Abstract
Background
The radiographic evaluation of the response to preoperative chemotherapy for bone and soft tissue sarcomas is based mostly on the change in primary tumor size before and after chemotherapy, as is done for many solid cancers. Its prognostic correlation, however, has hardly been validated.
Methods
We conducted a retrospective validation study of the Japanese Orthopaedic Association (JOA) radiographic response evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas as a JOA Committee on Musculoskeletal Tumors cooperative study. A total of 125 consecutive patients with high-grade bone (n = 77) and soft tissue (n = 48) sarcomas treated with neoadjuvant chemotherapy and definitive surgery in 25 tertiary referral hospitals were selected for the study. We investigated the correlation between the tumor size-based radiographic response evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas provided by the JOA Committee on Musculoskeletal Tumors (hereafter called the JOA criteria) and the patients’ overall survival using the Kaplan-Meier method and the log-rank test.
Results
The JOA criteria correlated relatively well with survival for malignant bone tumors (mostly comprising osteosarcoma and Ewing’s sarcoma) but not for soft tissue sarcomas, suggesting that the tumor size-based radiographic evaluation criteria for the response to preoperative chemotherapy in patients with soft tissue sarcomas is invalid.
Conclusions
The JOA criteria, based on the change in primary tumor size, is valid for malignant bone tumors but invalid for soft tissue sarcomas. Other new evaluation modalities of the response to preoperative chemotherapy using innovative functional imaging techniques are needed for soft tissue sarcomas.
Introduction
The multidisciplinary approach, including neoadjuvant chemotherapy and wide local excision of primary tumors with or without radiation therapy, has dramatically improved the prognosis of patients with malignant bone and soft tissue sarcomas, especially osteosarcoma1–3 and Ewing’s sarcoma.4–6 Furthermore, the limb-salvage procedure is now established as a standard treatment modality in this field. To accomplish limb-salvage surgery confidently, it is mandatory to evaluate the clinical response of primary tumors to preoperative chemotherapy.
We have been using radiographic and histological evaluation criteria to estimate the efficacy of preoperative chemotherapy for bone and soft tissue sarcomas.7,8 The radiographic response is evaluated based on the change in the primary tumor size before and after preoperative chemotherapy in the Japanese Orthopaedic Association (JOA) evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas (hereafter called the JOA criteria). This measure is done also for many solid cancers using World Health Organization (WHO) evaluation criteria or, more recently, the Response Evaluation Criteria in Solid Tumors (RECIST) in the oncology field worldwide.9 The prognostic significance of histological evaluation of response to preoperative chemotherapy assessed by the percent ratio of treatment-induced tumor necrosis in surgically resected tumor specimens has been well proven in patients with malignant bone tumors such as osteosarcoma10 and Ewing’s sarcoma,11,12 although it is still controversial for soft tissue sarcomas.13,14
There is generally no consensus that the radiographic evaluation of response to preoperative chemotherapy based on the change of tumor size correlates with survival in patients with bone and soft tissue sarcomas, and its prognostic implication has been validated only in a retrospective study of 65 patients with soft tissue sarcomas.15 Thus, we have conducted a retrospective validation study of the JOA criteria of preoperative chemotherapy for bone and soft tissue sarcomas as a cooperative study by the JOA Committee on Musculoskeletal Tumors.
Materials and methods
Patients
A total of 125 consecutive patients with high-grade bone and soft tissue sarcomas treated with neoadjuvant chemotherapy and definitive surgery in 25 tertiary referral cancer centers or university hospitals between January and December in 1997 were selected for the current study according to an inquiry conducted by the JOA Committee on Musculoskeletal Tumors in 2002. The cases included 77 primary malignant bone tumors and 48 soft tissue sarcomas.
The patient and tumor characteristics are summarized in Table 1. There were 75 male and 50 female patients, with ages ranging from 4 to 75 years (median 25 years). The location of the primary bone tumors were the femur in 32 patients, tibia in 19, pelvis in 11, humerus in 5, and other bones in 10; the soft tissue sarcomas were located in the lower extremity in 35, trunk in 8, and upper extremity in 5. Altogether, 104 patients had localized disease, and the other 21 (16.8%) had distant metastasis at presentation.
Table 1.
