Abstract
This study explored differences in intraindividual variability in three attention tasks across a large sample of healthy older adults and individuals with very mild dementia of the Alzheimer's type (DAT). Three groups of participants (healthy young adults, healthy older adults, very mild DAT) were administered three computerized tasks of attentional selection and switching (Stroop, Simon, Task Switching). The results indicated that a measure of intraindividual variability, coefficient of variation (CoV; SD/Mean) increased across age and early-stage DAT. The CoV in Stroop discriminated the performance of ε4 carriers from noncarriers in healthy older controls and the CoV in Task Switching was correlated with CSF biomarkers predictive of DAT.
Keywords: intraindividual variation, attention, aging, Alzheimer's disease
There has been considerable interest in the ability to diagnose dementia of the Alzheimer type (DAT) in the earliest possible stage of the disease, thus discriminating healthy aging from early-stage DAT. The elusive nature of the clinical detection of the early onset of DAT has been supported in longitudinal studies in presumed healthy older adults (e.g., Morris et al., 1996; Price & Morris, 1999; Rubin et al., 1998). These and other studies (e.g., Bennett et al, 2006) indicate that the Alzheimer's disease process may be present in the brain for years before the appearance of clinical symptoms. Thus, preclinical markers of the disease likely are present in some older individuals who appear to be clinically “normal”, underscoring the need to reliably identify more specific changes that could serve as additional antecedent markers for DAT.
Episodic memory loss has long been considered the primary marker for the first clinical manifestation of Alzheimer's disease (e.g., Albert et al., 2001; 2007; Rubin et al, 1998; Storandt et al., 2006). However, there has also been accumulating evidence documenting clear changes in components of attention in both healthy aging and in early-stage DAT (see reviews by Balota & Faust, 2001; Perry & Hodges, 1999). For example, in the classic Stroop task, Spieler, Balota, and Faust (1996) provided evidence that there is a disproportionate breakdown in the ability to inhibit the word code when naming colors in healthy older adults compared to young, and in DAT individuals, compared to age-matched controls. Furthermore, it has been argued that attentional breakdowns observed in healthy aging and in early-stage AD are likely to be related to the episodic memory changes in these individuals (e.g., Balota et al., 1999; 2002; Castel, Balota, & McCabe, 2009; Sommers & Huff, 2003). Memory researchers have long recognized the critical role of attention in declarative memory performance in both laying down distinct traces during encoding and directing search processes during retrieval (see, e.g., Craik & Lockhart, 1972; Jacoby, 1999).
The standard approach in documenting cognitive decline is to compare mean-level performance across groups of participants. For example, if one is interested in measuring a characteristic such as processing speed, one typically uses the mean-level reaction time RT performance across multiple observations within a given participant. Variability across trials is often simply considered error variance. However, recently there has been interest in examining within-individual changes in variability in reaction time (RT) across trials within a task or occasions of testing as an indicator of neurocognitive function (see Hultsch, Strauss, Hunter et al., 2008, for a review). Indeed, within-task variability can be viewed as consistent with a breakdown in an attentional control system that maintains the goals of a task across time and controls competing pathways (see West, 2001; West, Murphy, Armilio et al., 2002 for similar arguments). Recent work (Bunce, Anstey, Christensen et al., 2007; see also Murtha, Cismaru, Waechter et al., 2002) indicates that within-person variability in RTs is correlated with white matter hyperintensities in the frontal lobe, but not other brain regions (e.g., temporal or parietal areas). Stuss, Murphy, Binns et al (2003) also found that patients with frontal lobe lesions (with the exception of ventral medial/orbitofrontal region) showed increased inconsistency in task performance. These results are consistent with the possibility that increased variability may reflect a breakdown in executive control systems that are dependent upon the coordination of multiple brain areas (see MacDonald, Nyberg & Backman, 2006, for a review).
There is evidence that intraindividual variability in processing speed increases as a function of normal aging (e.g., Hultsch, MacDonald, & Dixon, 2002; Hultsch et al., 2008). Moreover, there is some evidence that intraindividual variability across trials can actually be a better predictor of group status (healthy control vs. mild DAT) than mean-level performance. Hultsch, MacDonald, Hunter et al. (2000) examined intraindividual standard deviations across trials and occasions for RT and memory tasks in healthy older adults, older adults with arthritis, and adults with mild dementia. They found increased intraindividual variability in the mildly demented group relative to the two neurologically intact groups regardless of physical health status (i.e., healthy vs. arthritis), and the measure of intraindividual variability predicted neurocognitive status independent of mean-level performance. Because means and standard deviations tend to be highly correlated (see Faust, Balota, Spieler et al., 1999), it is important that the measure of intraindividual variability takes into consideration overall differences in mean performance.
Because preclinical markers for Alzheimer's disease may be present in some individuals prior to a clinical diagnosis and presently undetected due to the subtle nature of the cognitive changes seen early on, intraindividual variability may serve as a useful cognitive marker for the early onset of the disease. Indeed, Christensen, Dear, Anstey et al. (2005) reported that a measure of intraindividual variability (mean independent variability; MIV) in both simple and choice RT tasks was greater for older adults (60-64 years) who met criteria for mild cognitive impairment (MCI) than healthy controls. In a more recent study, Dixon, Garrett, Lentz et al. (2007, see also Gorus, de Raedt, Lambert et al., 2008) also examined the utility of both speed (mean RT) and inconsistency (intraindividual standard deviation, ISD) in discriminating individuals with degrees of MCI (mild MCI and moderate MCI, based on individuals' performance relative to norms on a set of reference cognitive tasks) from healthy older adults. Importantly, logistic regression analyses indicated that ISDs predicted cognitive status (healthy adults vs. mild MCI and mild MCI vs. moderate MCI) above and beyond mean-level performance. In light of their results and those of Christensen and colleagues, Dixon et al. argue that intraindividual variability may serve as an important indicator of early cognitive impairment, especially in more cognitively demanding tasks and at later ages.
The major purpose of the present study was to further examine the utility of trial-to-trial intraindividual variability in processing speed in attentional task performance in discriminating healthy aging from the very earliest stages of DAT. In the present study, three groups of participants afforded an examination of intraindividual variability associated with both healthy aging (young vs. healthy older adults) and the onset of early-stage DAT (healthy older adults vs. very mild DAT). The clinical dementia rating (CDR) scale is used to identify individuals at the earliest detectable stages of DAT and is derived without knowledge of any independent cognitive testing. The power of the CDR in early diagnosis has been recently illustrated by Storandt et al. (2006) who compared the rate of progression of individuals who initially at enrollment met standard criteria for MCI (which presumes impairment, but does not yet meet criteria for dementia according to some), and individuals with a CDR of 0.5 (very mild DAT) who initially did not meet standard criteria for MCI. Interestingly, the rate of decline was reliably greater for the MCI group compared with the CDR 0.5 DAT group, using both a psychometric composite and time to reach a more advanced stage of DAT (i.e., CDR 1) as outcome measures. This study indicates that it is possible to detect very mild DAT with the CDR scale even prior to what is considered by some to be MCI without dementia. Thus, the present well-characterized sample can provide a more refined examination of within-person variability as an early marker for the onset of DAT.
We also examined the relation between the estimate of intraindividual variability and biological markers in healthy older adults. The two variables we have identified were the presence of the ApoE4 allele and the CSF biomarkers, Aβ42, tau, and phosphorylated tau (ptau). Of course, the presence of the ε4 allele is a well-established risk factor for DAT (e.g., Blacker, 1997; Corder et al., 1993; Henderson et al., 1995). Several studies have attempted to identify early cognitive markers for DAT by comparing healthy older adults who are at risk for DAT with those who are not. With respect to the present study, there has recently been increasing evidence suggesting that ε4 carriers, compared to ε4 noncarriers, produce some deficits in spatial attention and executive control systems (e.g., Parasuraman et al., 2002; Rosen et al., 2002). However, it should also be noted that other studies have failed to find ε4 differences in standard psychometric tests (e.g., Caselli et al., 2004; Wilson et al., 2002). In their metanalysis, Small et al. have argued that further work is needed in attentional/executive control measures to explore the influence of e4 status on cognitive performance.
