Abstract
Background
Inhibition of proteolytic MMP activity could be a therapeutic approach to prevent ventricular dilatation by diminishing collagen matrix turnover and interstitial fibrosis. We investigated the time-course of MMP/TIMP activity during transition from hypertrophy to ventricular dilatation in transgenic mice with myocyte overexpression of the human β1-adrenergic receptor (β1TG). These β1TG mice were studied at 3 (normal function), 5 (hypertrophy) and 12 (ventricular dilatation) months of age compared to age-matched controls (WT).
Methods
Picro Sirius red staining and real-time PCR were performed for total collagen and for collagen type I and III quantification, respectively. MMP-activity assays (zymography), immunoblotting and real-time PCR experiments were done for gelatinase- (MMP-2, -9), collagenase- (MMP-1, -13), membrane-type MMP- (MT1- MMP; MMP-14) and TIMP expression measurements. To investigate β1-integrin activity, integrin-linked kinase (ILK) expression was measured by immunoblotting.
Results
Compared to WT with normal cardiac function, interstitial collagen type I and III mRNA and protein expression increased 3.6-fold in β1TG at 5 months of age with moderate fibrosis and cardiomyocyte hypertrophy and 17-fold in β1TG at 12 months of age with severe fibrosis and ventricular dilatation. Protein expression of the collagenases MMP-1 and -13 as well as the gelatinase proMMP-2 increased in the β1TG group with cardiac hypertrophy. Maximal activity of the gelatinase MMP-2 (3.5-fold vs.WT) was measured in β1TG at 12 months of age with severe fibrosis and ventricular dilatation, accompanied by coexpression of MT1- MMP (3.8-fold vs.WT) colocalized to the cell membranes.
Conclusion
These data provide evidence that sympathetic overactivation can trigger interstitial matrix remodeling and fibrosis by induction of MMP/TIMP activity. In particular gelatinolytic MMP-2 activity accompanies ventricular dilatation and the development of heart failure.
Key words: heart failure, myocardial fibrosis, cardiac remodeling, β1-adrenergic receptors, matrix metalloproteinases
Introduction
Interstitial fibrosis, altered collagen cross-link formation and impairment of diastolic and systolic ventricular function are markers of advanced heart failure and are potentially influenced by neuroendocrine activation and β-adrenergic signal transduction changes, which go in parallel with a poor prognosis [5, 23, 30]. Enzymes known for degradation of collagen fibrils and extracellular matrix proteins are the MMP (matrix metalloproteinases) family. The isoforms are characterized by substrate specificity. Dysbalance of MMP activity and their endogenous MMP inhibitors (TIMPs) plays a pivotal role in myocardial interstitial matrix remodeling leading to enhanced collagen turnover and a reduction in fibrillar collagen cross-link formation and influences myocardial systolic and diastolic function [17, 31] Systolic heart failure in humans [13, 19] and hypertensive cardiac hypertrophy [16, 21, 27] or remodeling after myocardial infarction [21] in animal models are associated with enhanced MMP activity and reduced TIMP expression in left ventricular myocardium. It is important to understand MMP-isoform regulation by different stimuli, such as chronic sympathetic overactivation. MMPs are synthesized in cardiomyocytes [8] as well as fibroblasts [33]. Most of the MMP isoforms are stored as zymogens (proMMPs) in the interstitium before being activated [6]. The collagenase group of MMPs includes MMP-1 (interstitial collagenase) and MMP-13 (collagenase-3) cleaving triple helical cross-linked fibrillar collagens, such as types I, II and III. Gelatinases MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are involved in degrading gelatins (collagen fragments) and cleaving basement membrane matrix proteins, such as collagen types IV, V, VII. In addition these gelatinases cleave fibronectin at the amino acid sequence Arg-Gly-Asp (RGD sequence). Fibronectin is responsible for crosslinking of extracellular collagens to cell membrane integrins. The membrane-type MMPs (MT-MMPs) have been colocalized to the cell membrane in proximity to the ECM-binding integrins. α2β1 integrin is the major collagen type I receptor. Integrin-linked kinase (ILK) regulates integrin-mediated cellular processes like fibronectin matrix assembly, cell survival and proliferation [32]. Inhibition of MMP activity could be an interesting therapeutic approach by preventing ventricular dilatation in order to diminish collagen matrix turnover and fibrosis. The induction of MMP/TIMP species during transition to and from hypertrophy to ventricular dilatation is not known in detail. We investigated the time-course of MMP/TIMP activity related to the cardiac phenotype in transgenic mice with 15-fold α-MHC-targeted cardiomyocyte overexpression of the human β1-adrenergic receptor (β1TG) developing heart failure within one year. These transgenic mice were generated from FVB/N mice as reported earlier [11]. Investigations were performed at 3 (normal function), 5 (hypertrophy) and 12 (ventricular dilatation) months of age compared to age-matched controls (WT).
Methods
Picro Sirius red staining
Left ventricular tissue specimens obtained from β1TG mice and wild-type (WT) control animals were fixed in 4% formalin/PBS solution and processed for paraffin embedding. After hydration of the deparaffinized left ventricular circumferential sections (6µm) samples were incubated in 0.1 % Sirius red in a saturated solution of picric acid for 60 min. Unbound Sirius red was removed with 0.01 N HCl. Samples were examined and photographed at a magnification of 10× with a Nikon E600 microscope (Nikon, Düsseldorf, Germany). Five fields located in the middle third of the myocardium (covering the majority of the myocardium) were evaluated. Perivascular fibrosis was estimated from cross sections of coronary arteries. Picro sirius red stain highlights collagen type I by yellow-red birefringence and collagen type III by green birefringence (polarized light). Analysis and quantification of total interstitial fibrillar collagen content were performed with digital image software Lucia G (Nikon, Düsseldorf, Germany). Data are presented as fractional area of collagen content in % of myocardial tissue. A single investigator blinded to the experimental groups performed the analysis.