Patient and tumor characteristics (n = 125)
| Bone tumors | Soft tissue sarcomas | ||||
|---|---|---|---|---|---|
| Characteristics | Total or range | (n = 77) | (n = 48) | ||
| Sex (M:F) | 75:50 | 46:31 | 29:19 | ||
| Age (years) | |||||
| Range (median) | 4–75 (25) | 4–65 (16) | 11–75 (49) | ||
| Disease stage at presentation | |||||
| Localized (M0) | 104 | 67 | 37 | ||
| Metastatic (M1) | 21 | 10 | 11 | ||
| Primary location | |||||
| Femur | 32 | Lower extremitya | 35 | ||
| Tibia | 19 | Upper extremitya | 5 | ||
| Pelvis | 11 | Trunk | 4 | ||
| Humerus | 5 | Head and neck | 4 | ||
| Other bones | 10b | ||||
| Histological type | |||||
| Osteosarcoma | 53 | MFH | 11 | ||
| Ewing’s sarcoma | 11 | Liposarcoma | 7 | ||
| MFH | 4 | Synovial sarcoma | 6 | ||
| Postradiation sarcoma | 3 | Rhabdomyosarcoma | 5 | ||
| Others | 6c | MPNST | 3 | ||
| Extraskeletal chondrosarcoma | 3 | ||||
| Othersd | 13 | ||||
MFH, malignant fi brous histiocytoma; MPNST, malignant peripheral nerve sheath tumor
a Includes limb girdles
b Includes the ulna 2, rib 2, sacrum 2, fi bula 2, scapula 1, lumber spine 1
c Includes dedifferentiated chondrosarcoma 1, mesenchymal chondrosarcoma 1, angiosarcoma 1, parosteal osteosarcoma 1, fi brosarcoma 1, leiomyosarcoma 1
d Includes leiomyosarcoma 2, primitive neuroectodermal tumor 2, clear cell sarcoma 2, epithelioid sarcoma 2, postradiation sarcoma (MFH) 1, alveolar soft-part sarcoma 1, extraskeletal osteosarcoma 1, malignant rhabdoid tumor 1, undifferentiated sarcoma 1
Histological types among the bone tumors included osteosarcoma in 53 patients, Ewing’s sarcoma in 11, malignant fibrous histiocytoma (MFH) in 4, postradiation sarcoma in 3, and others in 6. Histological types of soft tissue sarcoma included MFH in 11, liposarcoma in 7, synovial sarcoma in 6, rhabdomyosarcoma in 5, malignant peripheral nerve sheath tumor (MPNST) in 3, extraskeletal chondrosarcoma in 3, and others in 13. All but eight patients received preoperative and postoperative neoadjuvant chemotherapy and subsequently underwent definitive surgery with/or without radiation therapy. The eight exceptions had metastatic disease at presentation (four with bone tumors, four with soft tissue sarcoma). One patient with localized rhabdomyosarcoma in the groin had a complete response to the induction chemotherapy. The neoadjuvant chemotherapy regimens are listed in Table 2.
Table 2.
Neoadjuvant chemotherapy regiments (n = 125)
| Disease | No. |
|---|---|
| Bone tumors (n = 77), osteosarcoma (n = 53) | |
| ADR/CDDP/MTX | 10a |
| ADR/CDDP/IFO | 8 |
| CDDP/MTX | 5 |
| ADR/CDDP | 4 |
| ADR/CDDP/IFO/MTX | 3 |
| NECO95J | 10 |
| NECO93J | 4 |
| Others | 9a |
| Ewing’s sarcoma (n = 11) | |
| VAdrC/IFO | 5b |
| VAdrC/IE/ICE | 3 |
| EVAIA | 1 |
| VAC/IE | 1 |
| VAIA + cyclosporin | 1 |
| Others (n = 13) | |
| ADR/CDDP | 3 |
| ADR/CDDP/MTX | 2 |
| Others | 8c |
| Soft tissue sarcomas (n = 48) | |
| IFO (high-dose) | 7 |
| ADR or THP/CDDP/IFO | 6 |
| ADR or THP/CDDP | 4 |
| MAID | 4 |
| CDDP/THP/IFO/ETP | 4d |
| K3 protocol | 4d |
| ADR/IFO | 3 |
| Others | 16d |
ADR, adriamycin; CDDP, cisplatin; MTX, methotrexate; IFO, ifosfamide; NECO93J/95J, multiinstitutional study of neoadjuvant chemotherapy using MTX/ADR/CDDP with/or without IFO for osteosarcoma in Japan; VAdrC, vincristine (VCR) + ADR + cyclophosphamide (CPA); IE, IFO + etoposide (ETP); ICE, IFO + carboplatin (CBDCA) + ETP; EVAIA, ETP + VCR + ADR + IFO + actinomycin D (ACD); VAC, VCR + ACD + CPA; VAIA, VCR + ADR + IFO + ACD; THP, pirarubicin; MAID, mesna + ADR + IFO + dacarbazine (DTIC); K3 protocol: chemotherapy regimen originally used in Kanazawa University
a–d Combined with caffeine in a seven cases, b one case, c two cases, and nine cases
Radiographic response evaluation of preoperative chemotherapy
To evaluate the response to preoperative chemotherapy radiographically, we have used the JOA criteria provided by the JOA Committee on Musculoskeletal Tumors, which was accordingly modified from the WHO/RECIST evaluation criteria (Table 3).7,8 These evaluation criteria are all fundamentally based on the radiographically determined change in size of the primary tumor from before to after chemotherapy estimated by computed tomography (CT) and/or magnetic resonance imaging (MRI) with contrast enhancement.