Regarding CSF biomarkers, Fagan, Roe, Xiong et al. (2007) reported decreased levels of CSF Aβ42 and increased levels of CSF tau and ptau in very mild DAT, consistent with prior studies of later stage DAT (see Sunderland et al., 2003 for a review), and the ratio of CSF tau/Aβ42 and ptau181/Aβ42 were predictive of conversion from healthy aging (CDR 0) to early-stage dementia (CDR > 0). In a recent study, Nordlund et al. (2008) found that MCI individuals with abnormally higher levels of tau and lower levels of Aβ42 performed more poorly on some tests of attention and memory than MCI individuals with normal levels of tau and Aβ42. In the present study, we examined the relationship between CSF biomarkers and variability in healthy older adults to assess whether intraindividual variability also changes as a function of particular CSF markers.
The present study focused on standard tasks that tap aspects of attentional selection and switching (Stroop, Simon, Task Switching) which appear to be particularly sensitive to early-stage DAT (Balota & Faust, 2001; Castel, Balota, Hutchison et al., 2007; Spieler et al., 1996). As Dixon et al. (2007) suggested, within-person variability may be a more precise marker when the attentional demands of the task are greater. Indeed, West et al. (2002) also found that performance variability increased as a function of increased response set (0- vs. 1-back) in the N-back task, especially for healthy older adults. Thus, the tasks we used also vary with respect to the demands placed on the attentional system; with the Simon task tapping spatial compatibility, the Stroop task tapping lexical compatibility, and the Switching task tapping potentially higher level control systems. The primary dependent variable in the present analyses is the coefficient of variation (CoV). The CoV is computed by dividing an individual's standard deviation by their mean (SD/Mean). This measure has the advantage of taking into consideration overall speed which is critical when comparing across age and dementia groups which vary substantially in terms of overall RT (see Hultsch et al., 2008 for an excellent review of alternative measures of variability).
Methods
Participants
A total of 291 individuals were recruited from the Washington University Alzheimer's Disease Research Center (ADRC) for this study. All ADRC participants were originally screened for depression, untreated hypertension, reversible dementias, and other disorders that could potentially produce cognitive impairment. The inclusionary and exclusionary criteria for DAT are consistent with the criteria for “probable AD” of the National Institute of Neurological and Communications Disorders and Stroke—Alzheimer's disease and Related Disorders Association (McKhann et al., 1984). The presence and severity of dementia were assessed according to the Washington University Clinical Dementia Rating (CDR) scale (Morris, 1993; Morris et al., 1988), with CDR 0, 0.5, 1, 2, and 3 representing no dementia, very mild dementia, mild dementia, moderate dementia, and severe dementia, respectively. The CDR is based on a 90-minute clinical interview that assesses the participant and also relies on information from their family members. This interview assesses potential changes in participants' cognitive and functional abilities in the areas of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care relative to previous behavior. The determination of a CDR score for each participant at baseline and at each annual assessment thereafter is made without reference to the psychometric performance of the individual. The recruitment and assessment methods permit the diagnosis of DAT in individuals who elsewhere may be characterized as MCI (see Berg et al., 1998; Morris et al., 2001 for details and see http://alzheimer.wustl.edu/cdr/PDFs/CDR_OverviewTranscript-Revised.pdf for a summary). Both the reliability of the CDR (Burke et al., 1988) and the validity of the diagnosis based upon autopsy by this research team have been excellent (93% accuracy), including individuals diagnosed with DAT in the CDR 0.5 stage (Berg et al.1998; Storandt et al., 2006).
We also recruited 35 healthy young adults from the undergraduate Psychology Department participant pool at Washington University (mean age = 20.29, SD = 1.07). Of the participants recruited via the ADRC, 220 were healthy older adults who were classified as non-demented, CDR 0 and 71 were older adults who were classified as very mild DAT, CDR 0.5 (see Table 1). There was a significant difference in both age [t (289) = 3.15, p = .002, ηp2 = .002] and MMSE scores [t (250) = 9.46, p < .001, ηp2 = .26] between the healthy older adults and very mild DAT individuals. As noted below, we covaried out age in all relevant analyses. This study was approved by the Institutional Review Board at Washington University and all participants provided their informed consent at the beginning of the study.
Table 1. Psychometric Means (SD) as a Function of Group.
| Healthy Old | Very Mild DAT | |
|---|---|---|
| Logical Memory | 12.09 (3.63) | 7.81 (4.60)* |
| Forward Digit Span | 6.61 (1.28) | 6.36 (1.20) |
| Backward Digit Span | 4.96 (1.30) | 4.41 (1.23)* |
| WMS Associate Recall | 14.68 (4.08) | 9.69 (3.95)* |
| Word Fluency S-P | 32.81 (10.81) | 26.03 (10.21)* |
| WAIS Information | 21.94 (4.17) | 18.25 (4.95)* |
| WAIS Digit Symbol | 48.85 (11.05) | 37.17 (11.32)* |
| WAIS Similarities | 25.81 (3.99) | 22.27 (5.48)* |
| Trailmaking A | 32.99 (13.13) | 43.17 (24.89)* |
| Trailmaking B | 86.78 (38.79) | 126.41 (54.82)* |
| Boston Naming | 56.10 (3.90) | 52.39 (7.89)* |
| Animal Fluency | 20.32 (5.92) | 15.05 (5.60)* |
| Selective Reminding | 30.46 (6.13) | 19.52 (9.00)* |
| MMSE | 29.09 (1.170 | 26.95 (2.36)* |
| Age | 71.75 (8.31) | 75.25 (7.68)* |
| Years of Education | 15.41 (2.71) | 14.67 (3.04) |
p < .05
Psychometric Testing
Each ADRC participant was administered a 2-hour standard neuropsychological battery in a separate testing session, by an examiner who was unaware of the participant's CDR score. Memory was assessed with Logical Memory, Forward and Backward Digit Span, and Associate Memory from the Wechsler Memory Scale (Wechsler & Stone, 1973) and the Selective Reminding Test (Grober, Buschke, Crystal et al., 1988). General intelligence was assessed with Information, Digit Symbol, and Similarities subtests of the Wechsler Adult Intelligence Scale (WAIS) (Wechsler, 1955). Visual perceptual-motor performance was assessed with Parts A and B of the Trail Making Test (Armitage, 1946). The Boston Naming Test (Goodglass & Kaplan, 1983a), the Word Fluency Test S-P (Thurstone & Thurstone, 1949), and the Animal Naming Test (Goodglass & Kaplan, 1983b) were administered as tests of semantic/lexical retrieval. The means and standard deviations of the psychometric measures for the healthy older adults and very mild DAT individuals are presented in Table 1. A series of t-tests indicated that performance on all of the measures was significantly different among groups (all ps < .05, ηp2 > .03), with the exception of WMS forward digit span (p = .19, ηp2 = .01) and years of education (p = .07, ηp2 = .01). Thus, the CDR classification of healthy control (CDR 0) vs. very mild DAT (CDR 0.5) is further supported by objective psychometric testing.
Genotyping and CSF biomarkers
Genotyping for the ApoE alleles (ε2, ε3, ε4) was available for 151 healthy older adults from the ADRC (n's for ε23= 22; ε33 = 82; ε24 = 4; ε34 = 38; ε44 = 5). Due to the small n's in some of the allele groups, participants were grouped according to the presence (ε4+; n = 47) vs. absence of at least one ε4 allele (ε4-; n = 104). There was no difference in education for ε4+ vs. ε4- participants, p= .89, ηp2 = .000, however the ε4+ group was slightly younger than the ε4- group, t (149) = 2.00, p = .047, ηp2 = .02. In addition, CSF biomarkers (Aβ42, Tau/Aβ42, and Ptau181/Aβ42) were available for 84 healthy older adults (see Fagan et al., 2007 for details on CSF collection, processing, and assessment).
Apparatus
A Pentium II IBM –compatible computer was used to control the display of the stimuli and to collect participants' responses. Display of all stimuli was synchronized with the vertical retrace of the monitor to control for presentation duration. The stimuli were displayed on a 15-inch monitor. A Gerbrands Model voice-operated relay was interfaced with the computer to measure voice onset latency in the Stroop task.
