Abstract
Objective. To assess gender differences in morbidity, mortality and patient management among adults born with a heart defect.
Methods and results. The database of the European Heart Survey on adult congenital heart disease was explored. This contains data on 4110 patients with one of eight congenital heart defects followed retrospectively for a median of 5.1 years. The existence of gender differences was assessed by considering mortality and a few ‘overall’ measures of morbidity. Adjusting for type of defect and age, it was found that cumulative mortality was greater in the male population (hazard ratio 1.63 (95% CI 1.12 to 2.38); p=0.011)). A significantly greater proportion of females had functional limitations (NYHA functional class >1; 37% vs. 29% of men; p=0.003). However, males were more likely to be on chronic medication during follow-up (59% vs. 55% of women; p=0.001), and males underwent diagnostic procedures more frequently (1.58/patient-year vs. 1.48/patient-year for women; p<0.02). There was no significant difference in the proportions of patients who underwent at least one intervention during follow-up, and rates of outpatient (re-)visits were not different between the sexes.
Conclusion. This exploratory assessment of a large international database found evidence that gender differences exist in morbidity and mortality among adult patients with congenital heart disease, as well as in medical management. Future studies in adult congenital heart disease should always take into account the effects of gender. (Neth Heart J 2009;17:414-7.)
Keywords: adult congenital heart disease, gender, gender differences, Euro Heart Survey
Within the field of cardiology the issue of gender differences has received attention since it was recognised that risk factors for cardiovascular disease were unevenly distributed according to sex. In recent years, it has been increasingly realised that not only does gender influence the manifestations of disease, but also that the management of men and women is often unequal.1 We used the database of the Euro Heart Survey (EHS) on adult congenital heart disease to explore the role of gender differences in adult congenital heart disease.
Methods
This study consisted of an analysis of data collected as part of the EHS on adult congenital heart disease. The methods used for this Survey have been extensively described elsewhere.2 Briefly, consecutive patients with one of eight congenital cardiac defects visiting an outpatient clinic of one of the participating centres in 1998 were identified, and their clinical course was documented in retrospect until April 2004. Data on medical history, results of diagnostic procedures and interventions were transcribed from patient records into an electronic case record file.
Statistical analysis
Descriptive statistics were used to estimate the following characteristics: the proportion of patients who were in New York Heart Association (NYHA) functional class greater than 1 at baseline (NYHA >1); the proportion of patients who were not on chronic medication during the follow-up period; the proportion of patients who underwent at least one intervention (surgery or a transcatheter intervention) during follow-up. These proportions were determined separately for men and women, both per defect and overall (aggregate). As it is known that sex distribution is unequal according to defect, logistic regression analysis was used to adjust for defect and age. In order to compare the numbers of outpatient (re-)visits (excluding the first visit) and diagnostic procedures, these were considered to be events per patient-year of follow-up and approximately distributed as a Poisson distribution. Rates were then compared using Mantel-Haenszel estimation to control for type of defect.3 Finally, survival analysis was performed to assess differences between men and women in the cumulative probability of death. Cox regression was used to adjust for type of defect and age. As a post-hoc analysis, we further analysed differences in the prevalence of arrhythmias between men and women. This was done using logistic regression, again to adjust for type of defect and age.
Results
Table 1 shows the distribution of patients according to sex and defect. Distribution is seen to be unequal: more females among patients with an atrial septal defect, but more males among patients with transposition of the great arteries (TGA) or aortic coarctation. In table 2 only the aggregate proportions of patients fulfilling the criteria tested are shown. Adjusting for type of defect, females were more likely to have functional limitations due to their cardiac defect (odds ratio (OR) 1.27 (95% CI 1.09 to 1.48); p=0.002), while males were more likely to be on chronic medication during follow-up (OR 1.26 (1.09 to 1.46); p=0.001). Males underwent diagnostic procedures more frequently, but there was no significant difference in the proportions of patients who underwent at least one intervention during follow-up. These differences in drug treatment and diagnostic procedure frequencies were found for all defects. Rates of outpatient (re-)visits were not significantly different between the sexes.
Table 1.
Sex distribution per defect.
| N | ||
|---|---|---|
| Females (%) | Males | |
| Atrial septal defect | 589 (67) | 293 |
| Ventricular septal defect | 332 (53) | 296 |
| Tetralogy of Fallot | 388 (48) | 423 |
| Aortic coarctation | 211 (38) | 340 |
| Transposition of the great arteries | 141 (39) | 222 |
| Marfan syndrome | 143 (50) | 144 |
| Fontan circulation | 89 (45) | 109 |
| Cyanotic defect | 241 (62) | 149 |
| Overall | 2134 (52) | 1976 |
Table 2.