Table 3.
JOA Committee on Musculoskeletal Tumors radiographic response evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas7,8 modified from the WHO/RECIST criteria for solid cancers9
| Malignant bone tumors | |
| • | Subjects: restricted to extraosseously involving lesions |
| • | Method: measured by the maximal distance (MD) from the surface of affected bone to the top of extraosseous mass lesion on CT and/or MRI images, then estimated by the tumor reduction ratio Tumor reduction ratio = (pretreatment MD — posttreatment MD)/(pretreatment MD) × 100 (%) |
| • | Categories of response evaluation |
| CR (complete response) — disappearance of the extraosseous mass lesion continued for ≥3 weeks | |
| PR (partial response) — at least 30% reduction of the extraosseous mass continued for ≥3 weeks | |
| NC (no change) — 10% expansion to lt30% reduction of the extraosseous mass continued for ≥3 weeks | |
| PD (progressive disease) — more than 10% expansion of the extraosseous mass or other lesions newly emerged | |
| Soft tissue sarcomas | |
| • | Subjects: all high-grade soft tissue sarcomas treated by neoadjuvant chemotherapy |
| • | Methods: estimated bidimensionally or unidimensionally by the change of maximal tumor size before and after preoperative chemotherapy using contrast-enhanced CT and/or MRI images. |
| Bidimensional measurement | |
| Tumor reduction ratio = (pretreatment MTD × VTD) − [posttreatment (MTD × VTD)/pretreatment (MTD × VTD)] × 100 (%) | |
| where MTD is the maximum tumor diameter, and VTD is the vertical tumor diameter | |
| Unidimensional measurement | |
| Tumor reduction ratio = (pretreatment MTD — posttreatment MTD)/(pretreatment MTD) × 100 (%) | |
| • | Categories of response evaluation |
| CR — disappearance of primary tumor continued for ≥4 weeks | |
| PR — at least 50% reduction in the size of the primary tumor measured in two dimensions or at least 30% reduction by single dimension continued for ≥4 weeks | |
| NC — from 25% (by two dimensions) or 10% (by single dimension) expansion of the primary tumor to <50% (by two dimensions) or 30% (by single dimension) reduction of primary tumor continued for ≥4 weeks | |
| PD — more than 25% (by two dimensions) or 10% (by single dimension) expansion of primary tumor or other lesions newly emerged | |
| MR (minor response) — lesions with PR effect lasting <4 weeks or with 25%–50% reduction in size continued ≥4 weeks were separately estimated as MR in soft tissue sarcomas | |
All patients were evaluated radiographically for the response to preoperative chemotherapy according to the criteria defined as follows: complete response (CR), disappearance of the extraosseous mass lesion continued for ≥3 weeks; partial response (PR), ≥30% reduction of the extraosseous mass continued for ≥3 weeks; no change (NC), from 10% expansion to <30% reduction of extraosseous mass continued for ≥3 weeks; progressive disease (PD), >10% expansion of extraosseous mass or other lesions newly emerged in the case of either bone tumors and soft tissue sarcomas; CR, disappearance of primary tumor continued for ≥4 weeks; PR, ≥50% reduction in the size of the primary tumor (two-dimensional) or ≥30% reduction (one-dimensional) continued for ≥4 weeks; NC, from 25% (two-dimensional) or 10% (one-dimensional) expansion of the primary tumor to <50% (two-dimensional) or 30% (one-dimensional) reduction of the primary tumor continued for ≥4 weeks; PD, >25% (two-dimensional) or 10% (one-dimensional) expansion of the primary tumor or other lesions newly emerged. The lesions with PR lasting <4 weeks or with 25%–50% reduction in size continued ≥4 weeks were separately estimated as a minor response (MR) in cases of soft tissue sarcoma. The radiographic response to preoperative chemotherapy should be evaluated more than 4 weeks after the end of the treatment, as originally defined in the WHO/RECIST criteria. The JOA criteria adopt the same interval of ≥4 weeks for soft tissue sarcomas. For bone tumors, however, the definitive surgery for primary tumors is generally performed within 3 weeks following the completion of preoperative chemotherapy. Consequently, the JOA evaluation criteria modified the interval from ≥4 weeks to ≥3 weeks to estimate the radiographic response to preoperative chemotherapy for bone tumors, and we accordingly use these modified criteria in Japan.