Stroop Task
The word stimuli consisted of four color words (red, blue, green, yellow) and four neutral words (bad, poor, deep, legal). The neutral words were chosen to match the color words in phoneme onset and word frequency. The task included a block of 104 word naming trials and a block of 104 color naming trials. There were 36 congruent, 32 neutral, and 36 incongruent trials in each block. In the congruent trials, each color word appeared nine times. In the incongruent trials, each color word appeared three times in each of three other colors (e.g., blue appeared in red, green, yellow three times). In the neutral trials, each word appeared two times in each of the four colors. The order of trials was randomized in each block and the order of blocks (word or color) was counterbalanced across participants.
Participants were given 16 practice trials before each block of trials. For the word (or color) naming trials, participants were instructed to read the words (or colors in which the word appeared) as quickly and as accurately as possible. At the beginning of each trial a fixation point appeared for 500 ms followed by a blank screen for 50 ms. The stimulus word then appeared on the screen for 5 seconds or until the participant responded. The experimenter recorded the response as correct, incorrect or a voice key error (e.g., stutters, false starts, or any noise that triggered the voice key). Participants were given breaks between trial blocks. Because there is relatively little attentional control exerted in the word-naming trials, we will focus on the color-naming trials in the present paper.
Simon Task
The stimulus display consisted of a white central fixation cross on the screen and white arrow stimuli (measuring approximately 4 cm in length and 2 cm in height) presented on a black background. The peripheral locations of the arrow (left and right) were situated 5° on the horizontal plane from the central fixation. Participants were told that they would be presented with an arrow pointing to either the left or right on the screen. The arrow could appear on the left half, right half, or center of the screen. Participants were told to ignore the arrow location on the screen and respond according to the arrow direction by pressing a key on either the left (i.e., q key) or the right side (i.e., p key) of the keyboard that corresponded to the arrow direction. In the congruent trials, the arrow direction corresponded to the arrow location (e.g., left facing arrow on the left side of the screen). In the incongruent trials, the arrow direction was opposite to the arrow location (e.g., left facing arrow on the right side of the screen). In the neutral trials, the arrow appeared at the center of the screen. Each trial began with a 500-ms central fixation cross, followed by the onset of an arrow, which stayed on the screen until the participant made a response or until 5 s had elapsed. Once a response was made, the screen cleared and an accuracy feedback was presented for 400 ms. There were 12 practice trials (4 congruent, 4 incongruent, and 4 neutral) and 120 experimental trials (40 congruent, 40 incongruent, and 40 neutral). The 40 neutral trials were included to ensure that participants would keep fixated at the center of the screen. These different trial types were randomly intermixed for each participant.
Switching Task
In this task, participants engaged in two different tasks on varying trials. On each trial, a stimulus pair (a letter and a number, e.g., A 3) was presented in the center of the screen with a cue (either OE or CV) at the top of the screen indicating if it is a “letter” or “number” trial. On letter trials, the participants made a decision as to whether the letter was a consonant or vowel (CV). On number trials, the participants made a decision as to whether the number was odd or even (OE). Participants pressed the d key when responding Consonant or Odd and pressed the k key when responding Vowel or Even.
Participants received 10 practice trials with feedback followed by a block of 48 “pure” letter trials (i.e., all CV trials) and then 48 “pure” number trials (i.e., all OE trials). After the pure blocks of trials, participants received 10 switch practice trials followed by a block of 60 switch/nonswitch trials presented using an alternate runs procedure, CV,CV,OE,OE,CV,CV,OE,OE etc. in which a given task (e.g., consonant or vowel decision) was performed on successive trials, but then switches to a different task (e.g., odd or even decision). This procedure allows one to compare switch and nonswitch trials within the same block. Thus, there were 30 switch trials (e.g., CV trial followed by OE trial) and 30 nonswitch trials (e.g., CV trial followed by CV trial). Feedback was not given on the pure or switch/nonswitch test trials. The stimulus display remained on the screen until the participants made a response and then the next stimulus display appeared immediately. Participants were instructed to respond as quickly and as accurately as possible and to try not to use the cues at the top of the screen, but instead try to keep track of the order of the trials.
Results
Although our focus is on the CoV as a measure of intraindividual variability, we first present the mean RT and error data for each attention task as a function of participant group and task condition to insure that these general indicators of task performance replicate prior literature. We also performed z-transformed RT analyses to determine if any condition effects were due to general slowing (Faust et al., 1999). For sake of brevity, we will not report these findings given that all crucial group × condition interactions remained statistically significant in the z-score analyses. The CoV data is presented for each task as a function of participant group and then as a function of ApoE status in the healthy older adults. We will first present the overall CoV (overall SD divided by overall mean) in each task to provide a more stable estimate of intraindividual variation and then in each task we will focus on the CoV in the incongruent/mixed-switch conditions in which the attentional control system was most heavily taxed. In all direct comparisons of healthy older adults with the very mild DAT, we covaried out age. Finally, the correlations among the CoV and CSF biomarkers will be presented. The partial eta-square (ηp2) indicates the effect size of our analyses.
Stroop Task Mean RT, Errors, and CoV
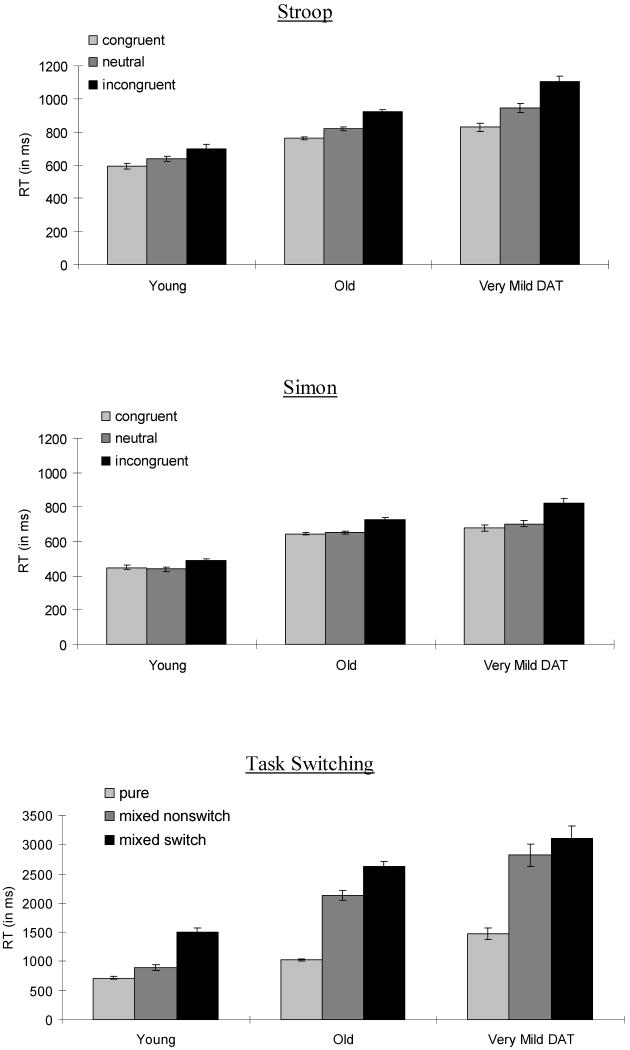
The mean RTs for correct color-naming trials as a function of group and congruency condition are displayed in Figure 1. Participants' mean RTs and errors were separately submitted to a 3 (group) × 3 (congruency: congruent, neutral, incongruent) mixed-factor Analysis of Variance (ANOVA). There were main effects of group [for RTs: F (2,300) = 37.47, MSe = 81389, p < .001, ηp2 = .20; for errors: F (2,300) = 7.74, MSe = 15.87, p = .001, ηp2 = .05] and congruency [for RTs: F (2,600) = 350.03, MSe = 3836, p < .001, ηp2 = .54; for errors: F (2,600) = 64.24, MSe = 9.19, p < .001, ηp2 = .18]. More important, there was a significant group × congruency interaction in both RTs and errors [for RTs: F (4,600) = 26.89, MSe = 3836, p < .001, ηp2 = .15; for errors: F (4,600) = 8.24, MSe = 9.19, p < .001, ηp2 = .05]. Post-hoc tests were conducted comparing the Stroop congruency effect (incongruent - congruent) across groups. The congruency effect was larger for very mild DAT group compared with the healthy older group [for RTs: p < .001, ηp2 = .17; for errors: p = .001, ηp2 = .06] and larger for the healthy older adults than the young adults in RTs (p = .003, ηp2 = .04). However, there was no age-related difference in the congruency effect in errors (p = .50, ηp2 = .002). Hence, we replicated the findings that indicated a breakdown in attentional control system due to healthy aging and DAT pathology (e.g., Spieler et al., 1996).