Tissue sampling
Similar amounts (35 mg) of left ventricular myocardium from β1TG at 3, 5 and 12 months of age as well as age-matched WT control animals were homogenized and lysed in extraction buffer containing 10 mmol/l cacodylic acid pH 5.0, 0.15 mol/l NaCl, 1 μmol/l ZnCl2, 20 mmol/l CaCl2, 1.5 mmol/l NaN3, and 0.01 % (v/v) Triton X-100. After incubation over night at 4 °C and centrifugation at 1200 g the supernatants were collected and pH was raised to 7.5 for zymography experiments. Protein concentrations were measured using a detergent compatible kit (DC protein assay, Bio-Rad, Munich, Germany). For zymography- and immunoblotting-electrophoresis, minigels of 0.8 mm thickness were used (Bio-Rad, Munich, Germany).
Immunoblotting
Cardiac tissue samples (25 µg protein/lane) were homogenized, denatured (95 °C) and separated on 12% (TIMP-1,TIMP-2) and 10% (MMPs, ILK) SDS-polyacrylamide gels, transferred to nitrocellulose membranes (ProtranR, Schleicher & Schuell GmbH, Dassel, Germany) by semi-dry electrophoretic blotting and subjected to Western blot analysis. Equal loading of total cardiac protein was controlled by Poinceau red staining and through calsequestrin immunoreactivity (PA1-913, Dianova, Hamburg, Germany). Membranes were blocked with Western Blocking Reagent (Roche, Mannheim, Germany) and incubated at 37°C with monoclonal mouse MMP-1 (IM 35 L, Calbiochem Bad Soden, Germany), −9 (IM 10 L, Calbiochem), −13 (IM 81, Calbiochem), MT1-MMP (IM 57 L, Calbiochem), TIMP-1 (IM 32 L, Calbiochem), TIMP-2 (IM 11 L, Calbiochem) and rabbit anti-ILK (06-592, Biomol, Hamburg, Germany) antibodies, all diluted at 1:1000. The interstitial collagenase antibody (MMP-1) recognizes an epitope from amino acid 349–357 of the human interstitial collagenase. The coding sequence of the murine counterpart for the interstitial collagenase is very similar to the human sequence [3]. This antibody detects latent MMP- 1 at 57/52 kD and the active form at 46/42 kD. Goat antimouse and goat anti-rabbit peroxidase labeled secondary antibodies were diluted 1:10.000 and incubated 90 min at room temperature (RT). Proteins were visualized by enhanced chemiluminescence (ECL) according to the manufacturer’s guidelines (Amersham Pharmacia Biotech, Freiburg, Germany). MMP control-1 (Sigma, Deisenhofen, Germany) was used as a qualitative positive control for MMP-1, -9, TIMP-1 and -2. Autoradiographs were quantified by imaging densitometry and analyzed by the “ImageQuant-TM” Software (Image Quant, Molecular Dynamics, Krefeld, Germany). Data are presented as optical density (OD) in percent of a control sample.
Zymography
Zymography detects degradative properties of gelatinases by integrating gelatin as a substrate into the gel. Gelatin type B from bovine skin (Sigma, Deisenhofen, Germany) was added to standard Laemmli acrylamide polymerization mixture at final concentrations of 1 mg/ml. Tissue extract (50 µg) was mixed with sample buffer (10% w/v SDS, 4% w/v sucrose, 0.1% w/v bromophenol blue) and loaded into slots of the 0.8 mm 10% acrylamide stacking gel. Gels were run at 20 mA and soaked in 2.5 % w/v Triton X-100 with gentle shaking for 60 min at RT. Gels were incubated over night at 37 °C in substrate buffer (50 mmol/l Tris-Cl, pH 8.0 and 5mmol/l CaCl2), stained in 0.05% Coomassie Brilliant Blue G-250 (Bio-Rad, Munich, Germany) diluted in ethanol, destained in acetic acid/ethanol and dried. Supernatant of HT 1080, a human fibrosarcoma cell line, was used as positive control for MMP-2 and -9. Quantification of the size-fractionated banding pattern was performed by densitometry. Each sample lane was scanned and presented as optical density (OD) in percent of control sample.
Reverse transcription
Total RNA from deep frozen left ventricular tissue of β1TG and WT controls at 3, 5 and 12 months of age was prepared according to the method of Chomczynski and Sacci [7] with PeqGOLD RNAPure® (Peqlab Biotechnology, Erlangen, Germany). RNA was quantified spectrometrically and ethidium bromide-stained agarose gels were used to check its integrity. Complementary DNA (cDNA) was transcribed from the RNA template using Sensiscript Reverse Trancriptase (Qiagen, Hilden, Germany) and random hexamer primers (Roche, Mannheim, Germany) according to the manufacturer’s protocol.
Real-time quantitative polymerase chain reaction (RT-PCR)
Real-time PCR (Applied Biosystems, Germany) was performed in 23 µl PCR Master Mix buffer volume (Sybr Green, dNTPs, AmpliTaqGold DNA polymerase), 2 µl (10 ng) template DNA and 200 nM of sense and antisense primer designed for conserved regions of the genes (Table 1). PCR was performed according to a two-step PCR protocol (5 min at 95 °C, 40 cycles for 15 s at 95 °C, 1 min at 59 °C). The ABI PRISM Sequence Detection System 7700 (Applied Biosystems) was used to detect the fluorescent (Sybr Green) signal which reflects the exponential accumulation of the PCR product. Threshold cycle (Ct) values were determined using the Sequence Detector 1.7 software. Dissociation curves were performed to control the specificity of the amplification product. Values are presented as the ratio of target mRNA to GAPDH of the same sample. An external GAPDH standard curve has been used to calculate Ct values in pg.