Statistical analysis
Overall survival rates of the patients were estimated by the method of Kaplan and Meier and were calculated from the date of the initial treatment (start of preoperative chemotherapy). A terminal point of overall survival was defined as the date of the patient’s death from the disease or from treatment-related toxicity. Deaths from any other concurrent causes were estimated as censored data in overall survival. The log-rank test was used to evaluate the significance of differences between the groups. Two-tailed P < 0.05 was considered statistically significant in comparison with survival curves. These statistical analyses were performed using the JMP version 5.01 statistical analysis software package for personal computers (SAS Institute, Cary, NC, USA).
Results
Radiographic response to preoperative chemotherapy
Table 4 shows the results of the radiographic responses to preoperative chemotherapy in 125 patients with bone and soft tissue sarcomas. There were 7 cases of CR, 26 PR, 27 NC, and 17 PD in patients with malignant bone tumors and 2 CR, 9 PR, 32 NC (including 9 MR), and 5 PD in those with soft tissue sarcomas. The objective response rates (patients with CR or PR/all patients) of patients with bone and soft tissue sarcomas were 43% (33/77) and 23% (11/48), respectively. The patients with CR included four with Ewing’s sarcoma, two with osteosarcoma, one with MFH of bone, and two with soft tissue rhabdomyosarcoma.
Table 4.
Results of radiographic response to preoperative chemotherapy (n = 125)
| Parameter | Malignant bone tumors (n = 77) | Soft tissue sarcomas (n = 48) |
|---|---|---|
| CR | 7a | 2b |
| PR | 26 | 9 |
| NC | 27 | 32c |
| PD | 17 | 5 |
| ORR | 33/77 (43%) | 11/48 (23%) |
ORR, objective response rate (% ratio of the number of CR + PR cases)
a Osteosarcoma 2/53 (ORR 4%); Ewing’s sarcoma 4/11 (ORR 36%); MFH of bone 1/4 (ORR 25%)
b Rhabdomyosarcoma 2/5 (ORR 40%)
c Including nine minor response cases
Correlation of radiographic response with survival in malignant bone tumors
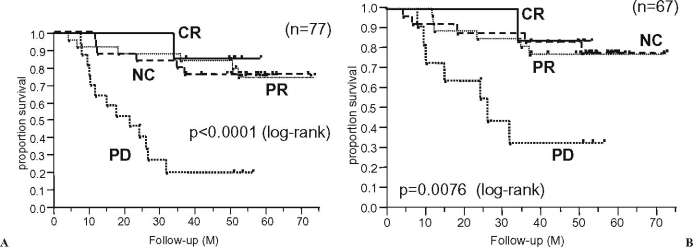
Figure 1 shows the overall survival curves according to the radiographic response to preoperative chemotherapy for all patients (n = 77) with malignant bone tumors and for 67 patients without metastasis at presentation. The radiographic response to preoperative chemotherapy correlated relatively well with the patients’ overall survival (P < 0.0001 for all patients and P = 0.0076 for the patients without metastasis at presentation, by log-rank test), although there seemed to be no prognostic correlation between the PR and NC groups.
Fig. 1.
Overall survival curves according to the radiographic response to preoperative chemotherapy for malignant bone tumors. CR, complete response; PR, partial response; NC, no change; PD, progressive disease. A All patients (n = 77). B Patients without metastasis at presentation (n = 67)
Figures 2 and 3 show the overall survival curves according to the radiographic response to preoperative chemotherapy in osteosarcoma patients (n = 53) and Ewing’s sarcoma patients (n = 11), respectively. In patients with both tumors, the JOA criteria were significantly associated with survival, suggesting their clinical validity in patients with malignant bone tumors.
Fig. 2.