Figure 1.
Mean RTs in Stroop, Simon and Task Switching as a function of condition and group. Error bars indicate standard errors of means.
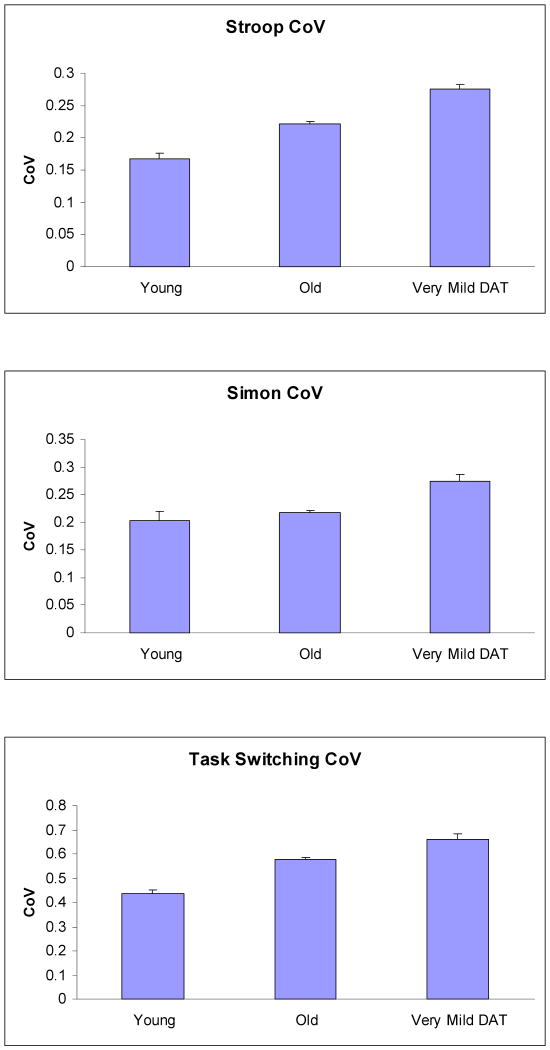
The overall CoV for color-naming trials as function of group is displayed in Figure 2. A one-way ANOVA indicated that the overall CoV increased across groups, F (2,300) = 47.07, MSe = .003, p < .001, ηp2 = .24. Post hoc tests indicated that the CoV increased with healthy aging (young vs. healthy older controls, p < .001, ηp2 = .10) and in very mild DAT (healthy older controls vs. very mild DAT, p < .001, ηp2 = .14). The CoV for incongruent trials was also computed for each individual to examine group differences in within-person variability in the more difficult task condition. A one-way ANOVA indicated that the incongruent CoV also increased across groups, F (2,300) = 23.68, MSe = .004, p < .001, ηp2 = .14. Post hoc tests indicated that the incongruent CoV was larger for healthy older adults (.205) than young adults (.154) (p < .001, ηp2 = .07) and larger for very mild DAT individuals (.244) than healthy older adults (.205) (p < .001, ηp2 = .06).
Figure 2.
Overall CoV in Stroop, Simon, and Task Switching as a function of group. Error bars indicate standard errors of means.
Simon Task Mean RT, Errors, and CoV
The mean RTs for correct trials as a function of group and congruency condition are displayed in Figure 1. Participants' mean RTs and errors were separately submitted to a 3 (group) × 3 (congruency: congruent, neutral, incongruent) mixed-factor ANOVA. There were main effects of group [for RTs: F (2,281) = 41.08, MSe = 58442, p < .001, ηp2 = .23; for errors: F (2,281) = 13.78, MSe = 19.02, p < .001, ηp2 = .09] and congruency [for RTs: F (2,562) = 208.39, MSe = 1905, p < .001, ηp2 = .43; for errors: F (2,562) = 60.53, MSe = 8.60, p < .001, ηp2 = .18]. More important, there was also a significant group × congruency interaction in both RTs and errors [for RTs: F (4,562) = 17.31, MSe = 1905, p < .001, ηp2 = .11; for errors: F (4,562) = 4.81, MSe = 8.60, p = .001, ηp2 = .03]. Post-hoc tests were conducted comparing the Simon congruency effect (incongruent - congruent) across groups. The congruency effect was larger for very mild DAT group than the healthy older group [for RTs: p < .001, ηp2 = .09; for errors: p = .003, ηp2 = .03], and larger for healthy older adults than for young adults in RTs (p < .001, ηp2 = .06) but not in errors (p = .75, ηp2 = .00). Hence, we replicated the findings of a breakdown in the attentional control system due to healthy aging and DAT pathology in the Simon task (e.g., Castel et al., 2007).
The overall CoV as function of group is displayed in Figure 2. A one-way ANOVA indicated that the overall CoV increased across groups, F (2,281) = 14.48, MSe = .006, p < .001, ηp2 = .09. Post hoc tests showed that the CoV was larger for the very mild DAT group than for the healthy older group (p < .001, ηp2 = .08). There was no significant difference in the overall CoV between young and healthy older adults (p = .309, ηp2 = .01). The CoV for incongruent trials was also computed for each individual. A one-way ANOVA indicated that the incongruent CoV also increased across groups, F (2,281) = 7.48, MSe = .006, p < .001, ηp2 = .05. Post hoc tests indicated that the incongruent CoV was larger for the very mild DAT group (.230) than the healthy older group (.188) (p = .001, ηp2 = .04). There was no difference in the incongruent CoV between young (.183) and healthy older adults (.188) (p = .71, ηp2 = .001).
Task Switching Mean RT, Errors, and CoV
Local Cost
The mean RTs for correct trials as a function of group and switch condition are displayed in Figure 1. To compute the “local cost” of switching, we compared switch and non-switch correct trials in the mixed block. Participants' mean RTs and errors were separately submitted to a 3 (group) × 2 (switch vs. non-switch trials) mixed-factor ANOVA. There were main effects of group [for RTs: F (2,283) = 28.46, MSe = 2363129, p < .001, ηp2 = .17; for errors: F (2,283) = 35.63, MSe = 112.88, p < .001, ηp2 = .20], and switch trials [for RTs: F (1,283) = 153.34, MSe = 124740, p < .001, ηp2 = .35; for errors: F (1,283) = 18.10, MSe = 12.86, p < .001, ηp2 = .06]. More important, there was a significant group × switch interaction in both RT and errors [for RTs: F (2,283) = 5.56, MSe = 124740, p = .004, ηp2 = .04; for errors: F (2,283) = 3.21, MSe = 12.86, p = .04, ηp2 = .02]. Post hoc tests were conducted comparing the local switch costs (switch - nonswitch) across groups. The local switch cost was larger for healthy older group than the very mild DAT group [for RTs: p = .01, ηp2 = .03; for zRTs: p < .001, ηp2 = .09; for errors: p = .03, ηp2 = .02]. However, there was no age-related difference in the local switch cost in RTs (p = .21, ηp2 = .01) or in errors (p = .53, ηp2 = .002).