Table 1.
Primer sequences used for real-time quantitative polymerase chain reaction
| 5′-3′ sequence | Accession number | |
|---|---|---|
| Pro-collagen type I (α2) | X58251 | |
| primer sense | GTAAACACCCCAGCGAAGAACTC | |
| primer antisense | TCAAACTGGCTGCCACCAT | |
| Pro-collagen type III (α1) | X52046 | |
| primer sense | ACCCAGAGATCCCATTTGGAG | |
| primer antisense | AGGAAGCACAGGAGCAGGTG | |
| Matrix metalloproteinase-2 | NM008610 | |
| primer sense | CGCTCAGATCCGTGGTGAG | |
| primer antisense | GGCTTGTCACGTGGTGTCAC | |
| Matrix metalloproteinase-13 | X66473 | |
| primer sense | AGAGGGTCTTCCCCGTGTTCT | |
| primer antisense | GGCTCTGAATGGTTATGACATTCTG | |
| MT1-MMP | U54984 | |
| primer sense | AACTTTGACACCGTGGCCA | |
| primer antisense | CAATGGGCATTGGGTATCC | |
| TIMP-1 | NM011593 | |
| primer sense | CACTCACTGTTTGTGGACGGA | |
| primer antisense | CAAGCAAAGTGACGGCTCTG | |
| TIMP-2 | NM011594 | |
| primer sense | GCAAGATGCACATTACCCTCTGT | |
| primer antisense | CAGGCTCTTCTTCTGGGTGATG | |
| GAPDH | M32599 | |
| primer sense | CCTGGACCACCCAGCCCAGCA | |
| primer antisense | TGTTATGGGGTCTGGGATGGA |
MT1-MMP membrane type 1-matrix metalloproteinase, TIMP-1 tissue inhibitor of matrix metalloproteinase-1, GAPDH glyceraldehydes-3-phosphate dehydrogenase
Statistical analysis
Data are expressed as mean±SEM. Statistical significance was estimated with one way analysis of variance and Bonferroni t-test. A probability value < 0.05 was considered significant.
Results
Increased interstitial collagen content
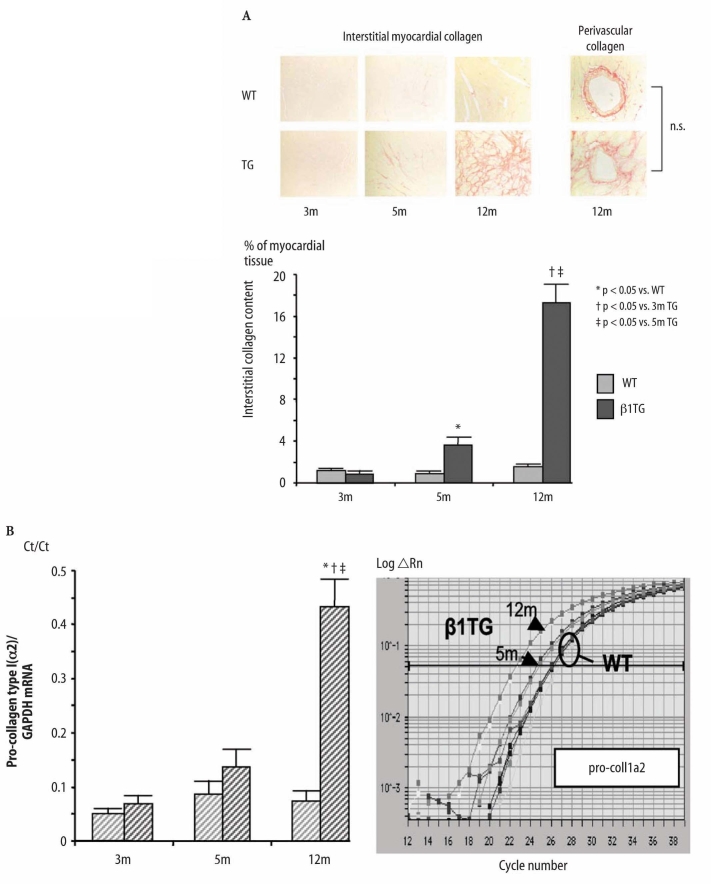
Fig. 1A shows increased interstitial collagen content in β1TG compared to WT mice. Interstitial total fibrillar collagens in β1TG increased from 0.9% (3 months) to 3.6% (5 months) and finally to 17% fractional area of left ventricular myocardial tissue at 12 months compared to age-matched WT animals with 1.5 % interstitial collagen fibers. The ratio of myocardial collagen type I to type III was unchanged (polarized light). Perivascular fibrosis was not significantly altered in β1TG compared to WT mice. The increase of collagen protein content was accompanied by a 4.5-fold increase of pro-collagen type I (α2) and type III (α1) mRNA expression measured by quantitative real-time PCR at 12 months of age (Fig. 1B).
Fig. 1.