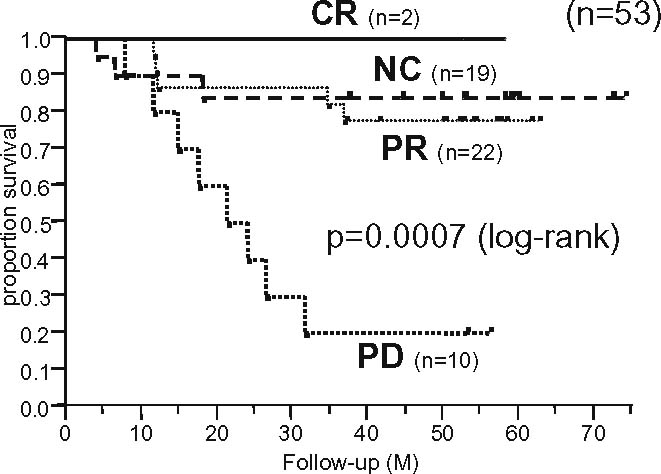
Overall survival curves according to the radiographic response to preoperative chemotherapy for osteosarcoma (n = 53)
Fig. 3.
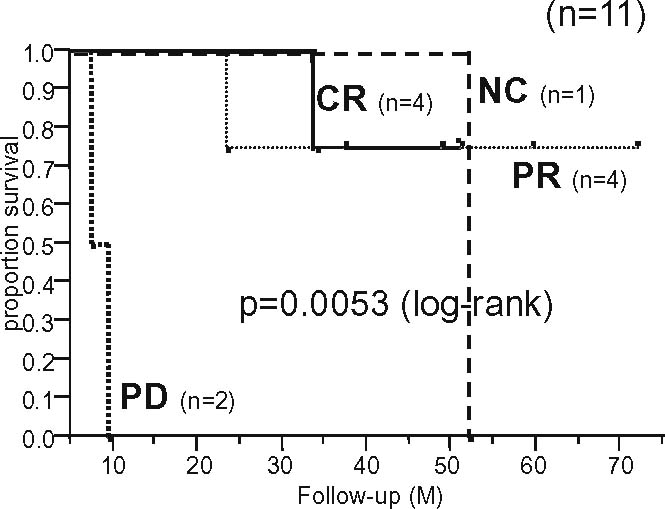
Overall survival curves according to the radiographic response to preoperative chemotherapy for Ewing’s sarcoma (n = 11)
Correlation of radiographic response with survival in soft tissue sarcomas
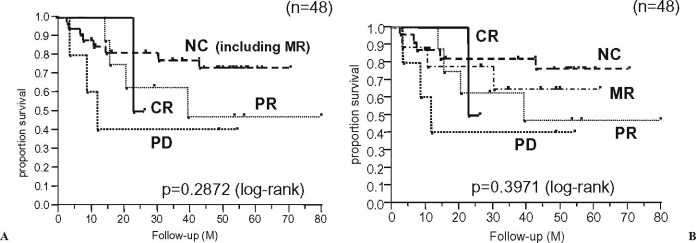
In patients with soft tissue sarcomas (n = 48), the radiographic response to preoperative chemotherapy did not significantly correlate with the patients’ survival (Fig. 4A). When the patients with NC were divided into NC and MR, there was also no correlation between the response and survival (Fig. 4B). Regarding soft tissue sarcomas, the patients with radiographically NC tended to have a better long-term survival than those with CR, PR, MR, or PD. Furthermore, there was also no significant association between the JOA criteria and survival even in patients without metastasis at presentation (n = 37) or in patients with soft tissue sarcomas excluding small round-cell sarcomas (five rhabdomyosarcomas and two primitive neuroectodermal tumors) (n = 41). In the seven patients with small round-cell sarcomas, there was no significant correlation between the radiographic response and survival because of the small number of these patients in the present series (data not shown).
Fig. 4.
Overall survival curves according to the radiographic response to preoperative chemotherapy for soft tissue sarcomas (n = 48). A CR vs. PR vs. NC vs. PD. B CR vs. PR vs. MR vs. NC vs. PD. MR, minor response especially
Discussion
The response to chemotherapy for solid cancers has been traditionally evaluated based on the radiographic change in tumor size before and after treatment. The WHO evaluation criteria were simplified and modified in 2000 to RECIST criteria,9 in which only one-dimensional measurement of the maximum tumor diameter in the axial plane of CT and/or MRI is used. Until now, the RECIST method has been widely used to evaluate the response to chemotherapy in the field of clinical oncology because of its simplicity and easy applicability. However, in nonspherical or irregularly shaped tumors such as esophageal cancer and malignant mesothelioma it is generally difficult to evaluate the response to chemotherapy accurately using the RECIST compared with relatively spherical tumors such as breast cancer, lymphoma, and metastatic lung/liver cancers.16 For these tumors, the RECIST is modified to fit the tumor, or it is even replaced by other biological modalities, such as serum tumor markers or glucose consumption measured by fl uorodeoxyglucose positron emission tomography (FDG-PET) scanning to estimate the response to chemotherapy.17–20 Interestingly, the tumors for which it was difficult to evaluate the chemotherapeutic response correctly by RECIST were reported to be nonspherical, irregularly shaped tumors even among breast cancers.21 In lymph node and skeletal metastases from various cancers (e.g., breast and prostate cancers) it is also difficult to evaluate the response to chemotherapy by a change in tumor size, and skeletal metastases have been primarily excluded from evaluable lesions by the RECIST method. Moreover, RECIST was originally developed as a tool for screening potential, new anticancer agents in clinical trials where response is the endpoint. RECIST was applied to define the clinical response rate, progression rate, and/or time to progression quickly, not necessarily to predict patients’ prognosis in clinical practice.
