Abstract
In this part of a series on founder mutations in the Netherlands, we review a Dutch family carrying the SCN5a 1795insD mutation. We describe the advances in our understanding of the premature sudden cardiac deaths that have accompanied this family in the past centuries. The mutation carriers show a unique overlap of long-QT syndrome (type 3), Brugada syndrome and progressive cardiac conduction defects attributed to a single mutation in the cardiac sodium channel gene SCN5a. It is at present one of the largest and best-described families worldwide and we have learned immensely from the mouse strains with the murine homologue of the SCN5a 1795insD mutation (SCN5a 1798insD). From the studies currently performed we are about to obtain new insights into the phenotypic variability in this monogenic arrhythmia syndrome, and this might also be relevant for other arrhythmia syndromes and the general population. (Neth Heart J 2009;17:422-8.)
Keywords: long-QT syndrome, Brugada syndrome, conduction disease, cardiac sodium channel disease, sudden cardiac death
Just over 50 years ago, in 1958, a 16-year-old male presented at one of our university hospitals (Groningen) because a routine sports examination had uncovered an abnormal electrocardiogram.1 This presentation eventually revealed a family accompanied by premature (mostly nocturnal) sudden cardiac deaths throughout the last two centuries and currently involves over 1000 individuals in ten generations. Moreover, in 1999 it became clear that these deaths and the electrocardiographic abnormalities could be attributed to a single mutation in the cardiac sodium channel gene SCN5a (1795insD).2 Of particular interest, carriers of the mutation had electrocardiographic features of long-QT syndrome, Brugada syndrome and/or progressive cardiac conduction defects, referred to as an ‘overlap syndrome’, which was not yet described for cardiac channelopathies at that time. As expected in a founder mutation, most mutation carriers clustered in a particular geographic region (in this instance in the provinces of Groningen and Friesland). However, the typical ECG characteristics were later recognised in other parts of the country and these patients appeared to be descendants from the same ancestors and to carry the same mutation. In the last decade we have performed several clinical and preclinical studies in the family carrying the SCN5a 1795insD mutation and in mice carrying a homologue mutation (SCN5a 1798insD).1–14 In this part of a series on founder mutations in the Netherlands we review what we have learned from these studies and look forward to more insights into the observed variability in this arrhythmia syndrome and possibly also in other populations.
The boy and the family
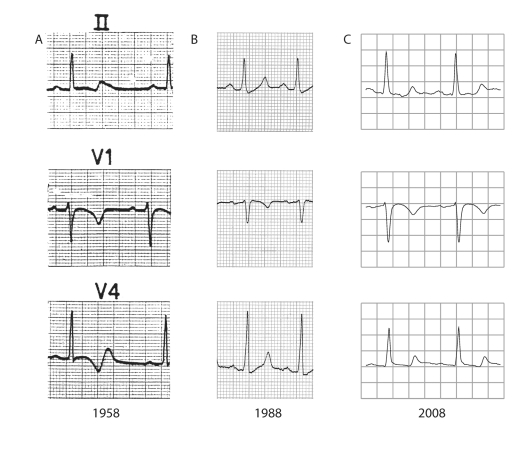
The ECG of the 16-year-old boy revealed an abnormal T-wave configuration (biphasic T waves) with a severely prolonged QTc interval (520 ms, figure 1A). Interestingly, it was only one year after the first description of long-QT syndrome with associated deafness by Jervell and Lange-Nielsen in 1957,15 and still prior to the description of common long-QT syndrome by Romano16 and Ward in the early 1960s.17 At that time the ECG of the boy did not show conduction defects but in the following 50 years he developed a first-degree AV block and QRS widening (figure 1B and 1C). Of particular interest in 1958 was his family history with the unexpected (nocturnal) death of his mother (aged 54 years), two sisters (aged 15 and 26 years) and a brother (aged 21 years). It was apparent that this was a familial disease with a disastrous outcome and therefore the boy's remaining family members were urged to visit the hospital. Soon it became clear that many of his family members displayed rather similar abnormal ECG findings. Notably, the most profound repolarisation abnormalities were always found at the lowest heart rates, suggesting a relation with the nocturnal deaths. However, at that time the exact origin of the premature deaths was unknown and preventive treatment was not possible.
Figure 1.
ECGs of the first family member identified throughout the past 50 years. Note the severe repolarisation abnormalities indicative of long-QT syndrome type 3 already apparent in the first ECG (A). During those 50 years the PQ and QRS interval clearly prolong, indicative of progressive cardiac conduction defects in both atria and ventricles (B and C).