A Myocardial interstitial and perivascular collagen. Picro sirius red (total collagen) stained circumferential midmyocardial sections of the left ventricle (magnification 10×) of β1-adrenergic receptor transgenic mice (β1TG) at 3 months (without fibrosis), 5 months (myocyte hypertrophy and moderate fibrosis) and 12 months of age (ventricular dilatation and severe interstitial fibrosis) compared to age-matched wild-type (WT) controls. Right: Perivascular fibrosis did not differ in β1TG compared to WT at any age. Analysis and quantification was performed with digital image software Lucia G (Nikon, Düsseldorf, Germany). Data are presented as fractional area of collagen content in % of myocardial tissue of n=5 per group. B Quantitative real-time PCR of pro-collagen type I (α2) mRNA. Threshold cycle (Ct) values are presented as a ratio of pro-collagen type I (α2) mRNA to GAPDH mRNA (left). Representative amplification plot (ABI Prism 7700 detection system) Rn normalized reporter signal (right)
Collagenases increased in cardiac hypertrophy Total protein expression of the collagenase MMP-13 (60 kD) significantly increased up to 3.6-fold in the β1TG group at 5 months of age in mice with myocardial hypertrophy and moderate interstitial fibrosis compared to mice without myocardial alterations. MMP-13 decreased in β1TG at 12 months of age with ventricular dilatation and severe fibrosis (MMP-13 [OD], WT: 3m 0.9±0.04, 5m 1.9±0.2, 12m 1.2±0.2, TG: 3m 1.0±0.1, 5m 3.7±0.3, 1m 1.8±0.6, n=6). MMP-13 antibody detects total protein expression (Fig. 2A).To confirm these results and for further differentiation in latent and active isoforms we used an antibody for interstitial collagenase MMP-1. The non-glycosylated latent proMMP-1 (57/52 kD) isoform also increased (4.6-fold) in β1TG mice at 5 months of age and decreased with 12 months (proMMP-1 [OD], WT: 3m 0.5±0.09, 5m 2.5±0.1, 12m 1.3±0.3, TG: 3m 0.9±0.1, 5m 4.0±0.4, 1m 1.5±0.4, n=6). Active MMP-1 (47 kD) continuously increased with disease progression from hypertrophy (2.3-fold) to ventricular dilatation (4.5-fold) in β1TG mice (active MMP-1 [OD], WT: 3m 0.8±0.06, 5m 0.9±0.09, 12m 0.8±0.2, TG: 3m 0.9±0.1, 5m 2.0±0.5, 1m 4.0±1.0, n=6; Fig. 2B). MMP-13 mRNA expression determined by real-time PCR was not different between all groups.
Fig. 2.
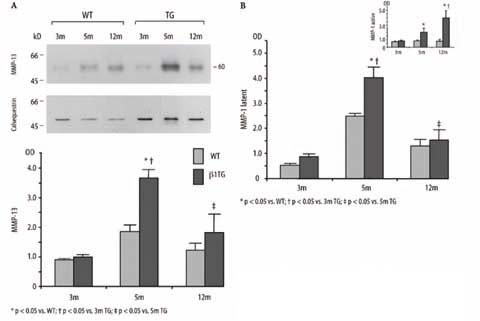
A Immunoblot analysis of MMP-13 collagenase protein expression and densitometric quantification. Corresponding blot of the housekeeping protein calsequestrin is shown below. B Densitometric quantification of the collagenase pro-MMP-1 (latent) and active MMP-1 protein expression measured by immunoblotting
Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) increased with aging
Protein expression of TIMP-1 (28 kD) increased in both, with aging in the WT groups and with disease progression in the β1TG groups. However, protein enhancement was more pronounced in the β1TG mice groups (TIMP-1 [OD]: WT: 3m 4.4±0.6, 5m 12.5±1.2, 12m 38.6±5.7, TG: 3m 6.6±0.9, 5m 22.8±4.3, 12m 44.9±6.6, n=5).
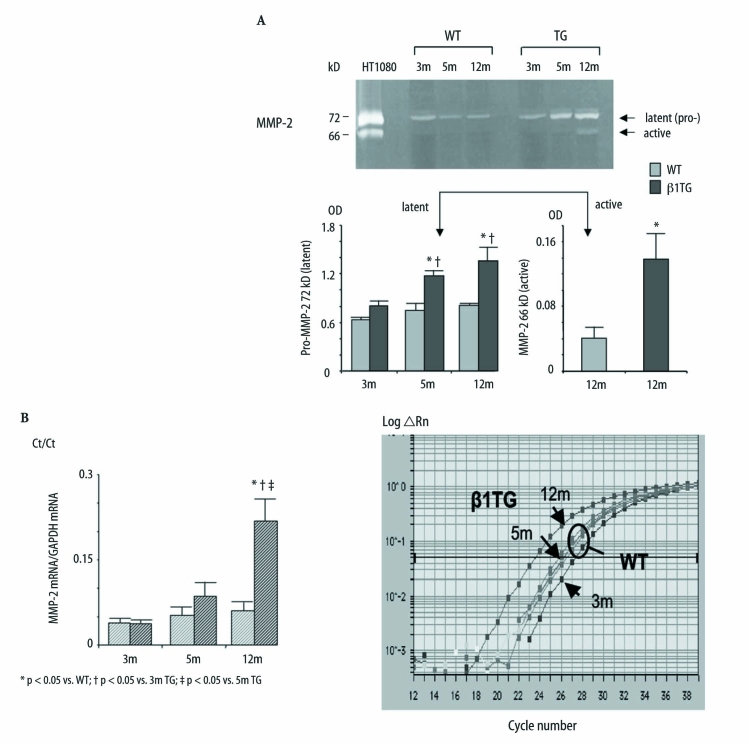
Gelatinolytic activity of MMP-2 was associated with ventricular dilatation
Zymography detects the latent (72/69 kD) and active (66/59 kD) form of gelatinase-A, MMP-2 (Fig. 3A). ProMMP-2 (72 kD) protein expression continuously increased in the β1TG group from 3 to 12 months of age compared to the age-matched WT control group.Active MMP-2 (66 kD) degrades gelatins and fibronectin, responsible for collagen crosslinking.MMP-2 activity has been detected in some of the β1TG at 5 months of age but significantly increased up to 3.5-fold (vs. WT) in the β1TG group at 12 months of age characterized by severe interstitial fibrosis and ventricular dilatation (proMMP-2 [OD] WT: 3m 0.6±0.02, 5m 0.75±0.08, 12m 0.8±0.02, TG: 3m 0.8±0.07, 5m 1.2±0.07.12m 1.4±0.1; MMP-2 (active): WT 12m 0.04±0.01, TG 12m 0.1±0.03, n=5). MMP-2 mRNA expression measured by real-time PCR was upregulated (4.4-fold) in β1TG mice at 12 months of age compared to β1TG and WT at 3- and 5 months of age (Fig. 3B).