With regard to bone and soft tissue sarcomas, the response to preoperative chemotherapy as “neoadjuvant” is routinely estimated by radiographic examinations including CT and/or MRI as well as by histopathological evaluation of the extent of tumor necrosis (i.e., the ratio of residual viable tumor cells) using surgically resected tumor specimens, especially for osteosarcomas10 and Ewing’s sarcoma.11,12 In malignant bone tumors, the JOA criteria are directed only at extraosseous lesions, modifying the WHO/RECIST criteria because the radiographic changes induced by chemotherapy reflect only the mixture of destructive change by tumors and reactive change by surrounding tissues, and the affected bone never returns to normal status. This modification of the evaluation criteria for malignant bone tumors is similar to that for malignant mesothelioma.22 Moreover, in soft tissue sarcomas, the JOA criteria discussed here are fundamentally based on the WHO/RECIST criteria and note that the necrotic area within the tumor after chemotherapy should also be interpreted as the effect of chemotherapy when the size of the tumor does not change before and after chemotherapy. The necrotic area is usually evaluated by gadolinium-enhanced MRI; however, it is frequently difficult to estimate clinically the precise area of intratumoral necrosis because of its complicated shape and/or distribution within the tumor and to distinguish necrosis following chemotherapy from that due to spontaneous tumor necrosis.
The present retrospective study has demonstrated that the JOA radiographic response evaluation criteria of preoperative chemotherapy — modifying the WHO/RECIST criteria based on the change in tumor size — correlate relatively well with the prognosis of patients with malignant bone tumors but not with that of patients with soft tissue sarcomas. Patients with soft tissue sarcomas whose disease was labeled radiographically as NC (defined as SD, or stable disease, in RECIST) tended to have better long-term survival than those whose disease was labeled CR, PR, MR, or PD. Similar results were indicated in patients with gastrointestinal stromal tumors (GISTs) by Choi and colleagues.23,24
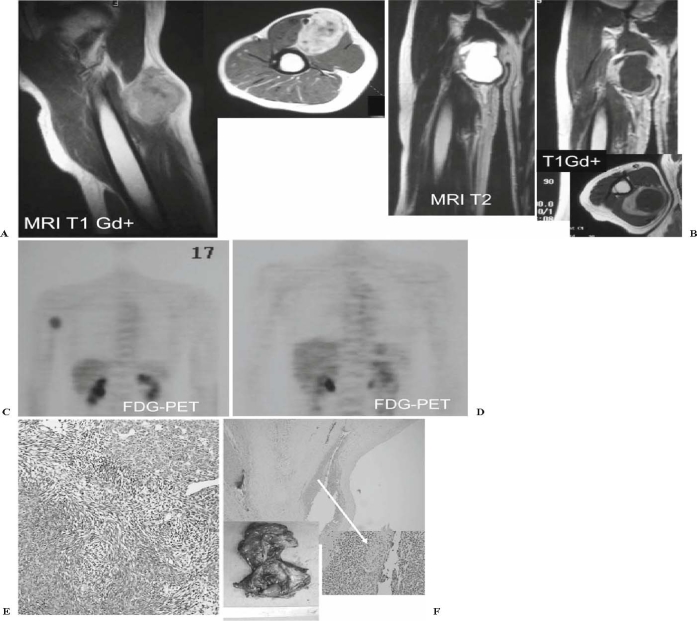
It has been pointed out that patients with various advanced cancers whose disease was estimated not only as CR or PR but also as NC/SD were considered to be able to have their survival prolonged in clinical trials using new molecular targeting agents that exhibited cytostatic rather than cytocidal activities on cancer cells.25 There are possibly two major reasons for this phenomenon in soft tissue sarcomas: (1) Tumor necrosis induced by preoperative chemotherapy does not necessarily reflect tumor shrinkage; instead, cystic change following tumor necrosis often results in temporary tumor enlargement (Fig. 5). (2) Soft tissue sarcomas show a relatively slower growing pattern than do osteosarcoma and Ewing’s sarcoma; thus, prompt tumor reduction in size is not expected only after several cycles of preoperative chemotherapy. Indeed, we recently treated a 20-year-old woman with a huge round-cell liposarcoma in her left leg associated with a solitary lung metastasis at presentation. She responded to 10 cycles of preoperative chemotherapy using high-dose ifosfamide (12.0–13.5 g/m2 per cycle with mesna) by finally being estimated as PR for the primary tumor and CR for the lung metastasis (data not shown). The discrepancy between the tumor size-based radiographic response evaluation criteria and the survival of patients with soft tissue sarcomas might be influenced by other factors, such as using different chemotherapeutic regimens and estimating the effect only by the changes in the size of the primary tumor.