After 20 years with a lack of treatment options, antibrady-pacing was introduced in the family in 1978. The implantation of pacemakers proved to provide tremendous relief as no more deaths occurred in patients treated with antibrady-pacing during 23 years of follow-up up to 2001.4 Also, the favourable effect of antibrady-pacing further validated the relationship between the repolarisation abnormalities at low heart rates and the premature deaths, although the exact mechanism was still not well understood.
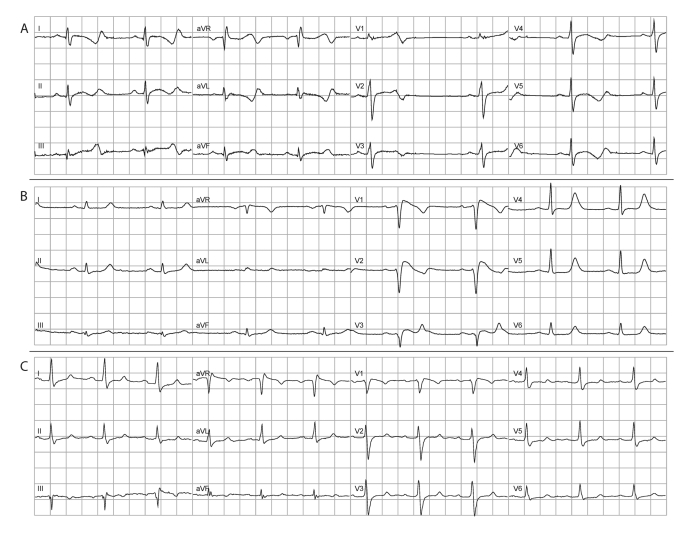
Another 20 years later, in 1998, the mutation linked to the ECG abnormalities was uncovered (SCN5a 1795insD).2 The mutation had, as expected, occurred in the cardiac sodium channel gene SCN5a. At that time SCN5a had just been reported to be implicated in long-QT syndrome (type 3),18 Brugada syndrome,19,20 and in progressive cardiac conduction defects.21 As the affected family members could display all three of these syndromes (figure 2) this was the first description of an arrhythmia overlap syndrome resulting from a single cardiac ion channel mutation. Moreover, the identification of the mutation enabled 100% specific presymptomatic screening of family members.
Figure 2.
ECGs of three different family members carrying the SCN5a 1795insD mutation where the huge phenotypic variability and overlap syndrome can be clearly appreciated. The presence of this overlap syndrome brought us to recognise several mutation carriers who were not earlier linked to the family. ECG A shows predominantly a long-QT syndrome type 3 phenotype with excessive QT prolongation during bradycardia. ECG B shows predominantly a Brugada syndrome phenotype with elevated coved-type ST segments, low initial R waves in the right precordial leads, broad P waves and QRS complexes, but also QT prolongation. ECG C shows a milder mixed phenotype with a first-degree AV block, wide QRS complexes, a coved-type like ST-segment in V1 and QT prolongation.