General Cost
The mean RTs for correct trials as a function of group and switch condition are displayed in Figure 1. To compute the “general cost” of switching, we compared the correct trials in the pure block and the non-switch correct trials in the mixed block. Participants' mean RTs and errors were separately submitted to a 3 (group) × 2 (pure vs. non-switch trials) mixed-factor ANOVA. There were main effects of group [for RTs: F (2,283) = 40.87, MSe = 958599, p < .001, ηp2 = .22; for errors: F (2,283) = 23.16, MSe = 64.67, p < .001, ηp2 = .14] and trial block [for RTs: F (1,283) = 146.77, MSe = 450398, p < .001, ηp2 = .34; for zRTs: F (1,283) = 50.62, MSe = .04, p < .001, ηp2 = .26; for errors: F (1,283) = 14.94, MSe = 23.33, p < .001, ηp2 = .05]. More important, there was a significant group × trial block interaction in both RTs and errors [for RTs: F (2,283) = 17.42, MSe = 450398, p < .001, ηp2 = .11; for errors: F (2,283) = 14.44, MSe = 23.33, p < .001, ηp2 = .09]. Post hoc tests were conducted comparing the general switch costs (nonswitch - pure trials) across groups. The general switch cost was not different between the two groups in RTs (p = .10, ηp2 = .01), but was larger for the very mild DAT group than the healthy older group in errors (p < .001, ηp2 = .08). The general switch cost was larger for healthy older adults than young adults [for RTs: p < .001, ηp2 = .13; for errors: p = .054, ηp2 = .02].
The overall CoV as function of group is displayed in Figure 2. A one-way ANOVA indicated that the overall CoV increased across groups, F (2,283) = 27.89, MSe = .02, p < .001, ηp2 = .17. Post hoc tests indicated that the CoV was larger for healthy older adults than for young adults (p < .001, ηp2 = .15) and for very mild DAT than for healthy older adults (p = .002, ηp2 = .04). The CoV for mixed-switch trials was also computed for each individual. A one-way ANOVA indicated that the mixed-switch CoV also increased across groups, F (2,283) = 34.64, MSe = .02, p < .001, ηp2 = .20. Post hoc tests indicated that the mixed-switch CoV was larger for healthy older adults (.277) than for young adults (.230) (p = .003, ηp2 = .04) and larger for very mild DAT individuals (.433) than for healthy older adults (.277) (p < .001, ηp2 = .15).
CoV and ApoE in Healthy Aging
We next examined the relationship between ApoE status and variability in the healthy older adults in each attention task to assess whether intraindividual variability can discriminate healthy individuals who are at risk vs. not at risk for DAT. First, it should be noted that there were no significant differences in psychometric performance as a function of ApoE status in the healthy older adults, with the exception of the Selective Reminding test (ε4- = 31.3; ε4+ = 29.0; p = .03, ηp2 = .03) and Trailmaking A (ε4- = 35.1; ε4+ = 30.0; p = .03, ηp2 = .04), where the ε4+ group actually showed better performance in this latter task. Also, the ε4+ group was slightly younger than the ε4- group (70.2 vs. 73.2, p = .047, ηp2 = .03). and so age was used as a covariate. We did not find any differences between ε4+ and ε4- individuals for the mean and standard deviation measures of all conditions across all three attention tasks (all ps > .17).
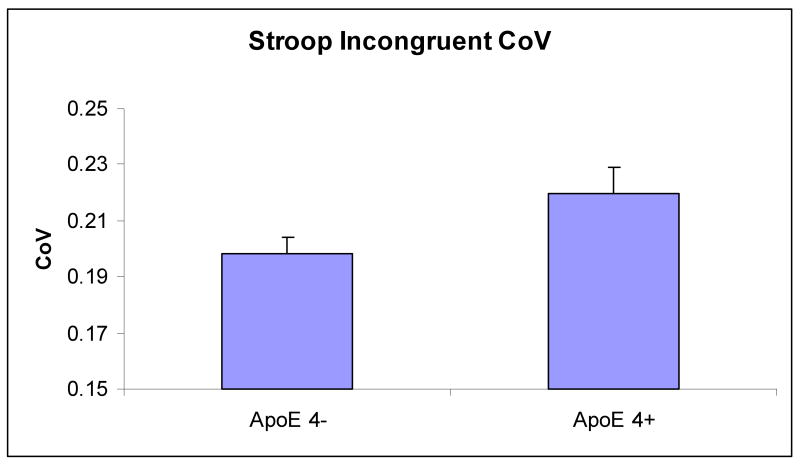
First, we examined the overall CoV in the Stroop task as a function of ApoE status in the healthy older group, controlling for age (by treating it as a covariate). There was no significant difference in the overall CoV between ε4+ (.230) and ε4- (.218) individuals, F (1,140) = 2.17, MSe = .002, p = .14, ηp2 = .02, although the means were in the predicted direction. Next, we examined the CoV for Stroop incongruent trials as a function of ApoE status. As can be seen in Figure 3, the CoV was larger in the ε4+ group (.220) than in the ε4- group (.199), F (1,140) = 4.46, MSe = .003, p = .037, ηp2 = .03. For the Simon Task, neither the overall CoV nor the CoV in the incongruent condition yielded a significant main effect of ApoE status, (p = .73, ηp2 = .001, and p = .83, ηp2 = .001, respectively), with age as a covariate. Similarly, for Task Switching neither the overall CoV (although the means were in the predicted direction) nor the CoV in the mixed-switch condition yielded a significant main effect of ApoE status, (p = .11, ηp2 = .01 and p = .936, ηp2 = .00, respectively), with age as a covariate. Hence, it appears that the CoV difference between ε4+ and ε4- individuals is slightly more sensitive to the conflict in the Stroop incongruent condition.
Figure 3.
Overall CoV and incongruent/mixed-switch CoV in Stroop, Simon, and Task Switching as a function of ApoE status in healthy older adults. Error bars indicate standard errors of means.
CoV differences beyond Standard Psychometrics
To examine whether the CDR status (i.e., 0 – healthy old vs. 0.5 – very mild DAT) could predict the CoV, above and beyond standard psychometric measures, we conducted a series of linear regression analyses. In each linear regression analysis, we entered the participants' age and one of 14 psychometric measures in Table 1 in the first and second steps of the linear regression model, respectively. Then, we entered CDR status in the last step of the model. The dependent measures of these linear regression models were Stroop incongruent CoV, Simon incongruent CoV or CVOE mixed-switch CoV. Table 2 presents the R-square changes and their significance levels of CDR status when it was entered in the last step of the linear regression analyses on predicting the incongruent CoV in the three attention tasks. There are a number of points to note here. First, although the Simon CoV clearly predicts unique performance compared to most of the psychometric measures, the Stroop CoV and the Switching CoV clearly do better overall. Second, the Simon task has particular overlap with the memory measures. Third, and most importantly, 38 out of the CDR-status predictors were significant in directional tests (i.e., p < .10), and 34 out of the 42 CDR-status predictors were significant, p < .05. We also performed the same regression analyses on mean performance in each of the comparable task conditions, and only 16 out of 42 CDR-status predictors reached significance in a directional test and only 11 reached the p < .05 level. Clearly, there are reliable differences in CoV as a function of CDR status above and beyond the standard psychometric measures, and this pattern is larger than the mean RT in each of the comparable conditions.
Table 2. The R-square changes CDR status (0 vs. 0.5) when it was entered in the last step of linear regression analyses on predicting the incongruent CoV.
| Stroop | Simon | CVOE | |
|---|---|---|---|
| Logical Memory | 0.06*** | 0.02** | 0.06*** |
| Forward Digit Span | 0.07*** | 0.03** | 0.14*** |
| Backward Digit Span | 0.05*** | 0.03** | 0.11*** |
| WMS Associate Recall | 0.02** | 0.01 | 0.04*** |
| Word Fluency S-P | 0.05*** | 0.02** | 0.12*** |
| WAIS Information | 0.03*** | 0.02** | 0.06*** |
| WAIS Digit Symbol | 0.03** | 0.00 | 0.05** |
| WAIS Similarities | 0.03*** | 0.02** | 0.08*** |
| Trailmaking A | 0.05*** | 0.02** | 0.13*** |
| Trailmaking B | 0.03** | 0.01 | 0.05*** |
| Boston Naming | 0.07*** | 0.03* | 0.11*** |
| Animal Fluency | 0.04*** | 0.02** | 0.09*** |
| Selective Reminding | 0.02* | 0.00 | 0.03*** |
| MMSE | 0.01* | 0.01 | 0.05*** |
p < .10,
p < .05,
p < .01
CoV and CSF Biomarkers
We now examine the association between the CSF biomarkers (Aβ42, Tau/Aβ42, Ptau181/Aβ42), which are predictive of the onset of DAT (see Fagan et al., 2007) in the healthy control (CDR = 0) individuals. Thus, we examined the correlations of these biomarkers with the CoV across the three attention tasks, while treating age and education as covariates1. Interestingly, in Task Switching, the overall CoV and the CoV on mixed-switch trials were significantly correlated with all of these CSF biomarkers, as displayed in Table 3. It should be noted that all of the correlations were in the predicted direction indicating increased individual variability with CSF biomarkers that suggest the presence of AD pathology (i.e., reduced Aβ42, and increased Tau/Aβ42 and Ptau181/Aβ42). However, these biomarkers were not reliably correlated with any CoV measures in the Stroop and Simon tasks with the full sample of healthy controls (all r's <.10)2.