Overall gender differences per category.
| Females | Males | P value | |
|---|---|---|---|
| Age, mean (SD) | 32.3 (13.1) | 30.1 (12.4) | <0.001 |
| Region (%) | |||
| - Eastern Europe | 20 | 16 | |
| - Northern Europe | 13 | 15 | |
| - Southern Europe | 30 | 30 | |
| - Western Europe | 36 | 39 | |
| Current smokers (%)* | 6 | 13 | < 0.001 |
| History of arrhythmias, overall (%) | 23 | 26 | 0.005 |
| - Supraventricular | 19 | 20 | |
| - Ventricular | 5 | 4 | |
| - Conduction disturbances | 3 | 5 | |
| Previous endocarditis (%) | 3 | 4 | |
| Previous CVA/TIA (%) | 4 | 5 | |
| Previous MI/PTCA/CABG (%) | 1 | 1 | |
| NYHA >1 | 37 | 29 | 0.003 |
| Chronic drug treatment | 55 | 59 | 0.010 |
| At least 1 intervention | 22 | 17 | 0.578 |
| # diagnostic procedures per patient-years (rate) | 14060/9497 (1.48) | 14074/8909 (1.58) | <0.02 |
| # re-outpatient visits per patient-years (rate) | 9723/9497 (1.02) | 8533/8909 (0.96) | 0.051 |
*Percentage calculated with only patients whose smoking history was known in the denominator. All p values were calculated adjusting for defect and age. CVA=cerebral vascular accident, TIA=transient ischaemic attack, MI=myocardial infarction, PTCA=percutaneous transluminal coronary angioplasty, CABG=coronary artery bypass graft.
Table 3 provides absolute numbers of all-cause deaths and cardiovascular deaths for males and females per defect. The last column displays five-year cumulative survival (Kaplan-Meier) estimates. Especially in TGA, Fontan circulation and in cyanotic patients, mortality was higher amongst males. For the category of cyanotic patients, mortality figures for the Eisenmenger patients were as follows: 22 females died vs. 18 males; Kaplan-Meier five-year survival estimates were 81% for men vs. 85% for women (non-significant).
Table 3.
Mortality.
| Overall (N) | Cardiovascular | KM 5-year survival estimates | ||||
|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females (%) | Males (%) | |
| Atrial septal defect | 4 | 5 | 2 | 3 | 98.8 | 98.1 |
| Ventricular septal defect | 6 | 2 | 5 | 1 | 97.6 | 99.3 |
| Tetralogy of Fallot | 3 | 8 | 2 | 6 | 98.9 | 97.8 |
| Aortic coarctation | 2 | 1 | 1 | 1 | 98.9 | 99.8 |
| Transposition of the great arteries | 1 | 8 | 1 | 8 | 99.0 | 95.8 |
| Marfan syndrome | 1 | 4 | 1 | 2 | 99.0 | 96.7 |
| Fontan circulation | 4 | 11 | 2 | 10 | 94.6 | 88.3 |
| Cyanotic defect | 29 | 24 | 22 | 22 | 86.4 | 81.3 |
| Overall | 50 | 63 | 36 | 53 | 96.9 | 96.1 |
KM= Kaplan-Meier.
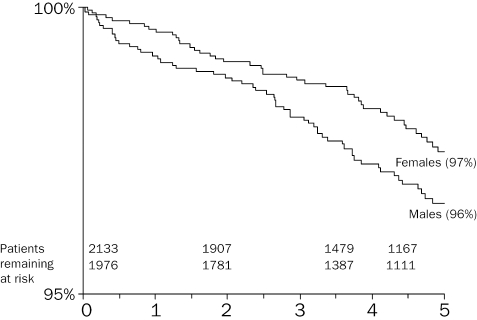
Figure 1 displays overall survival curves for females and males, showing higher mortality among males. Using Cox regression, males were found to be significantly more likely to die during follow-up than females (hazard ratio 1.63 (95% CI 1.12 to 2.38); p=0.011). Results of a post-hoc analysis (see above) showed that the prevalence of arrhythmias was significantly greater among males (OR 1.25 (1.07 to 1.46); p=0.005). Overall, the prevalence of arrhythmias was 26% amongst men vs. 23% for women.
Figure 1.
Crude five-year cumulative survival curves for men and women, aggregated over all defects.
Discussion
This analysis of data pertaining to a large European population of adult congenital heart disease patients reveals a few remarkable gender differences. A larger proportion of females were symptomatic, yet mortality over the five-year follow-up period was higher in males. More men received chronic medication and men underwent diagnostic procedures more frequently.
The role of gender has been hardly studied in adult congenital heart disease, apart, of course, from the risks associated with pregnancy. A published lecture by J. Somerville was devoted to gender issues in the care of adults with congenital heart disease.4 Although the main purpose of that report was to give advice to physicians, it also provided some data suggesting the existence of such gender differences as a higher female mortality among Eisenmenger patients, a less severe manifestation of aortic valve stenosis among females, and a greater longevity of pulmonary artery homografts in women. Recently, Verheugt and colleagues studied a large Dutch registry of adults with congenital heart disease (the CONCOR registry) to find significant gender differences in several outcomes. Women were found to be more prone to develop pulmonary hypertension, men had a higher risk of endocarditis and aortic complications (mainly aortic surgery). Men were also more likely to receive an ICD, while the frequency of ventricular arrhythmias was similar. The mortality rate was higher in men, although the difference in mortality was not significant, maybe due to the relatively small numbers of deaths.5,6 The database of the Euro Heart Survey on adult congenital heart disease offered an interesting opportunity to further explore the relevance of potential gender differences in this patient population.