Mutation-ECG relations
The relationship between long-QT syndrome and SCN5a mutations is persistent inward sodium current (gain-of-function) during the plateau phase of the cardiac action potential causing action potential prolongation and subsequent QT prolongation.18,22 In contrast, Brugada syndrome and conduction disease are related to SCN5a mutations following a decreased sodium inward current (loss-of-function) during the upstroke of the cardiac action potential causing a decrease of sodium current available for activation and subsequent conduction slowing.19,23 Only one year after the initial publication of the 1795insD SCN5a mutation we learned from HEK cell studies that the mutant channels indeed exhibited these two seemingly incompatible features of both gain- and loss-of-function.3 The SCN5a 1795insD mutation disrupts fast inactivation, causing sustained sodium current throughout the action potential plateau and prolonging cardiac repolarisation. While at the same time it augments slow inactivation, delaying recovery of sodium channel availability between stimuli and reducing the fast inward sodium current. Subsequent simulation studies and additional experimental studies in HEK cells further established the combination of these two seemingly distinct sodium channel characteristics in a single mutation.6,24 Finally we were able to establish a mouse strain carrying the homologue mutation (SCN5a 1798insD) which again showed the same properties of an overlap syndrome with both gain- and loss-of-function characteristics of the cardiac sodium channel.9
Only recently more insights are emerging on why the SCN5a 1795insD mutation would cause progressive cardiac conduction defects. This is different from functional causes of conduction defects due to loss-of-function sodium channel mutations (as already present in this family) or, e.g., sodium channel blocking drugs. Cardiac conduction defects in general develop with degenerative changes (fibrosis) of the cardiac conduction system as occurs during ageing. Progressive cardiac conduction defects, or Lev-Lenegre's disease, manifests when these degenerative changes present prematurely.25,26 Although histological studies of the family members are lacking, we were able to study this issue in detail in mice carrying SCN5a mutations. Mice carrying a loss-of-function SCN5a mutation may show profound and progressive cardiac conduction defects resulting from the initial loss of sodium channel function together with a progressive decrease in intracellular coupling due to progressive fibrotic invasion between the cardiomyoctes and altered gap junctions.27,28 Not surprisingly, we documented the same phenomena in the mice carrying the SCN5a 1798insD mutation.29 Of interest, both conduction abnormalities and histological changes are most prominently found in the right ventricle which recapitulates the pathophysiological substrate in Brugada syndrome.30,31
Clinical characteristics
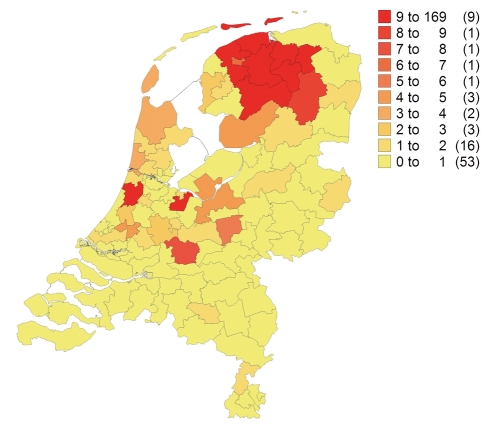
Currently, we have clinical data on 378 family members of whom 149 carry the SCN5a 1795insD mutation. As far as we are aware, the ancestral couple who started the family in the late eighteenth century married in Westerbroek, Groningen, and lived in Rottevalle, Friesland. Thus, as it is a founder mutation it is to be expected that the family is unevenly distributed over the Netherlands (figure 3). Only a few (<10) family members are known to live outside the Netherlands (mainly Germany and Australia). But even now, we are still discovering new branches of the family.
Figure 3.
Distribution of the family in the Netherlands. The founder effect can be appreciated with highest prevalence in the north (Friesland, Groningen).
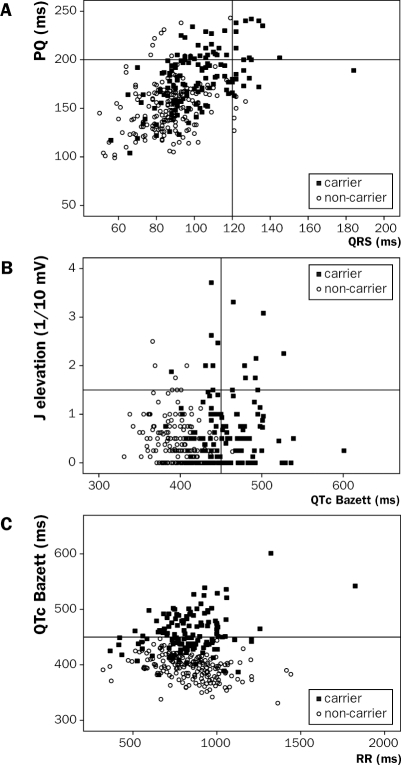
As was apparent from 1958 onward, mutation carriers may show a profoundly prolonged repolarisation, and frequently show atrial, AV and ventricular conduction slowing and ST-segment elevation. However, it also became apparent that this family was not spared from the genetic phenomenon of reduced penetrance with variable phenotypical expression of the trait. Hence, although virtually all mutation carriers primarily show some ECG abnormalities, there is huge overlap between carriers and non-carriers in both conduction and repolarisation indices (figure 4). In addition there is overlap between the different ECG features; whereas most mutation carriers show ECG features of long-QT syndrome (type 3), others rather display features of Brugada syndrome or conduction disease, and some may show a combination of the three phenotypes (figure 2). Holter studies showed that some mutation carriers exhibited sinus node dysfunction. Additionally, besides the relation between bradycardia and QT prolongation, there also seemed to be a role for the autonomic cardiac control with more severe QT prolongation during the night.4,10 Of interest, a similar effect, yet now as a protective mechanism, was found in a large founder family carrying the KCNQ1 A341V mutation which gives rise to long-QT syndrome type 1.32 In this type of long-QT syndrome, arrhythmias are associated with higher heart rates instead of lower heart rates, and lower heart rates are therefore protective.
