Abstract
Human papillomavirus type 16 (HPV16) is a 7.9 kb double stranded DNA virus that infects anogenital mucosal epithelia. In some rare cases, in women, infection can progress to cervical cancer. HPV16 gene expression is regulated through use of multiple promoters and alternative splicing and polyadenylation. The virus genome can be divided into an early and late coding region. The late coding region contains the L1 and L2 genes. These encode the virus capsid proteins L1 and L2 protein expression is confined to the upper epithelial layers and is regulated post-transcriptionally in response to epithelial differentiation. A 79 nt RNA regulatory element, the late regulatory element (LRE), involved in this regulation is sited at the 3′ end of the L1 gene and extends into the late 3′ untranslated region (3′ UTR). This element represses late gene expression in differentiated epithelial cells and may active it in differentiated cells. This article describes our current knowledge of LRE RNA/protein interaction and their possible functions.
Keywords: human papillomavirus type 16, late regulatory element, polyadenylation, RNA stability, RNA-protein interactions
Human papillomaviruses (HPVs) are small double stranded DNA viruses that cause mainly benign tumours of cutaneous and mucosal epithelia, commonly known as warts [1]. A significant subset of HPVs infects the mucosal epithelium of the anogenital tract. In some cases such persistent infection can progress to cancer, especially cervical cancer in women. The most prevalent cancer-causing HPV is HPV type 16 (HPV16). Although a prophylactic vaccine against HPV16 has been licensed for use in the UK, it will be over 50 years before the efficacy of the vaccine can be properly assessed [2]. In the meantime, there is a need to develop novel antiviral therapies. A thorough understanding of HPV16 gene regulation is required to facilitate this.
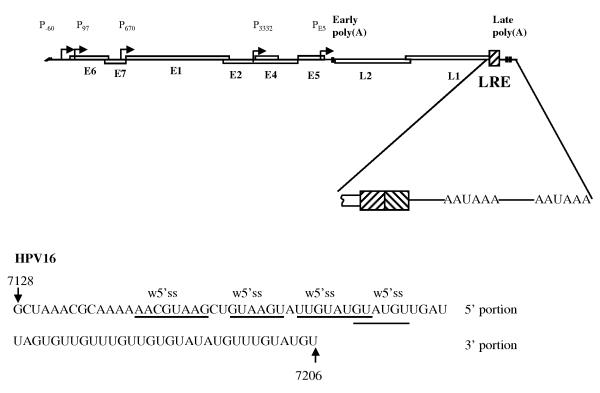
Epithelial infection leads to nuclear deposition of the 7.9 kb circular double stranded HPV16 genome. Transcription is polycistronic, yielding a complex set of mRNAs processed by alternative splicing and polyadenylation [3]. The virus genome has an early region containing six genes (E1-E7) encoding proteins involved in virus genome replication, transcriptional regulation, and cell transformation, a late region with two late genes encoding virus capsid proteins, L1 and L2, and a long control region (LCR). At its 5′ end the LCR contains RNA regulatory signals in the late 3′ UTR (Figure 1). The HPV life cycle is completely dependent upon epithelial differentiation. The early proteins are produced throughout the epithelium. In contrast, expression of the virus capsid proteins is restricted to terminally differentiated cells in the uppermost epithelial layer from where virus particles are shed [4]. The L1 and L2 capsid proteins are excellent targets for design of antiviral therapies because they are essential for virion formation and transmission.
Figure 1.