Fig. 3.
A Zymography of left ventricular homogenates comparing WT with β1TG at 3 months (m), 5 m and 12 m of age. Pro-MMP-2 (72 kD) expression continuously increased in β1TG mice with progressive interstitial fibrosis. MMP-2 activity (66 kD) is demonstrated in β1TG mice with ventricular dilatation and severe fibrosis at 12 m of age. HT1080, a fibrosarcoma cell-line, is used for MMP-2 latent/active positive control. n=5/group. B Quantitative real-time PCR of MMP-2 mRNA. Threshold cycle (Ct) values are presented as a ratio of MMP-2 mRNA to GAPDH mRNA (left). Representative amplification plot of MMP-2 mRNA (ABI Prism 7700 detection system) Rn normalized reporter signal (right)
TIMP-2 increased with progressive cardiac dysfunction
TIMP-2 (24 kD) protein level and mRNA expression continuously increased in the β1TG group from 3 months to 12 months of age with disease progression compared to age matched WT mice (TIMP-2 [OD] WT: 3m 2.0±0.46, 5m 6.97±0.98, 12m 6.0±1.3, TG: 3m 3.0±0.8, 5m 8.0±1.37.12m 12.2±1.2, n=5; Fig. 4).
Fig. 4.
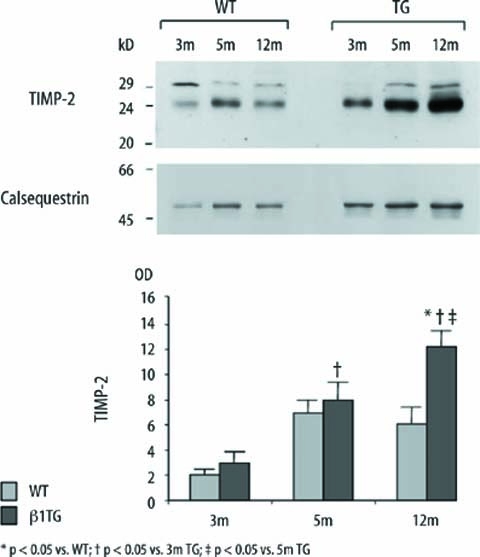
Immunoblot and densitometric quantification of tissue inhibitor of matrix metalloproteinase-2 (TIMP-2; 24 kD). TIMP-2 expression continuously increased in β1TG mice with progressive interstitial fibrosis and ventricular dysfunction. Corresponding blot of the housekeeping protein calsequestrin is shown below; n=5/group
MT1-MMP (MMP-14) expression increased with ventricular dilatation
MT1-MMP (58 kD) protein expression measured in left ventricular homogenates of β1TG mice at 5 months of age increased significantly (1.6-fold) compared to β1TG mice at 3 months of age and increased further up to 2.4-fold measured in the β1TG group at 12 months of age (MMP-14 [OD] WT: 3m 0.3±0.03, 5m 0.5±0.1, 12m 0.6±0.2,TG: 3m 0.3±0.03, 5m 0.5±0.07, 12m 1.2±0.13, n=5; Fig. 5A). In parallel MT1-MMP mRNA expression, measured by quantitative real-time PCR, increased significantly with disease progression in the β1TG group up to 2.8-fold at 12 months of age (Fig. 5B).
Fig. 5.
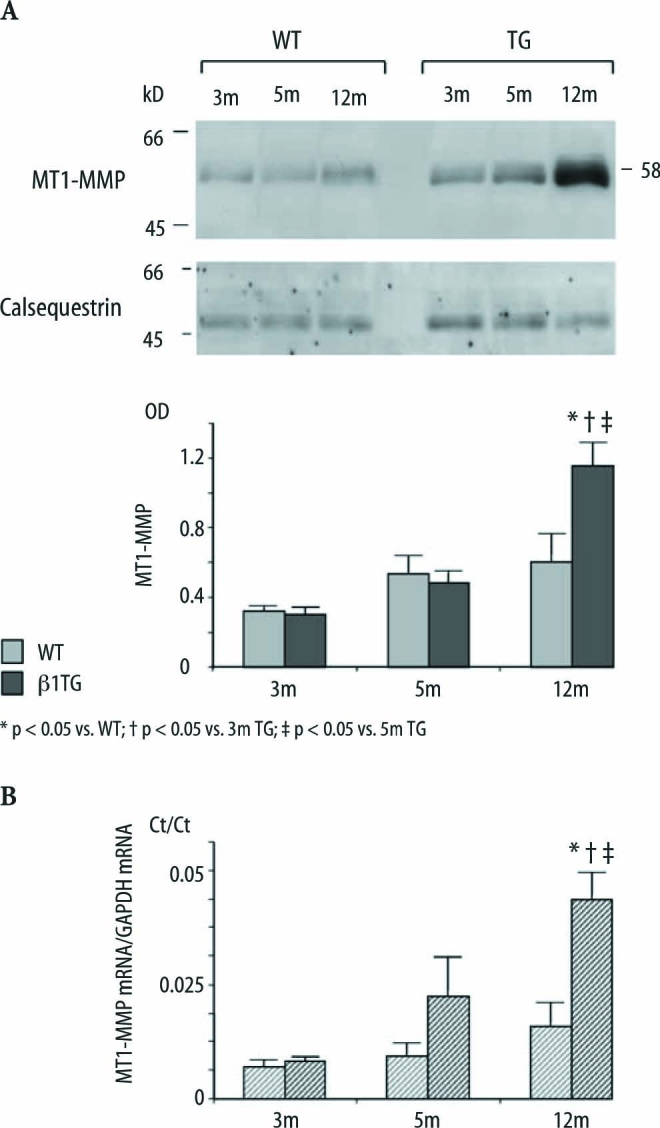
A Immunoblot and densitometric quantification of membrane-type-1 MMP protein (58 kD) expression of β1TG mice and WT. Corresponding blot of the housekeeping protein calsequestrin is shown below; n=5/group. B Quantitative real-time PCR of MT1-MMP mRNA. Ct values are presented as a ratio of MT1-MMP mRNA to the internal standard GAPDH mRNA
Integrin-linked kinase (ILK) decreased with ventricular dilatation
Protein expression of this serine/threonine kinase interacting with the β1 integrin cytoplasmic domain was significantly downregulated by 69 % in β1TG at 12 months of age with severe interstitial fibrosis and left ventricular dilatation compared to age-matched WT.ILK was not regulated in WT and younger β1TG mice before onset of ventricular dilatation and severe fibrosis (ILK [OD] WT: 3m 192.4±13.8, 5m 180.2±18.6, 12m 183.3±19, TG: 3m 169.7±10, 5m 143.9±11, 12m 66.6±6.6, n=5; Fig. 6).
