14 confers susceptibility to RA.11–14 MHC class II molecules present antigens to CD4+ T cells, suggesting an important role for T cells in the pathogenesis of RA. Moreover, the rheumatoid synovium contains activated T cells, providing further rationale for the proposal that T cells have an important role in RA.15,16 Antigen-presenting cells such as monocytes, macrophages, and dendritic cells are also present in the rheumatoid synovium,1 being activated and expressing both MHC class II and costimulatory molecules such as CD86 and CD80. These findings strongly suggest that the interaction between synovial T cells and APCs have a direct role in the progression of synovitis.2 Moreover, careful analysis of infiltrating synovial T cells has revealed a bias towards the TH1 phenotype.17,18 In particular, patients with autoimmune diseases such as multiple sclerosis, Graves’ disease, and RA have been found to have increased numbers of CD4+ CD26+ T cells in inflamed tissues as well as in their peripheral blood,19–22 with enhancement of CD26 expression in these autoimmune diseases correlating with disease severity. 19,20,23 In addition, we previously demonstrated that T cells migrating through endothelial cell monolayers in vitro express high levels of CD26.24 These findings imply that CD26+ T cells play an important role in the inflammation process and subsequent tissue damage in such diseases.
It is well established that T cells require at least two signals to be fully activated.25 The first signal is antigen-specific and is delivered by engagement of the T-cell receptor (TCR) complex with an MHC-peptide complex on APC. The second signal is exerted by the binding of a costimulatory receptor on T cells to a ligand on the APCs. A key costimulatory signal is provided by the interaction of CD28 on T cells with CD86 or CD80 on APCs. We showed previously that CD26 on T cells have a very strong costimulatory effect on CD4+ T-cell activation in response to memory antigen such as tetanus toxoid (TT).26–29 However, the molecular mechanism involved in the process of antigen-specific T-cell activation via CD26 has not been clearly elucidated. We recently demonstrated that caveolin- 1 on antigen-loaded monocytes is a binding partner of CD26 and that signaling downstream of caveolin-1 in APC is triggered by stimulation with exogenous CD26.30,31 Therefore, T-cell costimulation via CD26 as well as CD28 may have an important role in the pathophysiology of inflammatory diseases such as RA. In this review, we discuss various aspects of CD26 involvement in immune regulation and immune-mediated disorders such as RA, with a particular focus on the role of caveolin-1 as its key binding partner.
Structure and function of CD26
CD26 is a 110 kDa cell-surface glycoprotein that belongs to the serine protease family, and human CD26 is expressed on a variety of tissues including T lymphocytes, endothelial and epithelial cells. As shown in Fig. 1A, human CD26 is composed of 766 amino acids, including a short cytoplasmic domain of 6 amino acids, a transmembrane region of 24 amino acids, and an extracellular domain with dipeptidyl peptidase activity which selectively removes the N-terminal dipeptide from peptides with proline or alanine at the penultimate position (dipeptidyl peptidase IV, DPPIV).32 The amino acid sequence of human CD26 illustrates approximately 85% homology with the rat DPPIV enzyme and the mouse thymocyte activation molecule (THAM), the mouse homologue of human CD26.33 CD26 knockout (CD26-KO) mice with C57BL/6 background display an apparently normal phenotype.34,35 However, the percentage of CD4+ T cells is lower in the spleen lymphocyte population in the CD26-KO mice than in CD26-positive wild-type mice. After immunization of mice with PWM in vivo, serum levels of total IgG, IgG1, IgG2a and IgE were markedly decreased in CD26-KO mice than those in wild-type mice. Moreover, IL-4 and IL-2 level in sera of CD26-KO mice were decreased and production of interferon-gamma (IFN-γ) was delayed in response to PWM immunization. These results indicate that CD26 helps to regulate the development, maturation and migration of CD4+ T lymphocytes, cytokine secretion, T cell-dependent antibody production, and immunoglobulin isotype switching of B cells.34
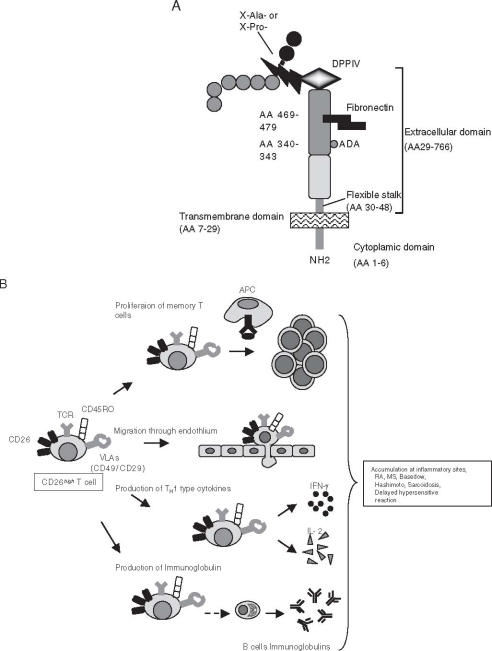
Fig. 1.