Table 3. Partial Correlations between CoV and CSF Biomarkers in Healthy Older Adults.
| Aβ42 | Tau/Aβ42 | Ptau181/Aβ42 | |
|---|---|---|---|
| Overall Sample | |||
| Task Switching | |||
| Overall CoV | -.32** | .28* | .27* |
| Mixed-Switch CoV | -.36** | .33** | .38** |
| ε4+ individuals only | |||
| Stroop | |||
| Overall CoV | -.32 | .42* | .45* |
| Incongruent CoV | -.26 | .40* | .40* |
| Task Switching | |||
| Overall CoV | -.37 | .44* | .30 |
| Mixed-Switch CoV | -.33 | .29 | .31 |
p < .05
p < .01
We next restricted our CoV-CSF biomarker analyses to ε4+ individuals only (i.e., those healthy older adults who are at risk for DAT; n=26). Due to the reduced sample size, some of the correlation coefficients did not reach significance. As shown in the bottom of Table 3, it is indeed the case for the overall CoV in the Switching task. In fact, even Stroop overall CoV and incongruent CoV were now correlated with CSF biomarkers in the predicted direction. Thus, this indicates that the magnitude of intraindividual variability is further accentuated for those healthy older individuals with at least one ε4+ allele.
Between Task Variability
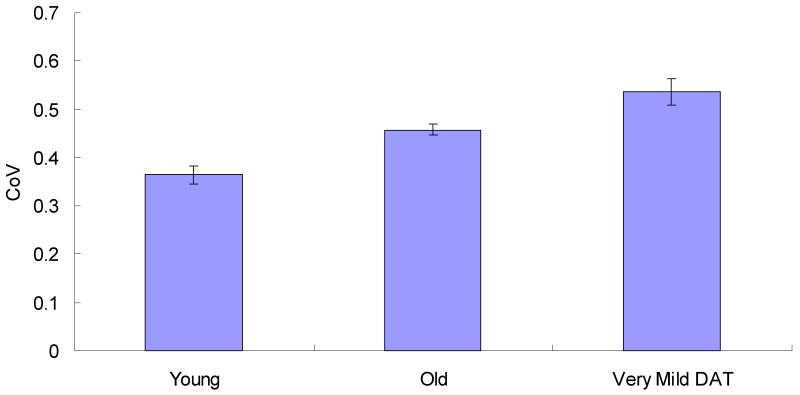
Finally, between-task variability across participant groups was examined by first computing each participant's overall mean in each task, then the standard deviation of these overall task means and in turn, the CoV (overall SD/overall tasks means) across the tasks (see Figure 4). This measure reflects the variability in performance across the tasks, above and beyond mean performance. A one-way ANOVA indicated that the between task CoV increased across groups, F (2,302) = 10.89, MSe = .03, p < .001, ηp2 = .07. Post hoc tests indicated that there was a significant increase in the task CoV with healthy aging (young vs. healthy older adults, p < .003, ηp2 = .04) and with the onset of dementia (healthy older adults vs. very mild DAT individuals, p = .002, ηp2 = .04). Therefore, in addition to the increase in variability across trials within a task, there is also evidence of an increase in variability across tasks within individuals across groups. These findings are consistent with a recent study by Holtzer, Verghese, Wang et al. (2008), who found that the risk of dementia increased as a function of higher “within-person across-neuropsychological test variability”, even after taking into account neuropsychological test performance. However, whereas we used three attention tasks with differential task demands in the current study, Holtzer et al. used three tests that represented different cognitive domains, including verbal intelligence (WAIS-R vocabulary subtest), attention control/executive function (WAIS-R digit symbol test), and memory (free and cued selective reminding test).
Figure 4.
Between-Task CoV as a function of group. Error bars indicate standard errors of means.
Discussion
The purpose of the present research was to provide a large scale examination of (a) the utility of trial-to-trial intraindividual variability in processing speed in three standard attention tasks in discriminating healthy aging from the very earliest stages of DAT; and (b) the relationship between ApoE4 status and variability in healthy older adults to determine whether within-person variability can discriminate healthy individuals at risk vs. not at risk for DAT, along with specific CSF biomarkers predictive of Alzheimer's disease.
Discriminating Healthy Aging and Very Mild DAT
The mean level performance in the present tasks are consistent with the notion of a breakdown in attentional control in early-stage DAT (see also Balota & Faust, 2001; Castel et al, 2007; Duchek & Balota, 2005; Spieler et al., 1996). First, in Stroop task, there was evidence for a larger congruency effect in RT and more errors in incongruent trials for very mild DAT individuals compared with healthy older adults. Thus, there is evidence for a disruption in the ability to control the prepotent “word” response in early-stage DAT. Similarly, in the Simon task, very mild DAT individuals showed a larger congruency effect in RTs and more errors in incongruent trials than healthy older adults, indicating more difficulty controlling the prepotent pathway when a conflict is presented in terms of incongruent mapping between a stimulus and the appropriate response. An interesting pattern of data emerged in Task Switching. The very mild DAT group actually showed smaller local switching costs (switch – nonswitch trials) in RTs than healthy older and young adults, yet more errors on switch trials, suggesting that they are less “tuned” to the constraints of the task and thus less affected by the switching within trial blocks, again suggesting a breakdown in attentional control systems that maintain the goals of the task. Importantly, all of these effects were also reliable in the z-score transformed data and thus are not simply a reflection of overall slowing (see Faust et al., 1999).
In terms of intraindividual variability, the results across the three attention tasks are also straightforward. We chose these tasks because they are particularly sensitive to early-stage DAT (e.g., Castel et al., 2007; Spieler et al., 1996). Indeed, there was a clear increase in within-person variability as a function of healthy aging (young vs. old) in both Stroop and Task Switching, and more importantly, as a function of DAT (healthy old vs. very mild DAT) in Stroop, Simon, and Task Switching. In all three tasks, relative to healthy older adults, very mild DAT individuals showed increased variability in overall performance as well as in incongruent/mixed-switch trials, indicating that the increase in intraindividual variability represents a general characteristic of overall performance and not just for the most difficult condition. Hence, these results are consistent with Christensen et al. (2005) and Dixon et al. (2007) who reported increased variability in MCI individuals and argued that inconsistency in performance may serve as a an additional marker for very early cognitive impairment.
As noted in the Introduction, there are numerous ways to measure variability, and we have chosen the relatively simple measure of the CoV, which is the ratio of the standard deviation and mean response latency. Because it is possible that the present results may be a reflection of this particular measure, we also used a covariance technique as converging evidence in which we partialed out the mean RT in an Analysis of Covariance to determine if there are reliable differences between the healthy old and the very mild DAT in the standard deviations. The results of this alternative approach are also quite clear. There is a reliable difference between the healthy control and very mild DAT groups for all three attention tasks. For example in the incongruent condition, after partialing out the mean RT for each of the three tasks, there was a significant group difference in the SD (Stroop, p = .003, effect size = .31; Simon, p = .047, effect size = .016; Task Switching, p < .001, effect size = .113).