Fig. 6.
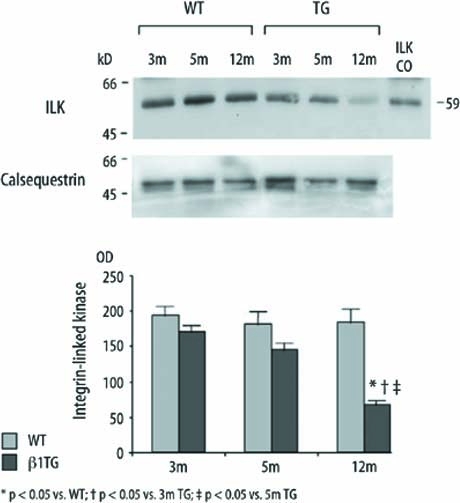
Immunoblot and densitometric quantification of integrin-linked kinase (ILK) protein expression of β1TG and age matched WT. ILK is recognized at 59 kD (ILK control) and significantly downregulated in mice with severe interstitial fibrosis and ventricular dilatation at 12 months of age. Corresponding blot of the housekeeping protein calsequestrin is shown below
Discussion
In this study interstitial matrix remodeling was explored in β1-adrenoceptor transgenic mice [11]. In β1TG, an increase in cardiac function is followed by ventricular hypertrophy at 5 months of age [11]. Transition from a compensated LV hypertrophic state to LV dilatation and dysfunction occurred in β1TG mice at the age of 5 to 9 months and is most pronounced in β1TG mice at the age of 12 months [32]. As shown by expression analyses (histology, real-time PCR, immunoblotting) and proteinase activity assays (zymography), β1-adrenoceptor transgenic mice (β1TG) developed progressive interstitial fibrosis that preceded systolic functional decline [11, 12] as studied earlier. Fibrosis was most pronounced at the age of 12 months.
Engelhardt et al. have shown an impairment of myocardial contractility associated with impaired Ca2+-handling in β1TG mice [11, 12]. Badenhorst et al. recently suggested that chronic sympathetic activation significantly contributes to progression from compensated left ventricular hypertrophy to cardiac dysfunction through deleterious cardiac matrix remodeling [1, 2]. Our data suggest that β-adrenergic overactivity by β1-adrenergic receptor overexpression is accompanied by interstitial matrix remodeling and turnover by induction of MMP/TIMP activity in parallel to cardiac phenotype changes. Increased gene expression of proMMP-2 and MT1-MMP (MMP-14) contributes to enhanced protein expression and activation of 72 KDa gelatinase (MMP-2). Gelatinase activity of MMP-2 is associated with interstitial fibrosis and ventricular dilatation. MMP-2 activity might be not contradictory to increased interstitial fibrosis because MMP-2 has been shown to degrade non triple-helical collagens like gelatins and fibronectin [29] which may promote cleavage of fibronectin from β1-integrins located at cardiomyocyte-matrix adhesions. Indeed, β1-integrin downstream signaling (integrin-linked kinase, ILK) was significantly impaired in failing β1-adrenoceptor transgenic mice.
In β1TG pro-collagen type-I and -III mRNA and interstitial collagen protein expression was progressively increased from four fold at 5 months and finally to 17-fold at 12 months compared to 3 months of age. In β1TG at 5 months of age with compensated cardiac hypertrophy, we observed an increased deposition of type-I and -III collagen fibers (polarization microscopy) surrounding the cardiomyocytes. Conversely, in the β1TG group at 12 months of age asymmetrical distribution of the collagen fibers with interruption of the collagen network structure was shown in Sirius red stained sections of the left ventricle. The turnover of collagens is regulated by proteolytic matrix metalloproteinase (MMP) activity and their endogenous tissue inhibitors (TIMPs). Progressive interstitial fibrosis was accompanied by increased expression levels of proMMP-2, MMP-2, TIMP- 2, the membrane bound MT1-MMP (MMP-14), and the collagen type I degrading enzymes MMP-1 and MMP- 13. Increased MMP activity has been indicated in both animal and human studies of heart failure with ventricular dilatation and fibrosis [19, 21, 26, 27]. In particular, these studies observed MMP/TIMP profiles which favor extracellular matrix (ECM) degradation. Since in these models increased myocardial fibrosis was observed, these data and those reported herein provide evidence for a dysbalance between formation and degradation of interstitial fibrous tissue.