A Schematic diagram of human CD26 structure. Adenosine deaminase (ADA) binding site at residues 340–343, fibronectin binding site at residue 469–479, and dipeptidyl peptidase IV (DPPIV) enzyme activity at Ser630. X-Ala- or X-Pro- denotes peptides containing any amino acid at N-terminal position with alanine or proline at the penultimate position. B Cellular function of CD26high T cell. See text for details. APC, antigen-presenting cell; TCR, T-cell receptor; IFN, interferon; IL, interleukin
In contrast to the function of murine CD26, human CD26+ T cells exert diverse effects.28,36,37 CD26 is a membrane-associated ectopeptidase with DPPIV activity, and possible substrates of CD26/DPPIV include several critical cytokines and chemokines. Activity of RANTES (regulated on activation, normal T-cell expressed and secreted; CCL5) is altered by the enzymatic cleavage of DPPIV, as CD26/DPPIV-processed RANTES affects important activities such as those implicated in monocyte chemotaxis and HIV-1 infection.38,39 Other important chemokines that appear to be substrates of DPPIV enzymatic activity include eotaxin (CCL11), macrophage-derived chemokine (MDC) (CCL22), interferon inducible chemokines (CXCL10), and other chemokines involved in the inhibition of HIV infection.39 In addition, recent work showed that CD26 plays an important role in the mobilization of hematopoietic stem cell (HSC) and hematopoietic progenitor cells (HPC) induced by granulocyte colony-stimulating factor (G-CSF).40 One of the substrates of CD26/DPPIV is CXCL12 (SDF-1α, stromal cell-derived factor 1 alpha), an important chemokine that serves as a chemoattractant for HSC/HPC.41,42 It has been shown that CXCL12 can be selectively truncated in vitro by CD26/DPPIV, and the truncated molecule lacks the ability to induce migration of hematopoietic cells isolated from mouse bone marrow. Furthermore, treatment of mice with CD26/DPPIV inhibitors during the process of G-CSF mobilization results in a significant reduction in the number of mobilized HPC.40,41 Other exciting development regarding DPPIV involves its role in glucose metabolism, since inhibition of endogenous glucagon-like peptide 1 (GLP-1) degradation by reducing DPPIV activity is an alternative strategy for improving the incretin action of GLP-1 in vivo and regulating glucose levels.43 Selective small molecule inhibitors of DPPIV are currently being investigated in clinical trials for the treatment of impaired glucose tolerance and type 2 diabetes.44
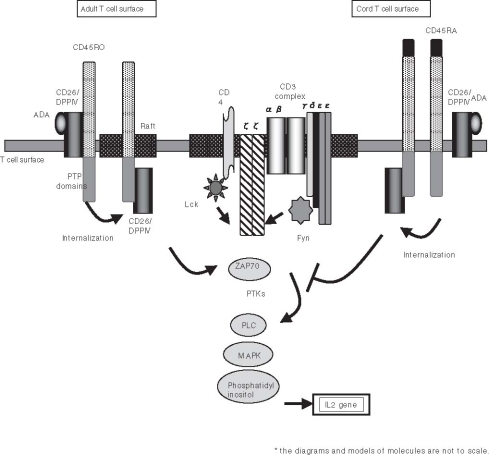
Besides its ability to regulate the effect of biological factors through DPPIV enzyme activity, CD26 has an essential role in human T-cell physiology, especially in response to memory antigens (Fig. 1B).28 Originally characterized as a T-cell differentiation antigen, CD26 is preferentially expressed on a specific population of T lymphocytes, the subset of CD4+ CD45RO+ memory T cells, and is upregulated following T-cell activation.29 Besides being a marker of T-cell activation, CD26 is also associated with T-cell signal transduction processes as a costimulatory molecule.27,37,45,46 In addition, CD26 serves as a functional collagen receptor with a role in T-cell activation, as well as having a potential role in thymic ontogeny (Fig. 2).26,46,47 The enzymatic activity of CD26 appears to be very important in enhancing cellular responses to external stimuli. For example, Jurkat cells transfected with wild type CD26 consistently demonstrate greater activation than parental CD26 negative Jurkat or cells transfected with CD26 mutated at the DPPIV enzymatic site.48 Furthermore, CD26 interacts with several molecules playing important roles in T-cell function. CD26 physically binds with adenosine deaminase (ADA), an enzyme that plays a key role in the development and function of lymphoid tissues.49–51 Adenosine deaminase is essential for purine metabolism, with the loss of ADA leading to a clinical syndrome characterized by severe immunodeficiency.52 When the ADA inhibitor pentostatin was used in the treatment of recurrent T-cell lymphomas, a significant reduction in circulating CD26+ T cells was observed in treated patients.53 This finding is consistent with the fact that there is a physical association between CD26 and ADA on the surface of T lymphocytes. CD26 also interacts with CD45RO, a tyrosine phosphatase with a critical role in T-cell signal transduction, at lipid rafts in peripheral blood T lymphocytes to modify cellular signaling events (Fig. 2).54,55 Interestingly, CD26 is associated with CD45 RA outside of lipid rafts in cord blood T cells, and the strong physical linkage of CD26 and CD45 RA may be responsible for the attenuation of cord blood T-cell activation signaling through CD26, which may in turn result in immature immune response and the relatively low incidence of severe graft-versus-host disease (GVHD) in cord blood transplantation (Fig. 2).56
Fig. 2.
Schematic diagram of CD26-associated molecules in T-cell receptor-mediated activation of human adult peripheral blood T cell and cord blood T cell
Since the 1970s, DPPIV-like activity has been reported in human serum. After identification of the ADA-binding protein of plasma as CD26, soluble form of CD26 protein was characterized in the serum and seminal fluids.57,58 In the previous report, we have shown that exogenous recombinant soluble CD26 (rsCD26) enhances the proliferative response of peripheral blood lymphocytes (PBLs) to stimulation with the soluble antigen tetanus toxoid (TT).59 More recently, we demonstrated that the target cells of rsCD26 are the CD14+ monocytes in the peripheral blood, and that rsCD26 upregulates CD86 expression, but not CD80 or HLA-DR antigen levels on monocytes.30 Significantly, mannose 6-phosphate/insulin-like growth factor II receptor (M6P/IGF-IIR) was identified as a platform molecule for CD26 interaction with APC.30 However, while both DPPIV-positive and DPPIV-negative rsCD26 are taken up by monocytes via M6P/IGF-IIR, only DPPIV-positive rsCD26 molecules affect CD86 upregulation on monocytes, thus suggesting that additional key factors may interact with CD26 in this process. We subsequently identified caveolin-1 on APC as a binding protein for CD26, and demonstrated that CD26 stimulation upregulates surface expression of CD86 on APC by means of caveolin-1 and enhances TT-mediated T-cell proliferation.31 In the next section of this review, we will focus on caveolin-1 as the binding protein of CD26 in the context of antigen-driven T-cell activation.