Regulation of papillomavirus late gene expression
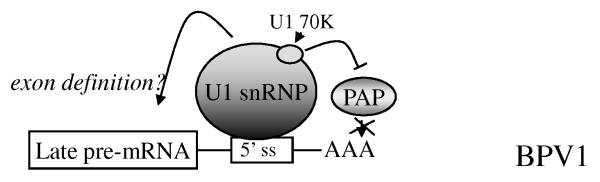
For HPV16 and other papillomaviruses, late gene expression is regulated post-transcriptionally via cis-acting RNA regulatory elements. The best-characterised element is the 53 nt bovine papillomavirus type 1 (BPV1) inhibitory element, which is located in the BPV1 late 3′UTR immediately upstream of the late polyadenylation site. This inhibitory element uses a good consensus 5′ splice donor site to base-pair with the 5′ end of cellular U1 small nuclear RNA (U1 snRNA), allowing assembly of U1 small nuclear ribonucleoprotein particle (U1 snRNP) [5,6]. The 70 kDa subunit of U1 snRNP (U170K) interacts with poly (A) polymerase (PAP) to inhibit polyadenylation [7]. Transcripts that are not polyadenylated are rapidly degraded in the nucleus and so cannot exported to the cytoplasm for translation. The inhibitory action of U170K has been mapped to four conserved motifs that are also found in the splicing proteins U1A and U2AF65. Inhibition of polyadenylation represses virus late gene expression in undifferentiated, BPV1-infected cells, but how suppression is released to allow capsid protein expression in terminally differentiated cells is unknown.
The HPV16 late regulatory element
The HPV16 late 3′ UTR contains a late regulatory element (LRE) that is structurally related to the BPV1 element. The 79 nt LRE overlaps the 3′ end of L1 gene and extends into the late 3′ UTR [8]. Two active polyadenylation sites are located 78 and 137 nts downstream [3]. The element efficiently represses gene expression in undifferentiated epithelial cells and the entire 79 nt sequence is required for this. However, repression is alleviated when the cells are differentiated [9]. Like BPV1, the 5′ portion of the element contains four 5′ splice sites. These are mostly poor consensus but they do bind a U1 snRNP-like complex [9]. Although U170K has not been identified in the U1 snRNP complex, U1A and U2AF65 (see below) that contain conserved PAP inhibitory motifs are present. It remains to be tested whether these proteins inhibit polyadenylation at either of the two downstream sites. The LRE also binds the polyadenylation factor CstF64 through its 3′ U-rich portion [10] (data not shown). In addition, the LRE has potential for substantial stem loop structure formation [9]. CstF64 binding and the LRE RNA secondary structure could sterically hinder polyadenylation complex formation in a similar manner to that observed for the unutilised polyadenylation site in the 5′ long terminal repeat (LTR) of HIV1 [11]. Such a mechanism could operate separately or in concert with U1 snRNP-mediated inhibition.
The HPV16 LRE and regulation of RNA processing
In in vitro polyadenylation experiments using HeLa cell extracts the LRE does not inhibit polyadenylation (data not shown). In contrast, Northern blotting revealed that in undifferentiated cervical epithelial cells harbouring nuclear copies of the HPV16 genome (W12 cells [12]) late RNAs were detected but these were nonpolyadenylated. Fully processed, polyadenylated mRNAs were found only in differentiated W12 cells [3]. This implies that there is a block to late RNA polyadenylation in less differentiated cells, but does not confirm that the LRE is involved.
The GU-rich 3′ LRE portion also has the capacity to bind the auxiliary splicing factor U2AF65 [13] partnered with the key SR protein SF2/ASF [14]. Taken together with U1snRNP binding, this means that the HPV16 LRE can bind a complex very similar to an early splicing complex. There are no splice acceptor sites downstream of the LRE and no mRNAs are produced from this 3′UTR, meaning that the LRE/protein complex is not involved in splicing. However, the terminal exons of virus late mRNAs are over 1.5 kb in length, so it is hypothesised that this element may act to define these for efficient splicing.
Cellular cytoplasmic proteins that bind the HPV16 LRE
Finally, the LRE binds two shuttling proteins with noted roles in the cytoplasm, hnRNP A1 and the elav-like HuR protein. In the nucleus, hnRNP A1 is an alternative splicing factor but also has roles in nuclear export, mRNA stability and translation. The protein binds the central portion of the LRE in cytoplasmic extracts [15]. Levels of hnRNP A1 increase in the upper epithelial layers where late gene expression is achieved [15], so we are currently elucidating its role in regulating late gene expression. HuR binds and stabilises mRNAs containing AU-rich elements (AREs) and accompanies them for export to the cytoplasm. HuR binds with high affinity the U-rich 3′ portion of the LRE that resembles a class III ARE. HuR binding was most efficient in the cytoplasm of differentiated W12 cells and siRNA-mediated knock-down of HuR levels resulted in abrogation of late protein expression (manuscript in preparation). Thus HuR may be a major cytoplasmic regulator of HPV16 late protein expression and a potential target for topically applied antiviral therapies.