In β1TG mice with hypertrophy, increased left ventricular protein expression of pro-MMP isoforms, such as the collagenases (MMP-1, MMP-13), the gelatinase proMMP-2 and the membrane bound MT1-MMP, preceded the development of ventricular dilatation. Compensated hypertrophy was associated with increased myocardial TIMP-1 and TIMP-2 levels, which would imply reduced MMP collagenase activity. This is in agreement with the concept that diminished myocardial MMP collagenase activity can facilitate collagen accumulation in developing hypertrophy [20, 21]. TIMP-1 protein expression also increased with age in the WT group but protein enhancement was more pronounced in β1TG mice. These data suggest a role for the control of cardiac MMP-1 and -13 proteolytic activity by TIMP- 1. The fact that protein expression of the collagenases MMP-1 and MMP-13 was accompanied by elevated TIMP-1 levels is compatible with results in chronic pressure- overload induced hypertrophy but contrasts to models with acute pressure-overload showing an increase in MMP activity [22].
β1TG mice developed dilatation and heart failure at the age of 12 months [11]. Total collagenase (MMP-1, MMP-13) protein expression decreased but membrane bound MT1-MMP (MMP-14), TIMP-2 protein expression and MMP-2 gelatinase activity significantly increased in β1TG mice at 5 and further at 12 months of age. We observed a weak signal of zymographic MMP-2 activity in some of the β1TG mice at 5 months of age preceding transition to ventricular dilatation. Gelatinolytic MMP-2 activity, measured by gelatin zymography, increased up to 3.6-fold in β1TG with decompensated heart failure symptoms at the age of 12 months. Our data support the hypothesis that activation of the gelatinase MMP-2 accompanies left ventricular remodeling and dilatation. TIMP-2 is known as an inhibitor of MMP-2 activity but also participates in proMMP-2 activation, involving binding of proMMP-2 to a membrane-bound MT1-MMP/TIMP-2 receptor complex [15, 33]. MT1- MMP has been localized to the cell membrane and in proximity to the ECM-binding integrins [24].α2β1 integrin is the major collagen type I receptor and located in focal adhesions in cardiomyocyte and fibroblast membranes [9].Myocardial fibrillar collagens, such as collagen types I and III, are essential for maintaining alignment of the myofibrils within the myocytes through a collagen-integrin-cytoskeletal myofibril relation [6, 17, 24]. Integrin signaling is important in the maintenance of normal myocardial structure and function [24]. MMP-2 activity may promote cleavage of fibronectin from β1-integrins located at cardiomyocyte-matrix adhesions. Indeed, β1-integrin activity measured by downstream signaling (integrin-linked kinase, ILK) was significantly impaired in failing β1-adrenoceptor transgenic mice at the age of 12 months. Since ILK is involved in cellular regulation of cell survival and proliferation [10, 32], disruption of cell-matrix adhesions could be involved in cardiomyocyte slippage, replacement fibrosis and finally ventricular dilatation. Sakata and coworkers [25] observed enhanced total MMP protein expression and activity in Dahl salt-sensitive rats (26 weeks) with LV dilatation and systolic dysfunction compared to earlier stages providing evidence that gelatinolytic activity precedes LV dilatation. Iwanaga et al. [16] showed, in Dahl salt-sensitive rats with compensated left ventricular hypertrophy (11 weeks), no changes in the hypertrophy stage, but increased MMP-2 mRNA, protein level and activity as well as TIMP-2 protein and mRNA levels after development of dilatation and dysfunction. In agreement targeted deletion of the MMP-2 gene reduced LV rupture and late remodeling in mice after myocardial infarction [14]. These findings supported the data of the present study. Spinale et al. [28] as well as Woodiwiss et al. [31] found that, irrespective of changes in myocardial collagen concentrations, a decrease in collagen cross-linking paralleled left ventricular dilatation in a rat model with pressure-overload and heart failure and in rats with left ventricular dilatation induced by a seven month-lasting enoceptor stimulation with isoproterenol. A potential role of β-adrenoceptor stimulation beyond replacement fibrosis has been provided by Coker et al. [8]. They stimulated myocytes with isoproterenol and demonstrated increased MMP-2 content and activity as well as increasedMT1-MMP expression. A positive correlation between noradrenaline and MMP-2 in patients with severe congestive heart failure and in vitro in human cardiac fibroblasts has been recently shown by Banfi et al. [4]. These data support the link between neurohormonal stimulation and collagen turnover. In conclusion the presented data provide evidence that gelatinase activity of MMP-2 accompanies replacement fibrosis and ventricular dilatation. MMP-2 activity may promote cleavage of fibronectin from β1-integrins located at cardiomyocyte-matrix adhesions in failing β1TG. The knowledge of the selective induction of MMPs within the myocardium might have particular relevance in future therapeutic approaches to prevent progression of chronic heart failure disease.
Acknowledgements
We thank Julia Michaely and Kirsten Schiller for excellent technical assistance. Experimental work was supported by grants from the Deutsche Forschungsgemeinschaft and the Bundesministerium für Bildung und Forschung (Kompetenznetz Herzinsuffizienz).