Structure and function of caveolin-1
Caveolin-1 was the first family member discovered, and demonstrated as a structural component and marker for caveolae and trans-Golgi derived transport vesicles.60,61 Caveolae were described as structures resembling “little caves” due to their appearance as 50- to 100-nm vesicular invaginations of the plasma membrane.62 Caveolin-1 is expressed in a wide variety of cell types, especially terminally differentiated cells such as endothelial cells, adipocytes, alveolar type I pneumocytes, macrophages, synoviocytes, and smooth muscle cells. Presently, caveolin-related proteins have been identified as caveolin-1, -2, and -3, all of which serve as protein markers for caveolae.63 The majority of caveolae in cells and tissues require only caveolin-1 expression for their proper formation, whereas caveolin-2 is not absolutely required, although the expression of caveolin-2 is tightly associated with the expression of caveolin-1.64,65 On the other hand, caveolin-3 is found in skeletal muscle tissue and cardiac myocytes.66 The three human genes encoding members of the caveolin family share significant homology. The caveolin-2 protein is approximately 38% identical and 58% similar to caveolin-1, while caveolin-3 is more closely related to caveolin-1, with 65% identity and 85% similarity.63 All three caveolins possess an invariant “FEDVIAEP” stretch within their hydrophilic N-terminal domains which are named the “caveolin signature motif.”67 Caveolin-1 is composed of 178 amino acid residues (Fig. 3A), and predominantly localized at the plasma membrane, demonstrating a punctuate staining patterns, and in Golgi-derived vesicles.60 Two isoforms of caveolin-1 (caveolin-1α and β) have been identified, with the β-isoform composed of 31 residue truncated N-terminus of caveolin-1α isoform.68 Caveolin-1 is composed of the N-terminal hydrophilic domain (residues 1–101), the oligomerization domain (residues 61–101), the scaffolding domain (SCD) (residues 82–101), the membrane spanning domain (residues 102–134), and the C-terminal lipid raft-anchoring domain (residues 135–178).63 As in trans-Golgi transport, caveolin-1 plays an important role in signal transduction via its SCD, which compartmentalizes a multitude of signaling molecules. 63,69 These include G proteins, epidermal growth factor receptor, insulin receptor, endothelial nitric oxide synthase (eNOS), nonreceptor tyrosine kinase (Src, Fyn, Yes), flotillins, Ser/Thr kinases (PKA, Raf, MAPK, PI3K, Grb2), and catenins.63,69 Other cellular functions of caveolin-1 are related to the lipid metabolism, especially to cholesterol scavenging in macrophages.70 However, it is unknown whether caveolin-1 also plays a role in signal transduction in APCs. Although CD26 was present in caveolae of fibroblast-like synoviocytes,71 direct CD26-cavolin-1 interaction and associated signaling events have not been demonstrated in immune cells. Interestingly, caveolin-1 knockout mice show defects in the angiogenic response to exogenous stimuli, such as Matrigel plugs containing angiogenic growth factors (bFGF) or tumors.72 In this context, angiogenic vessels density and penetration was significantly reduced in caveolin-1 null mice. Moreover, electron microscopic examination revealed incomplete de novo capillary formation in tumors implanted within caveolin-1 nullmice. Thus, it appears that caveolin-1 null mice have a defect in endothelial cell differentiation. This is consistent with in vitro observations demonstrating that overexpression of caveolin-1 enhances endothelial capillary-tube formation, while downregulation of caveolin-1 using an anti-sense approach blocks endothelial tube formation.73 With regards to inflammation and caveolin-1, a series of elegant experiments showed that caveolin-1 has a role in inflammation with association of eNOS.74 Using a cell permeable peptides link to the caveolin-1 scaffolding domain in aortic explants, the potent eNOS inhibiting activity of caveolin-1 was demonstrated. In vivo delivery of this peptide resulted in significant decreases in acute inflammation and edema resulting from vascular permeability. Taken together, these findings demonstrate an important relationship between caveolin-1 and vascularization, with implication for capillary formation in inflammatory processes.
Fig. 3.
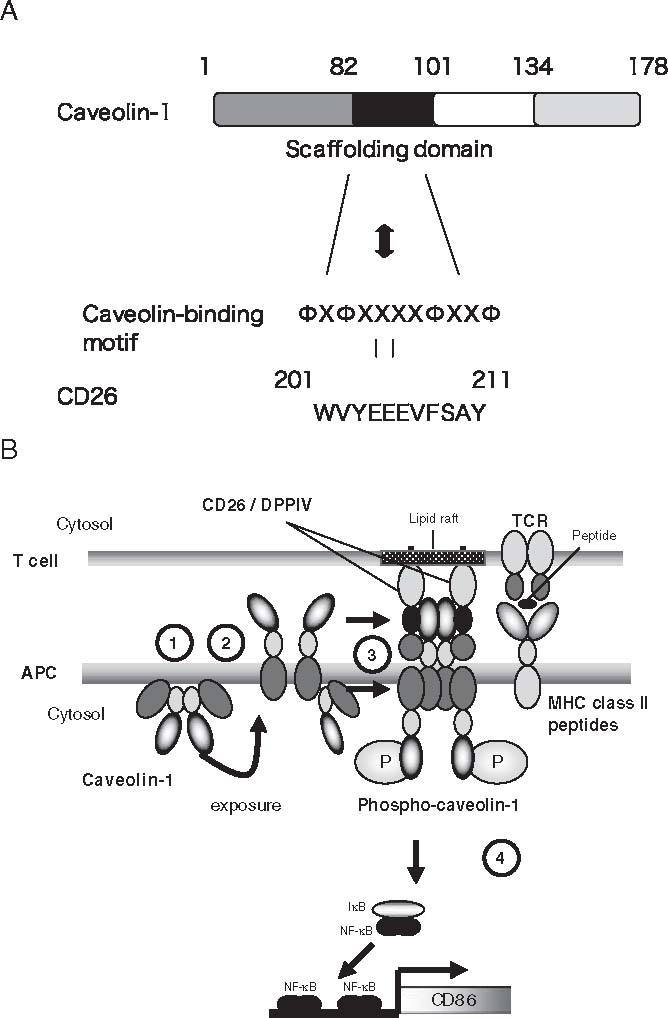