Fig. 5.
A 43-year-old man had a poorly differentiated synovial sarcoma in his right upper arm. A Magnetic resonance imaging (MRI) at his initial presentation. B MRI after four cycles of preoperative chemotherapy. There was marked cystic change in the tumor but no reduction in tumor size. C Fluorodeoxyglucose positron emission tomography (FDG-PET) at the initial presentation, showing abnormally hot uptake at the tumor. D FDG-PET after four cycles of preoperative chemotherapy showed disappearance of glucose uptake in the tumor. E Histology at biopsy (H&E). F Surgically resected specimen after preoperative chemotherapy, confirming the marked cystic change and only minimal residual viable tumor cells along the cyst wall (arrow)
The present study has strongly suggested that the tumor size-based radiographic response evaluation criteria for preoperative chemotherapy do not predict survival for patients with soft tissue sarcomas. Thus, we need to search for a more sensitive and specific evaluation method, such as FDG-PET20,26,27 or thallium (201Tl) scintigraphy,28,29 rather than the usual strictly size-based response evaluation criteria by CT and/or MRI to estimate the efficacy of newly developed chemotherapeutic (cytocidal) and molecular-targeting (cytostatic) agents for soft tissue sarcomas.
Choi et al. recently proposed novel response evaluation criteria for molecular-targeted anticancer drugs using a combination of reduced tumor size and decreased density on contrast-enhanced CT through their experiences in clinical trials of imatinib mesylate (Gleevec) for patients with GIST.23,24 Furthermore, several studies have suggested the usefulness of FDG-PET to estimate the response to preoperative chemotherapy in patients with osteosarcoma and Ewing’s sarcoma.26,27 However, FDG-PET has some limitations for evaluating tumor response to treatment, such as specificity, access, cost, and quantitative measurements; and at present it is not used universally to assess the response of various malignant tumors including GIST.
We plan to investigate a new method to evaluate the three-dimensional (3D) volumetric change of intratumoral necrosis, measured by density/intensity change on 3D-CT/or MRI scans. We think it may be a more sensitive, more specific modality for assessing the response of bone and soft tissue sarcomas to chemotherapy than the traditional but somewhat outdated size-based response evaluation criteria such as RECIST.
Conclusion
The JOA radiographic response evaluation criteria of preoperative chemotherapy based on the change of primary tumor size is valid for malignant bone tumors but invalid for soft tissue sarcomas. Other evaluation modalities to assess the response to preoperative chemotherapy using innovative functional imaging techniques, such as FDG-PET and contrast-enhanced 3D-CT/MRI, are needed for soft tissue sarcomas.
Acknowledgment
We gratefully appreciate that the following doctors also participated in this cooperative study: Satoshi Abe, MD, Teikyo University School of Medicine; Yutaka Nishimoto, MD, Gifu University School of Medicine; Jun Sonoda, MD and Atsumasa Uchida, MD, Mie University Postgraduate School of Medicine; Yoshihiro Nishida, MD, Nagoya University Graduate School of Medicine; Eiichi Sato, MD and Yoshiki Hamada, MD, University of Yamanashi, Faculty of Medicine; Masahiko Kanamori, MD, University of Toyama, Faculty of Medicine; Takeshi Minamizaki, MD, Tottori University Faculty of Medicine; Shoji Shimose, MD, Hiroshima University Graduate School of Biomedical Sciences; Hiroshi Kakizaki, MD, Hirosaki National Hospital; Hideomi Watanabe, MD, Gunma University Faculty of Medicine; and Koichi Saotome, MD, Dokkyo Medical University, Japan.