It should be emphasized that the current study provided a comparison of a well-characterized group of individuals in the earliest detectable stage of DAT with healthy older adults, free of any cognitive impairment. A designation of very mild DAT on the CDR scale (CDR 0.5) denotes cognitive impairment at an early stage comparable to MCI without dementia (Storandt et al., 2006). Dixon et al. (2007) did not use standard MCI diagnostic criteria, but instead defined MCI based on individuals' performance relative to norms on a set of reference cognitive tasks. Thus, it is not entirely clear how the level of impairment of their MCI groups compared with our very mild DAT group (although the MMSE scores for their MCI groups, ranged from 27.85 to 29.00, were slightly higher than our very mild DAT group, 26.95). It is interesting that Dixon et al. found the most reliable group discrimination of mild MCI and healthy aging among their older participants (74-92; mean age = 79.98), whereas our groups were slightly younger (healthy older adults = 71.75; very mild DAT = 75.25), and we still found that intraindividual variability discriminated healthy aging from very mild DAT.
Discriminating Healthy Controls at Risk
Turning to the results from the biomarkers in healthy control individuals, the overall Stroop CoV was numerically larger in ε4+ group, compared to e4- individuals, although this difference did not quite reach statistical significance (p = .12). However, the ε4+ individuals did show reliably greater within-person variability than ε4- individuals on the more demanding Stroop incongruent trials. It is important to note that there was no difference in mean RT in incongruent trials as a function of ApoE status (p = .66), nor were there any differences in psychometric test performance, with the exception of the Selective Reminding task. Thus, the variability in performance in the conflict condition of the Stroop task may potentially be a useful indicator for the risk for DAT.
These results are consistent with other studies in the literature where ε4+ related deficits are only apparent when experimental procedures are used that tap early aspects of visual attentional selection, rather than more global measures of cognition (e.g., Greenwood et al., 2005; Rosen et al., 2002, 2004). In a meta-analysis, Small et al. (2004) argued that the ε4 effect on cognitive performance in healthy aging is subtle and confined to more specific aspects of cognition, such as retrospective memory and executive function. Clearly, such executive control is paramount to effective performance in the Stroop task. In fact, Parasuraman et al. (2002) argue that attentional selection, in particular, is impaired in ε4 carriers and is an early sensitive marker for DAT. Of course, the variability in the Simon task was not sensitive to ApoE status. It is possible that the attentional selection involved in a relatively simpler spatial S-R compatibility task, such as the Simon task, is less demanding and so is not as sensitive to ApoE status in healthy older adults. Hence, the attentional selection component of the Stroop task, in particular, and the measure of intraindividual variability in attentional control mechanisms may provide an antecedent marker for the later onset of DAT (also see Balota, et al., submitted).
The potential for attention measures as an early marker for individuals at risk for developing DAT is also supported by the CSF biomarker data in our healthy older adults. As mentioned above, Fagan et al. (2007) and Li et al. (2007) found that the ratio of CSF tau/Aβ42 and ptau181/Aβ42 predicted conversion from healthy aging to early-stage dementia (after 3-4 years). In addition, cognitively normal individuals with cortical amyloid deposition can be identified by the presence of low CSF Aβ42 (Fagan et al., 2006, 2007). Our results indicate that greater within-person variability in Task Switching in healthy controls is modestly associated with decreased CSF Aβ42 and increased CSF tau/Aβ42 and ptau181/Aβ42, which are likely makers for the presence of amyloid deposition (Aβ42) and tangles (tau) in the brain. It is interesting that neither Stroop nor Simon performance correlated with the biomarkers when we examined the full sample of healthy older adults. However, when we restricted our analyses to the ε4+ individuals only (i.e., those at greater risk for the onset of DAT), we found that Stroop overall CoV and incongruent CoV were reliable correlated with the CSF biomarkers. Given that the Switching task was more difficult, as evidenced by the largest CoV across all groups and the slowest overall RT in this task, one might speculate that the relationship between these CSF biomarkers and task performance in healthy older adults will be apparent when greater demands are placed on the attentional system, as in the case of task switching3 (see West et al., 2002, for a similar argument) and/or when the risk for the onset of DAT is greater, as was the case in the Stroop task for the ε4+ individuals. To our knowledge, this is the first report of such a relationship between intraindividual variability, as a potential behavioral marker, and CSF biomarkers in healthy older adults. Of course given the relatively modest relationship we found in this study, further research is clearly warranted.
Based on the pattern of intraindividual variability for our healthy older adults, one might speculate that lower level attentional selection tasks, such as Stroop, may be more sensitive to ApoE 4 status. On the other hand, more complex attention tasks, such as Task Switching, may be more sensitive to CSF biomarkers as indicators for underlying pathology in healthy controls. Of course, the focus in this study has been on the biomarker relationships with attentional performance at the level of within-person variability, and not at the level of the sensitivity of the tasks themselves (e.g., congruency effects in RTs and errors in Stroop and Simon). In this light, it is important to note that in the current sample of healthy older adults, the congruency effects in errors in Stroop and the Switching task are both correlated with the ptau181/Aβ42 ratios, r = +.23, p < .05, and r = +.25, p < .03, respectively. Moreover, if one only considers the ε4+ individuals (N = 26), even the congruency effect sizes in the Simon task are also correlated with the ptau181/Aβ42 ratio in RTs, r = +.41, p = .03. Thus, effect sizes in these attention tasks may indeed be differentially sensitive to the underlying pathology identified by biomarkers.
At this point, it is important to note that numerous targeted analyses were conducted examining the relationships between ApoE status, CSF biomarkers and variability across the attention tasks. Thus one might be concerned that some of these relationships simply reflect capitalizing on chance due to multiple comparisons. Although this is a possibility, the ApoE effect in Stroop and the correlations with CSF biomarkers are in the predicted directions and consistent with other literature and hence are at least suggestive of the utility of examining within-person variability as a marker for early stage DAT. Indeed, although there were multiple reliable correlations in the predicted direction, there were no reliable correlations in the unpredicted direction. Clearly, further research is warranted and we are currently increasing our sample sizes to further explore these interesting relationships.
Possible Mechanisms
There are a number of potential mechanisms that could contribute to the observed changes in variability, including the speed of neural transmission, functioning of neurotransmitter systems, synchronicity of neutral activity, fatigue, stress, practice/learning, among many others (see Hultsch et al., 2008). As suggested by Holtzer et al. (2008), the increase in between-task intraindividual variability, which we also obtained in our study, may indicate an early decline in global cerebral integrity representing different sensitivities of numerous brain regions to the disease process rather than the disease's effect on a single brain region. Thus, it is likely that many of these mechanisms are intertwined. In addition to these neural accounts, at a higher cognitive level, we believe that the increase in variability in early-stage DAT and in individuals at risk for developing DAT is consistent within an attentional control framework (see Balota & Faust, 2001; Faust & Balota, 2007). Specifically, a major goal of the human cognitive system is to flexibly tune itself to current task demands and stay tuned to such demands across time. Indeed, it is the flexibility of the cognitive system that likely reflects current notions of executive/attentional control (see, e.g., Baddeley, Chincotta, & Adlam, 2001; Engle & Kane, 2004). As attentional control systems begin to deteriorate, these systems are no longer consistently tuned across time to the specific goals of the task. Hence, there is an increase in variability and indeed this variability in performance may serve as an additional antecedent marker for DAT. Of course, as noted earlier, it is unlikely that there will be one mechanistic explanation for such a pattern. It is much more likely that one will need multiple descriptions at multiple levels. In this light, the present work is a first step in attempting to integrate work on intraindividual variability across the wide range of domains of attention, healthy aging, DAT, genotype, and biomarkers for DAT.
Conclusion
In sum, the current study supports the utility of examining intraindividual variability as a discriminator of healthy aging and early-stage DAT and a potential early marker for the onset of the disease in healthy individuals (also see, Hultsch et al., 2008). Of course, how well such variability actually predicts conversion to early-stage DAT depends on a large scale longitudinal study of well-characterized healthy controls, which is currently underway. Finally, we argue that the clear increase in trial-to-trial variability in early-stage DAT is consistent with the notion of a breakdown in attentional control systems very early in the disease process.