A Schematic representation of human caveolin-1. Residues 1–81 comprise the N-terminal region (NT; striped rectangle), residues 82–101 comprise the scaffolding domain (SCD; black rectangle), residues 102–134 comprise the transmembrane region (memb; open rectangle), and residues 135–178 comprise the C-terminal region (dotted rectangle). CD26 contains a caveolin-binding motif (ΦXΦXXXXΦXXΦ; Φ and X depict aromatic residue and any amino acid, respectively), specifically WVYEEEVFSAY in CD26. B Model for CD26-caveolin-1 interaction leading to upregulation of CD86. (1) Caveolin-1 in monocytes (antigen-presenting cells; APC) resides at the inner membrane. (2) After uptake of tetanus toxoid into monocytes via caveolae, part of the population of caveolin-1 is exposed on the outer cell surface of tetanus toxoid (TT)-loaded monocytes. (3) Migration of CD26+ antigen-specific memory T cells to areas of antigen-loaded APCs results in contact with TT antigen-presenting APC, leading to the association of CD26 and caveolin-1. Aggregation of caveolin-1 in the contact area occurs, presumably by homo-oligomerization (via its residues 61–101), followed by its phosphorylation. (4) Phosphorylated caveolin-1 (phospho-caveolin-1) transduces signaling leading to activation of NF-κB, resulting in CD86 upregulation. DPPIV, dipeptidyl peptidase IV; TCR, T-cell receptor; MHC, major histocompatibility complex
Caveolin-1: CD26 binding protein in APC
Since CD26 on human T cells was identified as an activation antigen and costimulatory molecule of the TCR complex, several binding proteins to CD26 have been described. As described above, multiple chemokines interact with CD26/ DPPIV as its substrates, and other proteins such as ADA, fibronectin, thromboxane A2 receptor, and CXCR4 are shown to be associated with CD26.49,75–78 However, the precise mechanism involved in T-cell activation in response to memory antigen such as TT remains to be clearly characterized. Recently, we demonstrated that CD26 binds to caveolin-1 on APC, and that residues 201–211 of CD26 along with the serine catalytic site at residue 630, which constitute a pocket structure of CD26/DPPIV, contribute to binding to the caveolin-1 scaffolding domain (Fig. 3A).31 This region in CD26 contains a caveolin-binding domain (CBD) (ΦXΦXXXXΦXXΦ; Φ and X depict aromatic residue and any amino acid, respectively), specifically WVYEEEVFSAY in CD26.48,69 These observations strongly support the notion that DPPIV enzyme activity is necessary to exert TCR-costimulatory activation via CD26.48 In addition, following CD26-cavolin-1 interaction on TT-loaded monocytes, caveolin-1 is phosphorylated, with linkage to NF-κB activation, followed by upregulation of CD86. Finally, reduced caveolin-1 expression on monocytes inhibits CD26-mediated CD86 upregulation and abrogates CD26 effect on TT-induced T-cell proliferation (Fig. 3B). Taken together, these results strongly suggest that CD26-cavolin-1 interaction plays a role in the upregulation of CD86 on TT-loaded monocytes and subsequent engagement with CD28 on T cells, leading to antigen-specific T-cell activation.
Caveolin-1 has been reported to be an integral membrane protein with a cytoplasmic N-terminal domain and a cytoplasmic C-terminal domain.63 Our data showed that the N-terminal domain of caveolin-1 was expressed on the cell surface of monocytes 12–24h after tetanus toxoid was loaded (Fig. 4A). Since tetanus toxoid was trafficked in cells through caveolae,79,80 caveolin-1 may be transported along with the peptide-MHC complex in APC, and is then expressed on cell surface by the antigen-processing machinery for T-cell contact.80–82 The data shown in Fig. 4B indicated that CD26 on activated memory T cells directly faces caveolin-1 on TT-loaded monocytes in the contact area, which is the immunological synapse for T cell-APC interaction. It is conceivable that the interaction of CD26 with caveolin-1 on antigen-loaded monocytes results in CD86 upregulation, therefore enhancing the subsequent interaction of CD86 and CD28 on T cells to induce antigen-specific T-cell proliferation and activation.
Fig. 4.
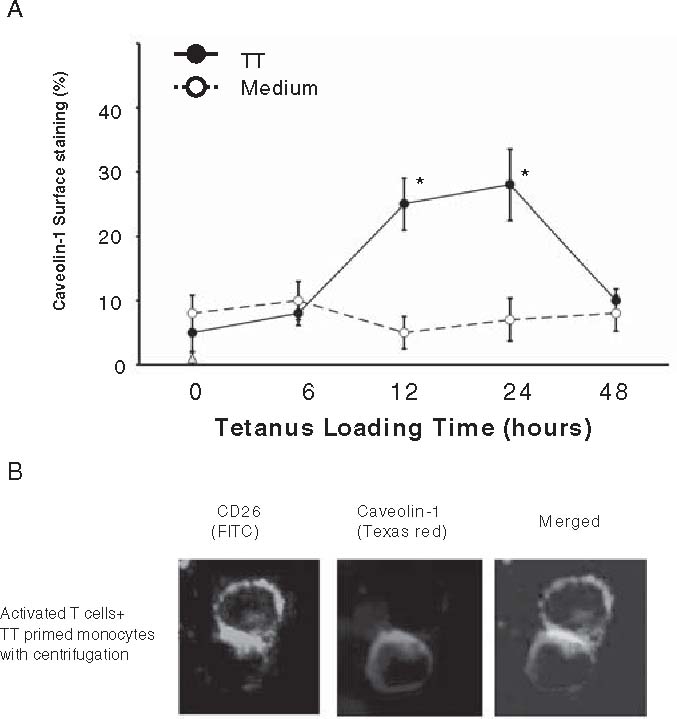
A,B. Immunocytochemical analysis of caveolin-1 and CD26 interaction. A Caveolin-1 in monocytes was exposed to cell surface after tetanus toxoid (TT) treatment, and interacted with CD26 on activated T cells. After purified monocytes were incubated with (solid circle) or without (open circle) TT, cell surface caveolin-1 was stained with anticaveolin-1 antibody detecting the N terminal region, and analyzed for % positive cells by flow cytometry. Data of % positive cells represent mean ± SE from five independent experiments. Asterisks indicate points of significant increase. B To form cell-cell conjugation, activated T cells and TT-loaded monocytes were mixed, followed by centrifugation. Conjugates were fixed without permeabilization, and stained with anti-CD26 (fluorescein isothiocyanate) and anti-caveolin-1 (Texas red) antibodies. Bars 10µm