References
- 1.Badenhorst D, Veliotes D, Maseko M, Tsotetsi OJ, Brooksbank R, Naidoo A, Woodiwiss AJ, Norton GR (2003) Betaadrenergic activation initiates chamber dilatation in concentric hypertrophy. Hypertension 41:499–504 [DOI] [PubMed]
- 2.Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, Woodiwiss AJ (2003) Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res 57:632–641 [DOI] [PubMed]
- 3.Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, Quesada V, Bordallo J, Murphy G, Lopez-Otin C (2001) Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem 276:10253–10262 [DOI] [PubMed]
- 4.Banfi C, Cavalca V,Veglia F, Brioschi M, BarcellaS, Mussoni L, Boccotti L, Tremoli E, Biglioli P, Agostoni PG (2005) Neurohormonal actvation is associated with increased levels of plasma matrix metalloproteinase-2 in human heart failure. Eur Heart J 26:481–488 [DOI] [PubMed]
- 5.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB (1982) Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211 [DOI] [PubMed]
- 6.Burlew BS, Weber KT (2000) Connective tissue and the heart, functional significance and regulatory mechanisms. Cardiol Clin 18:435–442 [DOI] [PubMed]
- 7.Chomczynski P, Sacci N (1987) Singlestep method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed]
- 8.Coker ML, Jolly JR, Joffs C, Etoh T, Holder JR, Bond BR, Spinale FG (2001) Matrix metalloproteinase expression and activity in isolated myocytes after neurohormonal stimulation. Am J Physiol Heart Circ Physiol 281:H543–H551 [DOI] [PubMed]
- 9.Communal C, Singh M, Menon B, Xie Z, Colucci WS, Singh K (2003) beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta-adrenergic receptor-stimulated apoptosis. J Cell Biochem 89:381–388 [DOI] [PubMed]
- 10.Dedhar S, Williams B, Hannigan G (1999) Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol 9:319–323 (Review) [DOI] [PubMed]
- 11.Engelhardt S, Hein L, Wiesmann F, Lohse MJ (1999) Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96:7059–7064 [DOI] [PMC free article] [PubMed]
- 12.Engelhardt S, Boknik P, Keller U, Neumann J, Lohse MJ, Hein L (2001) Early impairment of calcium handling and altered expression of junctin in hearts of mice overexpressing the beta1- adrenergic receptor. FASEB J 15:2718–2720 [DOI] [PubMed]
- 13.Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF Jr (1996) Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol 148:1639–1648 [PMC free article] [PubMed]
- 14.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A (2003) Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol 285:1229–1235 [DOI] [PubMed]
- 15.Hernandez-Barrantes S, Toth M, Bernardo MM,Yurkova M, Gervasi DC, Raz Y, Sang QA, Fridman R (2000) Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem 275:12080–12089 [DOI] [PubMed]
- 16.Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S (2002) Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol 39:1384–1391 [DOI] [PubMed]
- 17.Kato S, Spinale FG, Tanaka R, Johnson W, Cooper G IV, Zile MR (1995) Inhibition of collagen cross-linking: effects on fibrillar collagen and left ventricular diastolic function. Am J Physiol 269:H863–H868 [DOI] [PubMed]
- 18.Kim H.E, Dalal S.S, Young E, Legato M.J, Weisfeldt M.L, D’Armiento J (2000) Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest 106:857–866 [DOI] [PMC free article] [PubMed]
- 19.Li YY, Feldman AM, Sun Y, McTiernan CF (1998) Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation 98:1728–1734 [DOI] [PubMed]
- 20.Li H, Simon H,Bocan TMA,Peterson JT (2000) MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res 46:298–306 [DOI] [PubMed]
- 21.Mujumdar VS, Tyagi SC (1999) Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J Hypertens 17:261–270 [DOI] [PubMed]
- 22.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M,Spinale FG (2000) Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol 278:H151–H161 [DOI] [PubMed]
- 23.Packer M (1988) Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77:721–730 (Review) [DOI] [PubMed]
- 24.Ross RS, Borg TK (2001) Integrins and the myocardium. Circ Res 88:1112–1119 (Review) [DOI] [PubMed]
- 25.Sakata Y, Yamamoto K, Mano T, Nishikawa N,Yoshida J,Hori M,Miwa T, Masuyama T (2004) Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of Angiotensin-converting enzyme inhibitor. Circulation 109:2143–2149 [DOI] [PubMed]
- 26.Seeland U, Kouchi I, Zolk O, Itter G, Linz W, Böhm M (2002) Effect of ramipril and furosemide treatment on interstitial remodeling in post-infarction heart failure rat hearts. J Mol Cell Cardiol 34:151–163 [DOI] [PubMed]
- 27.Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L (1998) Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 82:482–495 [DOI] [PubMed]
- 28.Spinale FG, Zellner JL, Johnson WS, Eble DM, Munyer PD (1996) Cellular and extracellular remodeling with the development and recovery from tachycardiainduced cardiomyopathy: changes in fibrillar collagen, myocyte adhesion capacity and proteoglycans. J Mol Cell Cardiol 28:1591–1608 [DOI] [PubMed]
- 29.Steffensen B, Xu X, Martin PA, Zardeneta G (2002) Human fibronectin and MMP-2 collagen binding domains compete for collagen binding sites and modify cellular activation of MMP-2. Matrix Biology 21:399–414 [DOI] [PubMed]
- 30.Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ (1993) Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 87:454–463 [DOI] [PubMed]
- 31.Woodiwiss AJ, Tsotetsi OJ, Sprott S, Lancaster EJ, Mela T, Chung ES, Meyer TE, Norton GR (2001) Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation 103:155–160 [DOI] [PubMed]
- 32.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S,Mc Donald JA, Dedhar S (1998) Integrin- linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression and tumorigenicity. J Biol Chem 273:528–536 [DOI] [PubMed]
- 33.Zigrino P, Drescher C, Mauch C (2001) Collagen-induced proMMP-2 activation by MT1-MMP in human dermal fibroblasts and the possible role of alpha2beta1 integrins. Eur J Cell Biol 80: 68–77 [DOI] [PubMed]