Figure 4.
Scatterplots showing the overlap between mutation carriers (filled boxes) and non-carriers (open circles) for (A) PQ interval vs. QRS duration, (B) J elevation vs. QTc interval and (C) QTc interval vs. RR interval. Lines represent upper limits of normal values. It can be appreciated that mutation carriers show the worst phenotypes and that there is a positive relation between PQ and QRS prolongation and J elevation and QTc prolongation. Additionally the bradycardia dependent QTc prolongation can be appreciated.
During the study of the paediatric cohort within the family it became evident that several ECG features showed an age-dependant penetrance.7 Mutation carriers started to exhibit QT prolongation in the first year after birth with conduction disease appearing shortly thereafter, whereas ST-segment elevation only developed at over five years of age. From the current data we can appreciate that this age-dependency remains throughout life, recapitulating progressive cardiac conduction defects.33 Of particular interest and also similar to the mouse strains, the conduction slowing observed seems to be primarily located in the right ventricle with wider and deeper S waves in the inferior-lateral leads, lower initial R waves in the right precordial leads, and wider and taller terminal R waves in aVR.33 Again this recapitulates the pathophysiological substrate in Brugada syndrome.30,31
Treatment
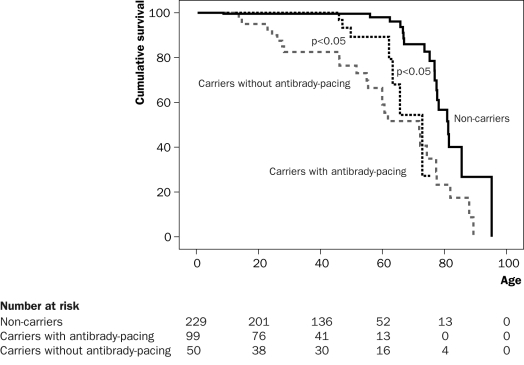
For 25 years our approach with antibrady-pacing was 100% successful. However, in the past six years we have had to acknowledge several sudden deaths in patients carrying a pacemaker. It is likely that these patients suffered from a tachyarrythmic event which could expectedly not be treated by the device. In all cases there was no (brady)arrhythmic event recorded by the device. Although our treatment with antibrady-pacing clearly shows survival benefit (figure 5), we are currently also using implantable cardioverter-defibrillators (ICD) in a subset of the patients. In retrospect it appeared that the patients who died suddenly with a pacemaker had more severe (ventricular) conduction abnormalities than most of the mutation carriers, while they did not have a distinct Brugada syndrome phenotype. It is possible that the tachyarrhythmic risk eventually dominated the bradyarrhythmic risk in these patients, and a causal role for increased conduction delay, creating a tachyarrhythmogenic substrate, seems likely. Hence, we now selectively use ICDs to treat both bradyarrhythmias as well as tachyarrhythmias while accepting the (much) higher complication rates of ICDs as compared with pacemakers.34
Figure 5.
Kaplan-Meier plot showing survival (mortality) with or without antibrady-pacing in mutation carriers vs. non-carriers. It can be appreciated that there is a clear benefit from antibrady-pacing. However, also in the group with antibrady-pacing, mortality is observed from the age of 45 years. The three deaths between 45 and 55 years of age in this latter group were siblings.