CD26 and caveolin-1 in synovitis
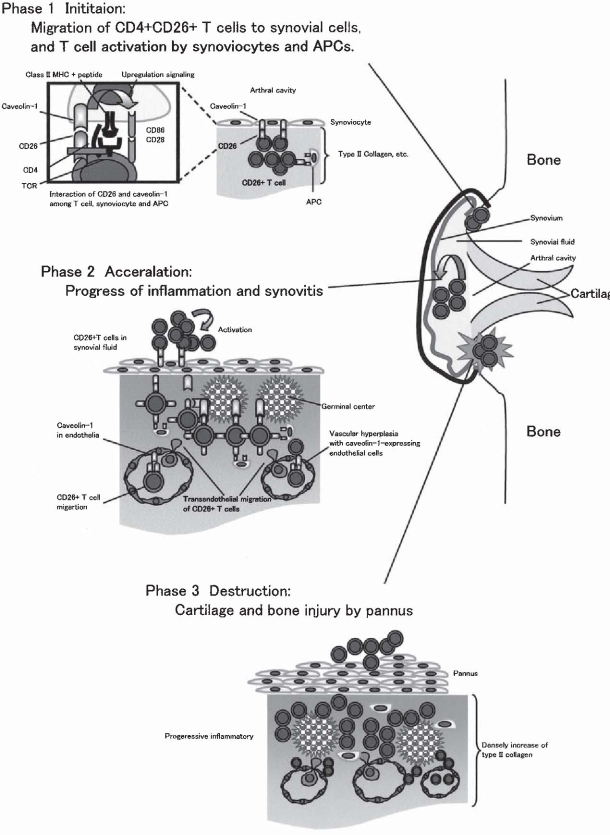
Rheumatoid arthritis is a classical example of an immune-mediated disease with chronically smoldering injury of the synovial joints resulting from infiltration of inflammatory cells, and synovitis of diarthrodial joints is its most visible manifestation. Although the observed architectures of rheumatoid synovitis vary in different individuals with RA as well as at various disease stages, the most frequent type of rheumatoid synovitis is a diffuse inflammatory infiltrate in which T cells, B cells, and macrophages are scattered around increased vasculature and synoviocytes. Meanwhile, in the remaining 40–50% of patients with RA, infiltrating inflammatory cells organize themselves into follicular structures.1 It is known that the inflammatory activation events in rheumatoid synovitis are dependent upon cell-cell contact among T cells, fibroblast-like synoviocytes, APCs, and regional tissues such as type II collagen and proteoglycan.83 Moreover, previous reports showed that CD26+ T cells exhibit strong migratory ability through endothelial cells, and are present at high levels in the rheumatoid synovium and the synovial fluids.20,22,23 These findings suggest that T cells with high levels of CD26 antigen may preferentially migrate into the rheumatoid synovium to induce inflammation and tissue destruction. To test this hypothesis, we examined the expression of CD26 and caveolin-1 in the rheumatoid synovium through immunohistochemistry. As shown in Fig. 5A, CD26+ lymphoid cells are clearly observed in diffuse synovitis. In follicular synovitis examined with the sequential sections, CD26+ lymphoid cells are infiltrated in the sublining area of caveolin-1-positive synovial cells (arrow in panel b of Fig. 5B), and are adjacent to caveolin-1-positive inflammatory cells (arrowheads in panel c of Fig. 5B). Moreover, the intimal lining layer is hyperplastic with multiple layers, and the synoviocytes in these layers highly express caveolin-1 (arrow in Fig. 5C). In addition, CD86 and caveolin-1 are coexpressed in the intimal lining synoviocytes and the sublining fibroblast-like synovial cells (black arrowhead in Fig. 5C). Furthermore, increased vascularization is seen in synovitis, and caveolin-1 is preferentially expressed in the luminar surface of endothelial cells in the rheumatoid synovium (white arrowheads in Fig. 5C). Taken together, we propose a model to describe the molecular events in monocytes leading to activation that are triggered by CD26-cavolin-1 interaction in rheumatoid synovium (Fig. 6). The initial step of inflammation in the synovium proceeds from activation of CD26+ T cells by APC and/or rheumatoid synoviocytes via presentation of a yet-to-be-identified antigen, concomitant with costimulation via such pairing as CD28-B7 and CD26-cavolin-1 (phase 1 in Fig. 6). With regard to the interaction between T cell-APC and the resultant immune response, entry of antigens via caveolae into APC leads to presentation of antigen peptides on MHC class II molecules and exposure of caveolin-1 (inside box in phase 1 of Fig. 6). APC thus induces the activation of memory T cells through the TCR and costimulatory molecules such as CD86/CD80-CD28, leading to the formation of mature immunological synapses. Following the association between caveolin-1 on APC and CD26 on memory T cells, CD86 is upregulated on APC surface, and memory T cells aresubsequently activated via the costimulatory effect of CD26 on TCR activation. By enhancing TCR activation via CD26-cavolin-1 interaction, prolongation of the immunological synapse may be maintained. CD86 upregulation therefore results in potent T cell-APC interaction, leading to the development of activated memory T cells locally and activation of the immune response, as well as the subsequent development of rheumatoid synovitis. After triggering inflammation of the synovium, memory T-cell activation leads to progression of inflammation in rheumatoid synovium, i.e., infiltration of inflammatory cells, increase of vascular vessels, formation of follicular germinal centers, and proliferation of synoviocytes (phase 2 in Fig. 6). Destructive inflammation then progresses to cartilage and bone injury by pannus (phase 3 in Fig. 6). As a result, progressive inflammation leads to synovial membrane invasion of bone, loss of cartilage and bone destruction in joints.
Fig. 5.