Acknowledgments
This work was supported by NIA PO1 AGO3991, P50AGO5681, and PO1 AGO26276. The first two authors contributed equally to this project. Thanks are extended to the clinicians at the Washington University Alzheimer's Disease Research Center (ADRC) for their careful recruitment and description of the healthy older adult and DAT participant groups, Martha Storandt and the Psychometrics Core for the neuropsychological data, the Genetics Core for the genotyping of the participants, and the Biomarkers Core for their assays and provision of the CSF data. We also thank Meredith Minear for help with the Switching Task, Keith Hutchison with the Stroop and Simon tasks, and Brian Weber and Elizabeth Hemphill for their help in collecting data at various stages of this project. Correspondence should be sent to Janet M. Duchek (jduchek@wustl.edu) Department of Psychology, Washington University in St. Louis, St. Louis, MO 63130.
Footnotes
The scatterplots were examined and any data points that were 3 SD units or more from the regression line were identified as outliers and removed from the correlational analysis. All reported effects were also reliable when these outliers were not removed from the correlational analyses.
It should be noted that the overall mean RT was not significantly correlated with the CSF biomarkers for any of the attention tasks (all p's > .07).
It Indeed the correlations with these CSF biomarkers are present in a different version of the switching task with this participant sample.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
References
- Albert M, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss MB, Blacker D, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used in evaluation of brain injury. Psychological Monographs. 1946;60(1, Whole No. 277):1–48. [Google Scholar]
- Baddeley A, Chincotta D, Adlam A. Working memory and the control of action: Evidence from task switching. Journal of Experimental Psychology: General. 2001;130:641–657. [PubMed] [Google Scholar]
- Balota D, Burgess G, Cortese M, Adams D. The word-frequency mirror effect in young, old, and early-stage Alzheimer's disease: Evidence for two processes in episodic recognition performance. Journal of Memory and Language. 2002;46(1):199–226. [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, III, McDermott KB, Yerys BE. Veridical and false memories in healthy older adults and in dementia of the Alzheimer's type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Balota DA, Faust ME. Attention in dementia of the Alzheimer's type. In: Bolla F, Cappa S, editors. Handbook of neuropsychology: Vol 6. Aging and dementia. 2nd. New York: Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Balota DA, Tse CS, Hutchison K, Spieler DH, Duchek JM, Morris JC. Predicting Conversion to Dementia of the Alzheimer Type in a Healthy Control Sample: The Power of Stroop Performance. Submitted. 2008 doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating. Psychopharmacology Bulletin. 1988;24:637–639. [PubMed] [Google Scholar]
- Berg L, McKeel DW, Miller PJ, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Blacker D, Haines JL, Rodes L, Terwedow H, Go RC, Harell LE, et al. APOE-4 and age at onset of Alzheimer's Disease: The NIMH genetics initiative. Neurology. 1997;48:139–147. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, Christensen H, Dear K, Wen W, Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60-64 years. Neuropsychologia. 2007;45:2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek JM, et al. The reliability of the Washington University Clinical Dementia Rating. Archives of Neurology. 1988;45:31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer's disease: Evidence for disproportionate selection impairments in the Simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DP. Aging, memory efficiency and the strategic control of attention at encoding: Selective impairments of value-directed remembering in Alzheimer's Disease. Neuropsychology. 2008 doi: 10.1037/a0014888. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Dear KBG, Anstey KJ, Parslow RA, Sachdev P, Jorm AF. Within-Occasion Intraindividual Variability and Preclinical Diagnostic Status: Is Intraindividual Variability an Indicator of Mild Cognitive Impairment? Neuropsychology. 2005;19:309–317. doi: 10.1037/0894-4105.19.3.309. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning & Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Dixon RA, Garrett DD, Lentz TL, MacDonald SWS, et al. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA. Failure to control prepotent pathways in early-stage dementia of the Alzheimer type: Evidence from dichotic listening. Neuropsychology. 2005;19:687–695. doi: 10.1037/0894-4105.19.5.687. [DOI] [PubMed] [Google Scholar]
- Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In: Ross B, editor. The Psychology of Learning and Motivation. Vol. 44. NY: Elsevier; 2004. pp. 145–199. [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of Neurology. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid-sub-4-sub-2 ratio as a prediction of cognitive decline in nondemented older adults. Archives of Neurology. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Faust M, Balota D. Inhibition in cognition. Washington, DC US: American Psychological Association; 2007. Inhibition, facilitation, and attentional control in dementia of the Alzheimer's type: The role of unifying principles in cognitive theory development; pp. 213–238. [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: Implications for group differences in response latency. Psychological Bulletin. 1999;125:779–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test. PA: Lea & Febiger; 1983a. [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination Booklet. PA: Lea & Febiger; 1983b. [Google Scholar]
- Gorus E, De Raedt R, Lambert M, Lemper J, Mets T. Reaction times and performance variability in normal aging, mild cognitive impairment, and Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology. 2008;21(3):204–218. doi: 10.1177/0891988708320973. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Bushke H, Crystal HA, Bang S, et al. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Easteal S, Jorm AF, Mackinnon AJ, Korten AE, Christensen H, et al. Apolipoprotein-E allele epsilon-4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387–1390. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. Journal of the American Medical Association. 2008;300:823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danzinger W, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. Journals of Gerontology. 2002;57B:P101–P115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Strauss E, Hunter MA, MacDonald S. Intraindividual variability, Cognition, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd. New York: Psychology Press; 2008. pp. 491–556.pp. 491–556. [Google Scholar]
- Hultsch DF, MacDonald SWS, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14:588–598. doi: 10.1037//0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. Deceiving the elderly: Effects of accessibility bias in cued-recall performance. Cognitive Neuropsychology. 1999;16:417–436. [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- MacDonald S, Nyberg L, Bäckman L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences. 2006 Aug;29(8):474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Jr, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer's disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in ‘normal’ aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Murtha S, Cismaru R, Waechter R, Chertkow H. Increased variability accompanies frontal lobe damage in dementia. Journal of International Neuropsychological Society. 2002;8:360–372. doi: 10.1017/s1355617702813170. [DOI] [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Klang O, Lind K, Pedersen M, Blennow K, Edman A, Hansen S, Wallin A. Episodic memory and speed/attention deficits are associated with Alzheimer-typical CSF abnormalities in MCI. Journal of the International Neuropsychological Society. 2008;14:582–590. doi: 10.1017/S135561770808079X. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16:254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Annals of Neurology. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Bergeson JL, Putman K, Harwell A, Sunderland T. Working memory and apolipoprotein E: What's the connection? Neuropsychologia. 2002;40:2226–2233. doi: 10.1016/s0028-3932(02)00132-x. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Sunderland T, Levy J, Harwell A, McGee L, Hammond C, et al. Apolipoprotein E and category fluency: Evidence for reduced semantic access in healthy normal controls at risk for developing Alzheimer's disease. Neuropsychologia. 2004;43:647–658. doi: 10.1016/j.neuropsychologia.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, Berg L. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Huff LM. The effects of age and dementia of the Alzheimer's type on phonological false memories. Psychology and Aging. 2003;18:791–806. doi: 10.1037/0882-7974.18.4.791. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer's type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathological outcomes in original vs. revised MCI and in preMCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Storandt M, Hill RD. Very mild senile dementia of the Alzheimer type: II. Psychometric test performance. Archives of Neurology. 1989;46:383–386. doi: 10.1001/archneur.1989.00520400037017. [DOI] [PubMed] [Google Scholar]
- Stuss D, Murphy K, Binns M, Alexander M. Staying on the job: The frontal lobes control individual performance variability. Brain: A Journal of Neurology. 2003 Nov;126(11):2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, et al. Decreased {beta}-Amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone LG. Examiner manual for the SRA Primary Mental Abilities Test. Chicago: Science Research Associates; 1949. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale (Manual) San Antonio, TX: Psychological Corp.; 1955. [Google Scholar]
- Wechsler D, Stone CP. Wechsler Memory Scale (Manual) San Antonio, TX: Psychological Corp.; 1973. [Google Scholar]
- West R. The transient nature of executive control processes in younger and older adults. European Journal of Cognitive Psychology. 2001;13:91–105. [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FIM, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, et al. The apolipoprotein E e4 allele and decline in different cognitive systems during a 6-year period. Archives of Neurology. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]