Another issue in this family, and other families with cardiac ion channel mutations, is preventive treatment by the avoidance of certain drugs with cardiac ion channel blocking effects (e.g. antiarrhythmic drugs, antibiotics and psychotropic drugs). Because this family shows the unique combination of long-QT syndrome and Brugada syndrome, we advise them to avoid drugs associated with long-QT syndrome,35 as well as drugs associated with Brugada syndrome.36
Genetic modification of the arrhythmic substrate
As mentioned earlier there is a largely unexplained but extensive phenotypic variability between the mutation carriers and also between the mutation non-carriers. Together with the recent failure of treatment in several patients this urged us to better explain this variability. It is to be expected that genetic modifiers play an important role in determining the ultimate arrhythmic substrate the patients face.37 In a complex trait as present in this family it is a daunting task to define markers which can ultimately guide preventive treatment for the individual. However, within the mouse strains (the 129P2 strain and the FVB/N strain) we have recently discovered that genetic background indeed plays a significant role in determining the ultimate phenotypic expression of the disease.13 In mice from the 129P2 strain the SCN5a 1798insD mutation resulted in more severe conduction slowing, particularly in the right ventricle, as compared with mice from the FVB/N strain. Additionally, pan-genomic mRNA expression profiling in the two mouse strains uncovered a drastic reduction in mRNA encoding the cardiac sodium channel auxiliary subunit β4 (SCN4b) in the 129P2 mice as compared with the FVB/N mice. Hence, genetically determined differences in sodium current characteristics on the cardiomyocyte level can modulate disease severity. Currently we are performing genotyping studies to find genes modulating disease severity in the family. Hopefully this will help us to identify those patients at highest risk of bradyarrhythmic and/or tachyarrhythmic events, allowing appropriate preventive measures to be taken. Ultimately, the discovery of genetic modifiers in this arrhythmia syndrome may have similar effects in other populations where they can be either antiarrhythmic or proarrhythmic. It logically follows that the relevance of providing insight into the genetic modulation of one arrhythmia syndrome carries much further than this exceptional family.
Acknowledgements
We gratefully acknowledge the cooperation of all the family members throughout the years without whom it would not have been possible to perform the past and current studies mentioned in this manuscript. Also, we would like to thank Tineke van der Laan for her genealogical research, the previous and current participants in the 1795insD and 1798insD projects, and all referring physicians.
References
- 1.van den Berg MP, Viersma JW, Beaufort-Krol GC, Bink-Boelkens MT, Bezzina CR, Veldkamp MW, et al. A large family characterised by nocturnal sudden death. Neth Heart J. 2002;10:304–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Bezzina C, Veldkamp MW, van den Berg MP, Postma AV, Rook MB, Viersma JW, et al. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999;85:1206–13. [DOI] [PubMed] [Google Scholar]
- 3.Veldkamp MW, Viswanathan PC, Bezzina C, Baartscheer A, Wilde AA, Balser JR. Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ Res. 2000;86:E91–E97. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg MP, Wilde AA, Viersma TJW, Brouwer J, Haaksma J, van der Hout AH, et al. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:630–6. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan PC, Bezzina CR, George AL Jr, Roden DM, Wilde AA, Balser JR. Gating-dependent mechanisms for flecainide action in SCN5A-linked arrhythmia syndromes. Circulation. 2001;104:1200–5. [DOI] [PubMed] [Google Scholar]
- 6.Veldkamp MW, Wilders R, Baartscheer A, Zegers JG, Bezzina CR, Wilde AA. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ Res. 2003;92:976–83. [DOI] [PubMed] [Google Scholar]
- 7.Beaufort-Krol GC, van den Berg MP, Wilde AA, van Tintelen JP, Viersma JW, Bezzina CR, et al. Developmental aspects of long QT syndrome type 3 and Brugada syndrome on the basis of a single SCN5A mutation in childhood. J Am Coll Cardiol. 2005;46:331–7. [DOI] [PubMed] [Google Scholar]
- 8.Wilde AA, van den Berg MP. Ten years of genes in inherited arrhythmia syndromes: an example of what we have learned from patients, electrocardiograms, and computers. J Electrocardiol. 2005;38:145–9. [DOI] [PubMed] [Google Scholar]
- 9.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–94. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg MP, Haaksma J, Veeger NJ, Wilde AA. Diurnal variation of ventricular repolarization in a large family with LQT3-Brugada syndrome characterized by nocturnal sudden death. Heart Rhythm. 2006;3:290–5. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg MP, Haaksma J, Wilde AAM. T-wave alternans in a patient with long-QT syndrome type 3. Neth Heart J. 2006;14:152–3. [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg MP, Haaksma J. Rhythmic ECG Changes in a patient with long QT syndrome type 3. J Cardiovasc Electrophysiol. 2007;18:1342–3. [DOI] [PubMed] [Google Scholar]