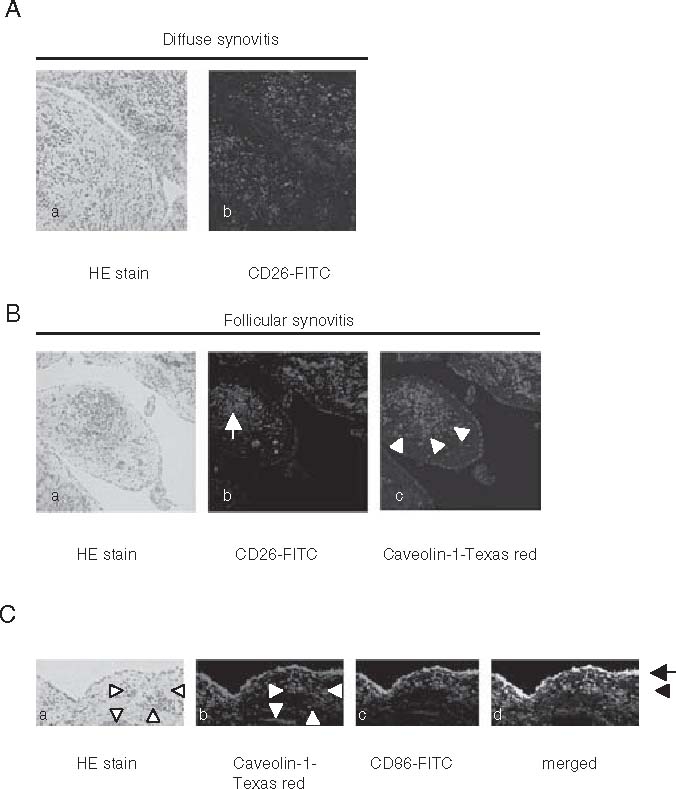
A–C. Architecture and immunohistochemistry of rheumatoid synovitis. APanel a shows H&E-stained histology of diffuse synovitis with inflammatory cells intermingled with fibroblast-like synoviocytes (×100). Panel b shows immunohistochemistry of the sequential section of panel a, which was stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD26 antibody (×100). BPanel a shows H&E-stained histology of follicular synovitis with germinal center formation (×100). Panel b shows immunohistochemistry of the sequential section of panel a, which was stained with FITC-conjugated anti-CD26 antibody. This reveals that CD26-positive lymphoid cells are scattered around the follicles (arrow) (×100). Panel c shows immunohistochemistry of the sequential section of panel b, which was stained with anti-caveolin-1 (Texas red). This reveals that the intimal lining synoviocytes and the sublining fibroblast-like synoviocytes adjacent to CD26+ lymphoid cells (arrow head) express caveolin-1 (×100). CPanel a shows synovial histology of rheumatoid arthritis with H&E staining (×100). Panels b and c show immunohistochemistry of the sequential section of panel a which was stained with caveolin-1 (Texas red) and CD86 (FITC), simultaneously. Panel d shows the merged view of panels b and c. Arrow shows the intimal lining synoviocytes, and black arrowhead shows the sublining fibroblast-like synoviocytes. White arrowheads show the increased vascularization in synovitis
Fig. 6.
Schematic diagram of inflammatory progress in rheumatoid synovitis. See text for details
Molecular-targeted therapeutic strategies in RA
Although the specific antigens responsible for the pathogenesis of RA have not been identified, T-cell activation via interaction with APCs plays a pivotal role in disease development. In this regard, therapeutic strategies have targeted cellular pathways in RA. In addition to anti-cytokine reagents, impressive therapeutic effect has been recently reported following the blocking of CD28-mediated costimulation by the use of cytotoxic T-lymphocyte-associated antigen 4-IgG1 (CTLA4Ig).84,85 Expressed on T cells within hours to days after activation,86,87 CTLA4 is the high-avidity receptor for both CD80 and CD86, and inhibits T-cell proliferation and IL-2 production.88,89 A fusion protein, CTLA4Ig binds both CD80 and CD86 on APCs, thereby preventing these molecules from engaging CD28 on T cells.90 By blocking the engagement of CD28, CTLA4Ig prevents the delivery of the second costimulatory signal that is required for optimal activation of T cells. The successful usage of this agent therefore demonstrates that blocking costimulatory signal to inhibit T-cell activation is a novel and promising therapeutic concept for selected autoimmune diseases.84,85 In this regard, since we showed that CD26-cavolin-1 interaction may play a pivotal role in rheumatoid synovitis, reagents capable of blocking CD26-cavolin-1 interaction in synovitis may be potentially useful in the treatment of patients with RA.
Conclusions
Our results may thus provide a new approach to the treatment of autoimmune diseases or other immune-mediated disorders by directly intervening with the interaction between activated T-cell and APC. Targeting the binding of the pocket structure of CD26 and the scaffolding domain of caveolin-1 may lead to novel therapeutic approaches, including the utilization of antagonists that regulate antigenspecific immune response in immune-mediated disorders such as RA.
Acknowledgment
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture (K.O. and C.M.), and Ministry of Health, Labor, and Welfare, Japan (C.M.).
References
- 1.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 2.Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol. 2005;115(2):118–28. doi: 10.1016/j.clim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Yamada R, Tanaka T, Unoki M, Nagai T, Sawada T, Ohnishi Y, et al. Association between a single-nucleotide polymorphism in the promoter of the human interleukin-3 gene and rheumatoid arthritis in Japanese patients, and maximum-likelihood estimation of combinatorial effect that two genetic loci have on susceptibility to the disease. Am J Hum Genet. 2001;68(3):674–85. doi: 10.1086/318789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344(8930):1105–10. doi: 10.1016/S0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 5.Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41(12):2196–204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337(3):141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 8.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 9.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343(22):1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 10.Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130(6):478–86. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 11.Nepom GT, Byers P, Seyfried C, Healey LA, Wilske KR, Stage D, et al. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- 12.Gao XJ, Olsen NJ, Pincus T, Stastny P. HLA-DR alleles with naturally occurring amino acid substitutions and risk for development of rheumatoid arthritis. Arthritis Rheum. 1990;33(7):939–46. doi: 10.1002/art.1780330704. [DOI] [PubMed] [Google Scholar]
- 13.Nakai Y, Wakisaka A, Aizawa M, Itakura K, Nakai H, Ohashi A. HLA and rheumatoid arthritis in the Japanese. Arthritis Rheum. 1981;24(5):722–5. doi: 10.1002/art.1780240516. [DOI] [PubMed] [Google Scholar]
- 14.Ohta N, Nishimura YK, Tanimoto K, Horiuchi Y, Abe C, Shiokawa Y, et al. Association between HLA and Japanese patients with rheumatoid arthritis. Hum Immunol. 1982;5(2):123–32. doi: 10.1016/0198-8859(82)90057-X. [DOI] [PubMed] [Google Scholar]
- 15.Fox DA. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997;40(4):598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- 16.Goronzy JJ, Weyand CM. T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16(3):212–7. doi: 10.1097/00002281-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104(10):1393–401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101(4):746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eguchi K, Ueki Y, Shimomura C, Otsubo T, Nakao H, Migita K, et al. Increment in the Ta1+ cells in the peripheral blood and thyroid tissue of patients with Graves’ disease. J Immunol. 1989;142(12):4233–40. [PubMed] [Google Scholar]