Footnotes
The *former and **present chairmen of the JOA Committee on Musculoskeletal Tumors
References
- 1.Uchida A, Myoui A, Araki N, Yoshikawa H, Shinto Y, Ueda T. Neoadjuvant chemotherapy for pediatric osteosarcoma patients. Cancer 1997;79:411–415. [DOI] [PubMed]
- 2.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol 1998;16:2452–2458. [DOI] [PubMed]
- 3.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities and trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–790. [DOI] [PubMed]
- 4.Paulussen M, Ahrens S, Dunst J, Winkelmann W, Exner GU, Kotz R, et al. Localized Ewing tumor of bone: final results of the Cooperative Ewing’s Sarcoma Study CESS 86. J Clin Oncol 2001;19:1818–1829. [DOI] [PubMed]
- 5.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694–701. [DOI] [PubMed]
- 6.Obata H, Ueda T, Kawai A, Ishii T, Ozaki T, Abe S, et al. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: the Japanese Musculoskeletal Oncology Group Cooperative Study. Cancer 2007;109:767–775. [DOI] [PubMed]
- 7.Committee on Musculoskeletal Tumors. General rules for clinical and pathological studies on malignant bone tumors. 3rd edn. Tokyo: Kanehara; 2000. p. 67–68 (in Japanese).
- 8.Committee on Musculoskeletal Tumors. General rules for clinical and pathological studies on malignant soft tissue tumors. 3rd edn. Tokyo: Kanehara; 2002. p. 84–85 (in Japanese).
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed]
- 10.Huvos AG. Osteogenic sarcoma: pathologic assessment of preoperative (neoadjuvant) chemotherapy. In: Bone tumors: diagnosis, treatment, and prognosis. 2nd edn. Philadelphia: Saunders; 1991. p. 122–128.
- 11.Picci P, Rougraff BT, Bacci G, Neff JR, Sangiorgi L, Cazzola A, et al. Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing’s sarcoma of the extremities. J Clin Oncol 1993;11:1763–1769. [DOI] [PubMed]
- 12.Wunder JS, Paulian G, Huvos AG, Heller G, Meyers PA, Healey JH. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am 1998;80:1020–1033. [DOI] [PubMed]
- 13.Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 2001; 19:3203–3209. [DOI] [PubMed]
- 14.Menendez LR, Ahlmann ER, Savage K, Cluck M, Fedenko AN. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin Orthop 2006;455:219–224. [DOI] [PubMed]
- 15.Meric F, Hess KR, Varma DG, Hunt KK, Pisters PW, Milas KM, et al. Radiographic response to neoadjuvant chemotherapy is a predictor of local control and survival in soft tissue sarcomas. Cancer 2002;95:1120–1126. [DOI] [PubMed]
- 16.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 2006;42:1031–1039. [DOI] [PubMed]
- 17.Scher HI, Morris MJ, Kelly WK, Schwartz LH, Heller G. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res 2005;11:5223–5232. [DOI] [PMC free article] [PubMed]
- 18.Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer 2006;6:409–414. [DOI] [PubMed]
- 19.Moon L, McHugh K. Advances in paediatric tumour imaging. Arch Dis Child 2005;90:608–611. [DOI] [PMC free article] [PubMed]
- 20.Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol 2006;24:3245–3251. [DOI] [PubMed]
- 21.Prasad SR, Saini S, Sumner JE, Hahn PF, Sahani D, Boland GW. Radiological measurement of breast cancer metastases to lung and liver: comparison between WHO (bidimensional) and RECIST (unidimensional) guidelines. J Comput Assist Tomogr 2003;27:380–384. [DOI] [PubMed]
- 22.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257–260. [DOI] [PubMed]
- 23.Choi H. Critical issues in response evaluation on computed tomography: lessons from the gastrointestinal stromal tumor model. Curr Oncol Rep 2005;7:307–311. [DOI] [PubMed]
- 24.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760–1764. [DOI] [PubMed]
- 25.Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol 2004;22:4442–4445. [DOI] [PubMed]
- 26.Aoki J, Endo K, Watanabe H, Shinozaki T, Yanagawa T, Ahmed AR, et al. FDG-PET for evaluating musculoskeletal tumors: a review. J Orthop Sci 2003;8:435–441. [DOI] [PubMed]
- 27.Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU 3rd, et al. [18F]fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828–8834. [DOI] [PubMed]
- 28.Menendez LR, Fideler BM, Mirra J. Thallium-201 scanning for the evaluation of osteosarcoma and soft tissue sarcoma: a study of the evaluation and predictability of the histological response to chemotherapy. J Bone Joint Surg Am 1993;75:526–531. [DOI] [PubMed]
- 29.Goto Y, Ihara K, Kawauchi S, Ohi R, Sasaki K, Kawai S. Clinical significance of thallium-201 scintigraphy in bone and soft tissue tumors. J Orthop Sci 2002;7:304–312. [DOI] [PubMed]