- 13.Remme CA, Scicluna BP, Verkerk AO, Amin AS, van Brunschot S, Beekman L et al. Genetically determined differences in sodium current characteristics modulate conduction Disease Severity in Mice With Cardiac Sodium Channelopathy. Circ Res. 2009;104:1283–92. [DOI] [PubMed] [Google Scholar]
- 14.Tobe TJ, de Langen CD, Bink-Boelkens MT, Mook PH, Viersma JW, Lie KI, et al. Late potentials in a bradycardia-dependent long QT syndrome associated with sudden death during sleep. J Am Coll Cardiol. 1992;19:541–9. [DOI] [PubMed] [Google Scholar]
- 15.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54:59–68. [DOI] [PubMed] [Google Scholar]
- 16.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age. II. Syncopal attacks due to paroxysmal ventricular fibrillation [Italian]. Clin Pediatr(Bologna). 1963;45:656–83. [PubMed] [Google Scholar]
- 17.Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc. 1964;54:103–6. [PubMed] [Google Scholar]
- 18.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–11. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–6. [DOI] [PubMed] [Google Scholar]
- 20.Rook MB, Bezzina Alshinawi C, Groenewegen WA, van Gelder I, van Ginneken AC, Jongsma HJ, et al. Human SCN5A gene mutations alter cardiac sodium channel kinetics and are associated with the Brugada syndrome. Cardiovasc Res. 1999;44:507–17. [DOI] [PubMed] [Google Scholar]
- 21.Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, et al. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet. 1999;23:20–1. [DOI] [PubMed] [Google Scholar]
- 22.Bennett PB, Yazawa K, Makita N, George AL Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995; 376:683–5. [DOI] [PubMed] [Google Scholar]
- 23.Tan HL, Bink-Boelkens MT, Bezzina CR, Viswanathan PC, Beaufort-Krol GC, van Tintelen PJ, et al. A sodium-channel mutation causes isolated cardiac conduction disease. Nature. 2001;409:1043–7. [DOI] [PubMed] [Google Scholar]
- 24.Clancy CE, Rudy Y. Na(+) channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002;105:1208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lev M. The pathology of complete atrioventricular block. Prog Cardiovasc Dis. 1964;6:317–26. [DOI] [PubMed] [Google Scholar]
- 26.Lenegre J. Etiology and pathology of bilateral bundle branch block in relation to complete heart block. Prog Cardiovasc Dis. 1964;6:409–44. [DOI] [PubMed] [Google Scholar]
- 27.van Veen TA, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, et al. Impaired impulse propagation in Scn5aknockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation. 2005;112:1927–35. [DOI] [PubMed] [Google Scholar]
- 28.Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, et al. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res. 2009;83:52–60. [DOI] [PubMed] [Google Scholar]
- 29.Remme CA, Engelen MA, van Brunschot S, van Ginneken AC, Belterman CN, van Rijen HV, et al. Severity of conduction disease and development of cardiac structural abnormalities in sodium channel disease depends on genetic background [Abstract]. Heart Rhythm. 2007;4:S60–1. [Google Scholar]
- 30.Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJ, Verkerk AO, de Groot JR, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–77. [DOI] [PubMed] [Google Scholar]
- 31.Postema PG, van Dessel PF, de Bakker JM, Dekker LR, Linnenbank AC, Hoogendijk MG, et al. Slow and discontinuous conduction conspire in brugada syndrome: a right ventricular mapping and stimulation study. Circ Arrhythm Electrophysiol. 2008;1:379–86. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz PJ, Vanoli E, Crotti L, Spazzolini C, Ferrandi C, Goosen A, et al. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol. 2008;51:920–9. [DOI] [PubMed] [Google Scholar]
- 33.Postema PG, Grundeken M, Kilicarslan M, Bezzina CR, Wilde AA, van den Berg MP. Age-dependent right ventricular conduction defects in a SCN5a overlap syndrome [Abstract]. Eur Heart J. 2008;29:34 [Google Scholar]
- 34.Towbin JA. Ventricular tachycardia or conduction disease: what is the mechanism of death associated with SCN5A? J Cardiovasc Electrophysiol. 2001;12:637–8. [DOI] [PubMed] [Google Scholar]
- 35.List of Drugs that Prolong the QT Interval and/or Induce Torsades de Pointes Ventricular Arrhythmia. University of Arizona Health Sciences Center 2009;Available from: URL: httpwww.qtdrugs.org
- 36.Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations and an up-to-date website (www.brugadadrugs.org). Heart Rhythm. 2009;6:1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scicluna BP, Wilde AW, Bezzina CR. The primary arrhythmia syndromes: same mutation, different manifestations. Are we starting to understand why? J Cardiovasc Electrophysiol. 2008;19:445–52. [DOI] [PubMed] [Google Scholar]