- 20.Gerli R, Muscat C, Bertotto A, Bistoni O, Agea E, Tognellini R, et al. CD26 surface molecule involvement in T-cell activation and lymphokine synthesis in rheumatoid and other inflammatory synovitis. Clin Immunol Immunopathol. 1996;80(1):31–7. doi: 10.1006/clin.1996.0091. [DOI] [PubMed] [Google Scholar]
- 21.Hafler DA, Fox DA, Manning ME, Schlossman SF, Reinherz EL, Weiner HL. In vivo activated T lymphocytes in the peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. N Engl J Med. 1985;312(22):1405–11. doi: 10.1056/NEJM198505303122201. [DOI] [PubMed] [Google Scholar]
- 22.Mizokami A, Eguchi K, Kawakami A, Ida H, Kawabe Y, Tsukada T, et al. Increased population of high fluorescence 1F7 (CD26) antigen on T cells in synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 1996;23(12):2022–6. [PubMed] [Google Scholar]
- 23.Muscat C, Bertotto A, Agea E, Bistoni O, Ercolani R, Tognellini R, et al. Expression and functional role of 1F7 (CD26) antigen on peripheral blood and synovial fluid T cells in rheumatoid arthritis patients. Clin Exp Immunol. 1994;98(2):252–6. doi: 10.1111/j.1365-2249.1994.tb06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuyama J, Berman JS, Cruikshank WW, Morimoto C, Center DM. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992;148(5):1367–74. [PubMed] [Google Scholar]
- 25.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161–80. doi: 10.1034/j.1600-065X.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 26.Dang NH, Torimoto Y, Shimamura K, Tanaka T, Daley JF, Schlossman SF, et al. 1F7 (CD26): a marker of thymic maturation involved in the differential regulation of the CD3 and CD2 pathways of human thymocyte activation. J Immunol. 1991;147(9):2825–32. [PubMed] [Google Scholar]
- 27.Dang NH, Torimoto Y, Sugita K, Daley JF, Schow P, Prado C, et al. Cell surface modulation of CD26 by anti-1F7 monoclonal antibody. Analysis of surface expression and human T cell activation. J Immunol. 1990;145(12):3963–71. [PubMed] [Google Scholar]
- 28.Morimoto C, Schlossman SF. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065X.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto C, Torimoto Y, Levinson G, Rudd CE, Schrieber M, Dang NH, et al. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989;143(11):3430–9. [PubMed] [Google Scholar]
- 30.Ohnuma K, Munakata Y, Ishii T, Iwata S, Kobayashi S, Hosono O, et al. Soluble CD26/dipeptidyl peptidase IV induces T cell proliferation through CD86 up-regulation on APCs. J Immunol. 2001;167(12):6745–55. doi: 10.4049/jimmunol.167.12.6745. [DOI] [PubMed] [Google Scholar]
- 31.Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Yoshikawa N, Shimizu N, et al. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci USA. 2004;101(39):14186–91. doi: 10.1073/pnas.0405266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T, Camerini D, Seed B, Torimoto Y, Dang NH, Kameoka J, et al. Cloning and functional expression of the T cell activation antigen CD26. J Immunol. 1992;149(2):481–6. [PubMed] [Google Scholar]
- 33.Naquet P, MacDonald HR, Brekelmans P, Barbet J, Marchetto S, Van Ewijk W, et al. A novel T cell-activating molecule (THAM) highly expressed on CD4-CD8- murine thymocytes. J Immunol. 1988;141(12):4101–9. [PubMed] [Google Scholar]
- 34.Yan S, Marguet D, Dobers J, Reutter W, Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur J Immunol. 2003;33(6):1519–27. doi: 10.1002/eji.200323469. [DOI] [PubMed] [Google Scholar]
- 35.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA. 2000;97(12):6874–9. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischer B. CD26: a surface protease involved in T-cell activation. Immunol Today. 1994;15(4):180–4. doi: 10.1016/0167-5699(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 37.von Bonin A, Huhn J, Fleischer B. Dipeptidyl-peptidase IV/CD26 on T cells: analysis of an alternative T-cell activation pathway. Immunol Rev. 1998;161:43–53. doi: 10.1111/j.1600-065X.1998.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 38.Oravecz T, Pall M, Roderiquez G, Gorrell MD, Ditto M, Nguyen NY, et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186(11):1865–72. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proost P, Struyf S, Schols D, Opdenakker G, Sozzani S, Allavena P, et al. Truncation of macrophage-derived chemokine by CD26/dipeptidyl-peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J Biol Chem. 1999;274(7):3988–93. doi: 10.1074/jbc.274.7.3988. [DOI] [PubMed] [Google Scholar]
- 40.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 41.Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26-/- mice. Exp Hematol. 2003;31(11):1126–34. doi: 10.1016/j.exphem.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169(12):7000–8. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 43.Wiedeman PE, Trevillyan JM. Dipeptidyl peptidase IV inhibitors for the treatment of impaired glucose tolerance and type 2 diabetes. Curr Opin Invest Drugs. 2003;4(4):412–20. [PubMed] [Google Scholar]
- 44.Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7(6):692–8. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 45.Dang NH, Torimoto Y, Deusch K, Schlossman SF, Morimoto C. Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol. 1990;144(11):4092–100. [PubMed] [Google Scholar]
- 46.Dang NH, Torimoto Y, Schlossman SF, Morimoto C. Human CD4 helper T cell activation: functional involvement of two distinct collagen receptors, 1F7 and VLA integrin family. J Exp Med. 1990;172(2):649–52. doi: 10.1084/jem.172.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz P, Zacharievich N, Hao L, Viciana AL, Shenkin M. Human thymocyte dipeptidyl peptidase IV (CD26) activity is altered with stage of ontogeny. Clin Immunol Immunopathol. 1998;88(2):156–68. doi: 10.1006/clin.1998.4550. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Kameoka J, Yaron A, Schlossman SF, Morimoto C. The costimulatory activity of the CD26 antigen requires dipeptidyl peptidase IV enzymatic activity. Proc Natl Acad Sci USA. 1993;90(10):4586–90. doi: 10.1073/pnas.90.10.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261(5120):466–9. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 50.Dong RP, Kameoka J, Hegen M, Tanaka T, Xu Y, Schlossman SF, et al. Characterization of adenosine deaminase binding to human CD26 on T cells and its biologic role in immune response. J Immunol. 1996;156(4):1349–55. [PubMed] [Google Scholar]
- 51.Dong RP, Tachibana K, Hegen M, Munakata Y, Cho D, Schlossman SF, et al. Determination of adenosine deaminase binding domain on CD26 and its immunoregulatory effect on T cell activation. J Immunol. 1997;159(12):6070–6. [PubMed] [Google Scholar]