Unequal sex distributions per defect largely reflect reported prevalences at birth, for which there is currently no explanation. As we performed our analyses controlling for type of defect, it is unlikely that the differences we found were due to that source of confounding. The fact that gender distribution remains roughly stable from birth into adulthood suggests a more or less equal mortality rate among the sexes. The finding in our study that mortality was lower among females, despite the hazards of pregnancy, is therefore surprising and requires further study. Especially in TGA and Fontan circulation, but also amongst cyanotic patients, relatively more men seemed to die. Does this mean that the more severe the defect the more vulnerable men are? As far as TGA is concerned, it could be that the higher mortality among males is partly related to smoking.7 One direction we were able to explore to find possible explanations for the excess mortality was the more frequent occurrence of arrhythmias among males. At the same time, more females had functional limitations. This seems somewhat paradoxical. However, it could be that a higher mortality due to arrhythmiacaused sudden death amongst men leaves a larger proportion of female survivors with relatively high morbidity. Although NYHA classification has a subjective component and partly reflects patients' and physicians' interpretations of symptoms, the fact that more males received chronic medication and that men underwent diagnostic procedures more frequently might indicate a certain degree of undertreatment of females. Alternatively, it cannot be excluded that the greater prevalence of functional limitations among women is actually a result of insufficient or inadequate treatment.
In general cardiology, the suggestion that in similar physical conditions females are less likely to be referred, undergo diagnostic investigation and receive treatment has been labelled the Yentl syndrome.8 Studies to assess the reality of the phenomenon have produced contradictory results. Thus, Roeters van Lennep and colleagues found no evidence for a different approach to women compared with men.9 However, a recent analysis of data from a Euro Heart Survey on stable angina did find gender differences in patient management and outcomes.10 The evidence for the existence of gender differences in morbidity, mortality and medical management that we found in the data of the Euro Heart Survey and are reporting here seems to provide some support for the relevance of the character of Yentl to adult congenital heart disease.
In conclusion, the data discussed in this article indicate that men and women born with a heart defect differ in mortality, morbidity and, to some extent, the way they are managed. Whether this relates to inherent biological differences, the smaller size of women's vessels, or intrinsic genetic differences remains uncertain. Genetic polymorphisms may have different expressions in men and women.11 Sex hormones may influence the expression of genes and their potential to modify cellular function. In future studies, investigation of different genetic susceptibility and reporting of gender differences should be done more systematically. With increased knowledge, a sex-specific approach may improve the outcomes for the estimated more than 25,000 adults in the Netherlands, and over 1.2 million adults in Europe, with congenital heart disease.
References
- 1.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, et al., on behalf of the Euro Heart Survey investigators. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–8. [DOI] [PubMed] [Google Scholar]
- 2.Engelfriet PM, Boersma E, Oechslin E, Tijssen JGP, Gatzoulis MA, Thilén U, et al. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period–The Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2005;26:2325–33. [DOI] [PubMed] [Google Scholar]
- 3.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins, 1998, pp. 269–70. [Google Scholar]
- 4.Somerville J. The Denolin Lecture: The woman with congenital heart disease. Eur Heart J. 1998;19:1766–75. [DOI] [PubMed] [Google Scholar]
- 5.Verheugt CL, Uiterwaal CSPM, van der Velde ET, Meijboom FJ, Pieper PG, Vliegen HW, et al. Gender and Outcome in Adult Congenital Heart Disease. Circulation. 2008;118:26–32. [DOI] [PubMed] [Google Scholar]
- 6.Van der Velde ET, Vriend JWJ, Mannens MMAM, Uiterwaal CSPM, Brand R, Mulder BJM. CONCOR, an initiative towards a national registry and DNA-bank of patients with congenital heart disease in the Netherlands: Rationale, design, and first results. Eur J Epidemiol. 2005;20:549–57. [DOI] [PubMed] [Google Scholar]
- 7.Engelfriet P, Drenthen W, Pieper PG, Tijssen JGP, Yap SC, Boersma E, et al. Smoking and its effects on mortality in adults with congenital heart disease. Int J Cardiol. 2008;127:93–7. [DOI] [PubMed] [Google Scholar]
- 8.Healy B. The Yentl syndrome. N Engl J Med. 1991;325:274–6. [DOI] [PubMed] [Google Scholar]
- 9.Roeters van Lennep JE, Zwinderman AH, Roeters van Lennep HWO, Westerveld HE, Plokker HWM, Voors AA, et al. Gender differences in diagnosis and treatment of coronary artery disease from 1981 to 1997. No evidence for the Yentl syndrome. Eur Heart J. 2000;21:911–8. [DOI] [PubMed] [Google Scholar]
- 10.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, et al. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:467–9. [DOI] [PubMed] [Google Scholar]
- 11.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nature Rev Genet. 2008;9:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]