Figure 2.
Abbreviations
- HPV
human papillomavirus
- BPV
bovine papillomavirus
- UTR
untranslated region
- LCR
long control region
- LRE
late regulatory element
- snRNA
small nuclear RNA
- snRNP
small nuclear ribonucleoprotein particle
- PAP
poly (A) polymerase
- LTR
long terminal repeat
- ARE
AU-rich element
References
- 1.Howley PM. Papillomaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Lippincott-Raven Publishers; Philadelphia, Pa.: 1996. pp. 2045–2076. [Google Scholar]
- 2.Stanley MA. Human papillomavirus vaccines. Rev. Med. Virol. 2006;16:139–149. doi: 10.1002/rmv.498. [DOI] [PubMed] [Google Scholar]
- 3.Milligan SG, Veerapraditsin T, Ahamat B, Mole S, Graham SV. Analysis of novel human papillomavirus type 16 late mRNAs in differentiated W12 cervical epithelial cells. Virology. 2007;360:172–181. doi: 10.1016/j.virol.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 2004;68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furth PA, Baker CC. An element in the bovine papillomavirus late 3′ untranslated region reduces polyadenylation cytoplasmic RNA levels. J. Virol. 1991;65:5806–5812. doi: 10.1128/jvi.65.11.5806-5812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furth PA, Choe W-T, Rex JH, Byrne JC, Baker CC. Sequences homologous to 5′ splice sites are required for the inhibitory activity of papillomavirus late 3′ untranslated regions. Mol. Cell. Biol. 1994;14:5278–5289. doi: 10.1128/mcb.14.8.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson SI, Polycarpou-Schwarz M, Mattaj IW. U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol. Cell. 1998;1:255–264. doi: 10.1016/s1097-2765(00)80026-x. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy IM, Haddow JK, Clements JB. Analysis of human papillomavirus type 16 late mRNA 3′ processing signals in vitro and in vivo. J. Virol. 1990;64:1825–1829. doi: 10.1128/jvi.64.4.1825-1829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cumming SA, McPhillips MG, Veerapraditsin T, Milligan SG, Graham SV. Activity of the human papillomavirus type 16 late negative regulatory element is partly due to four weak consensus 5′ splice sites that bind a U1 snRNP-like complex. J. Virol. 2003;77:5167–5177. doi: 10.1128/JVI.77.9.5167-5177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koffa MD, Graham SV, Takagaki Y, Manley JL, Clements JB. The human papillomavirus type 16 negative regulatory element interacts with three proteins that act at different posttranscriptional levels. Proc. Natl. Acad. Sci. USA. 2000;97:4677–4682. doi: 10.1073/pnas.070049097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klasens BIF, Thiesen M, Virtanen A, Berhout B. The ability of the HIV-1 AAUAAA signal to bind polyadenylation factors is controlled by local RNA structure. Nucleic Acids Res. 1999;27:446–454. doi: 10.1093/nar/27.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley MA, Browne HM, Appleby M, Minson AC. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int J Cancer. 1989;43:672–676. doi: 10.1002/ijc.2910430422. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich-Goetz W, Kennedy IM, Levins B, Stanley MA, Clements JB. A cellular 65kDa protein recognizes the negative regulatory element of human papillomavirus late mRNA. Proc. Natl. Acad. Sci. USA. 1997;94:163–168. doi: 10.1073/pnas.94.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPhillips MG, Veerapraditsin T, Cumming SA, Karali D, Milligan SG, Boner W, Morgan IM, Graham SV. SF2/ASF binds the human papillomavirus type 16 late RNA control element and is regulated during epithelial differentiation. J. Virol. 2004;78:10598–10605. doi: 10.1128/JVI.78.19.10598-10605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheunim T, Zhang J, Milligan SG, McPhillips MG, Graham SV. The alternative splicing factor hnRNP A1 is up-regulated during virus-infected epithelial cell differentiation and binds the human papillomavirus type 16 late regulatory element. Virus Res. 2007 Oct 19; doi: 10.1016/j.virusres.2007.09.006. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]