- 52.Goldblum RM, Schmalstieg FC, Nelson JA, Mills GC. Adenosine deaminase (ADA) and other enzyme abnormalities in immune deficiency states. Birth Defects Orig Artic Ser. 1978;14(6A):73–84. [PubMed] [Google Scholar]
- 53.Dang NH, Hagemeister FB, Duvic M, Romaguera JE, Younes A, Jones D, et al. Pentostatin in T-non-Hodgkin’s lymphomas: efficacy and effect on CD26+ T lymphocytes. Oncol Rep. 2003;10(5):1513–8. [PubMed] [Google Scholar]
- 54.Torimoto Y, Dang NH, Vivier E, Tanaka T, Schlossman SF, Morimoto C. Coassociation of CD26 (dipeptidyl peptidase IV) with CD45 on the surface of human T lymphocytes. J Immunol. 1991;147(8):2514–7. [PubMed] [Google Scholar]
- 55.Ishii T, Ohnuma K, Murakami A, Takasawa N, Kobayashi S, Dang NH, et al. CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci USA. 2001;98(21):12138–43. doi: 10.1073/pnas.211439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi S, Ohnuma K, Uchiyama M, Iino K, Iwata S, Dang NH, et al. Association of CD26 with CD45RA outside lipid rafts attenuates cord blood T-cell activation. Blood. 2004;103(3):1002–10. doi: 10.1182/blood-2003-08-2691. [DOI] [PubMed] [Google Scholar]
- 57.Iwaki-Egawa S, Watanabe Y, Kikuya Y, Fujimoto Y. Dipeptidyl peptidase IV from human serum: purification, characterization, and N-terminal amino acid sequence. J Biochem (Tokyo) 1998;124(2):428–33. doi: 10.1093/oxfordjournals.jbchem.a022130. [DOI] [PubMed] [Google Scholar]
- 58.Durinx C, Lambeir AM, Bosmans E, Falmagne JB, Berghmans R, Haemers A, et al. Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem. 2000;267(17):5608–13. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka T, Duke-Cohan JS, Kameoka J, Yaron A, Lee I, Schlossman SF, et al. Enhancement of antigen-induced T-cell proliferation by soluble CD26/dipeptidyl peptidase IV. Proc Natl Acad Sci USA. 1994;91(8):3082–6. doi: 10.1073/pnas.91.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264(34):20163–6. [PubMed] [Google Scholar]
- 61.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68(4):673–82. doi: 10.1016/0092-8674(92)90143-Z. [DOI] [PubMed] [Google Scholar]
- 62.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1(5):445–58. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19(11):7289–304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276(41):38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 65.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22(7):2329–44. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276(24):21425–33. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 67.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93(1):131–5. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270(27):16395–401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 69.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272(10):6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 70.Gargalovic P, Dory L. Caveolins and macrophage lipid metabolism. J Lipid Res. 2003;44(1):11–21. doi: 10.1194/jlr.R200005-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Riemann D, Hansen GH, Niels-Christiansen L, Thorsen E, Immerdal L, Santos AN, et al. Caveolae/lipid rafts in fibroblast-like synoviocytes: ectopeptidase-rich membrane microdomains. Biochem J. 2001;354(Pt 1):47–55. doi: 10.1042/0264-6021:3540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woodman SE, Ashton AW, Schubert W, Lee H, Williams TM, Medina FA, et al. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003;162(6):2059–68. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Wang XB, Park DS, Lisanti MP. Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem. 2002;277(12):10661–8. doi: 10.1074/jbc.M110354200. [DOI] [PubMed] [Google Scholar]
- 74.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6(12):1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 75.Cheng HC, Abdel-Ghany M, Elble RC, Pauli BU. Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J Biol Chem. 1998;273(37):24207–15. doi: 10.1074/jbc.273.37.24207. [DOI] [PubMed] [Google Scholar]
- 76.Cheng HC, Abdel-Ghany M, Pauli BU. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem. 2003;278(27):24600–7. doi: 10.1074/jbc.M303424200. [DOI] [PubMed] [Google Scholar]
- 77.Wrenger S, Faust J, Mrestani-Klaus C, Fengler A, Stockel-Maschek A, Lorey S, et al. Down-regulation of T cell activation following inhibition of dipeptidyl peptidase IV/CD26 by the N-terminal part of the thromboxane A2 receptor. J Biol Chem. 2000;275(29):22180–6. doi: 10.1074/jbc.m002338200. [DOI] [PubMed] [Google Scholar]
- 78.Herrera C, Morimoto C, Blanco J, Mallol J, Arenzana F, Lluis C, et al. Comodulation of CXCR4 and CD26 in human lymphocytes. J Biol Chem. 2001;276(22):19532–9. doi: 10.1074/jbc.M004586200. [DOI] [PubMed] [Google Scholar]
- 79.Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296(5858):651–3. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 80.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3(5):311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 81.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285(5425):221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 82.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–7. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 83.Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BM, Feldmann M. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: involvement of phosphatidylinositol 3-kinase and nuclear factor kappaB pathways in tumor necrosis factor alpha production in rheumatoid arthritis. Arthritis Rheum. 2002;46(1):31–41. doi: 10.1002/1529-0131(200201)46:1<31::AID-ART10029>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 84.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 85.Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46(6):1470–9. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- 86.Lindsten T, Lee KP, Harris ES, Petryniak B, Craighead N, Reynolds PJ, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151(7):3489–99. [PubMed] [Google Scholar]
- 87.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262(5135):909–11. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 88.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily — CTLA-4. Nature. 1987;328(6127):267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 89.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801. doi: 10.1016/S1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 90.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G, et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993;262(5135):907–9. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]