Abstract
A growing body of evidence indicates that resolution of acute inflammation is an active process1,2. Resolvins are a new family of lipid mediators enzymatically generated within resolution networks that possess unique and specific functions to orchestrate catabasis2,3. Resolvin D2 (RvD2) was originally identified in resolving exudates, yet its individual contribution in resolution remained to be elucidated. Here, we established RvD2’s potent stereoselective actions in reducing excessive neutrophil trafficking to inflammatory loci. RvD2 decreased leukocyte:endothelial interactions in vivo by endothelial-dependent nitric oxide production, and direct modulation of leukocyte adhesion receptor expression. In microbial sepsis initiated by cecal ligation and puncture (CLP), RvD2 sharply decreased both local and systemic bacterial burden, excessive cytokine production and neutrophil recruitment, while increasing peritoneal mononuclear cells and macrophage phagocytosis. These multi-level pro-resolving actions of RvD2 translate to increased survival from CLP-induced sepsis and surgery. Together, these results identify RvD2 as a potent endogenous regulator of excessive inflammatory responses that acts via multiple cellular targets to stimulate resolution and preserve immune vigilance.
Ungoverned inflammation is an underlying component of many pathologies, such as cardiovascular disease, diabetes and sepsis4,5. It’s now recognized that resolution of inflammation is an active program controlled by temporal and spatial production of specialized chemical mediators2,3,6. Recently, autacoids endogenously generated from omega-3 essential fatty acids, namely resolvins, were identified during the resolution phase of inflammation that actively promote catabasis via potent pro-resolving and anti-inflammatory actions2,3. Resolvin D2 (RvD2), biosynthesized from docosahexaenoic acid (DHA), was originally identified during resolution3. Its complete stereochemistry and actions remained of interest. To this end, we investigated whether RvD2 preserves host immune function to facilitate resolution of inflammatory sepsis.
First, the complete stereochemistry of endogenous RvD2 was determined by physical matching with compounds prepared by total organic synthesis (Fig. S1a) from enantiomerically and geometrically pure starting materials in accordance with the basic structure determined in resolving exudates3 (Fig. 1). This approach was needed because the nanogram amounts of endogenous RvD2 isolated precluded direct NMR analysis. The double bond geometry of synthetic material was validated by 1H NMR (Fig. S1b). The biosynthesis of RvD2 involves 17-lipoxygenation of DHA to 17S-hydroperoxy-4Z, 7Z, 10Z, 13Z, 15E, 19Z-docosahexaenoic acid (17-HpDHA) that’s enzymatically transformed to a 7(8)epoxide-containing intermediate3,7 in human leukocytes. This enzymatic activity involves 5-lipoxygenase (LOX) and its epoxide generating activity 8. These steps can occur within a single cell type or via transcellular biosynthesis. For example, eosinophils, rich in 15-LOX, can convert DHA to 17-HpDHA that PMN can convert to RvD2 (Fig. 1a). Actively phagocytosing polymorphonuclear neutrophils (PMN) converted resolvin precursor 17-HpDHA to RvD2 as determined by LC-MS/MS-based lipidomics. A total ion chromatogram (m/z 375 [M-H]) of human leukocyte-derived RvD2 is shown (Fig. 1b), with characteristic conjugated tetraene UV-chromophore (λmax at 301nm with shoulders at 289 and 315). Synthetic material showed an exclusive and prominent peak with retention time (RT) and UV-spectrum essentially identical to leukocyte-derived RvD2 (Fig. 1c). Co-injection of synthetic and leukocyte-derived RvD2 led to an increase in intensity and co-elution (Fig. 1d). To further establish the physical properties, their tandem mass spectra (MS) were analyzed, with essentially identical MS fragmentation, and diagnostic ions in agreement with original assignments for endogenous RvD2 (Fig. 1e & f)3. To validate the biosynthetic pathway, activated human PMN were incubated with deuterium labelled 17S-HpDHA-d5 or DHA-d5; RvD2 containing the d5 label was biosynthesized (Fig. 1g), the parent ion increased to m/z 380 [M-H], and neutral loss ions reflective of d5-containing fragments (Fig. S2). Next, to further confirm the structural assignment, derivatized RvD2 was subjected to GC/MS. Derivatized RvD2 C-value was 25.2 ± 0.1 and its spectrum (Fig. S3) showed diagnostic ions at m/z 479, 435, 229 and 1713. Collectively, matching of synthetic and leukocyte-derived RvD2 (by RT, diagnostic MS ions and derivatization) established the complete stereochemistry and double bond geometry of endogenous RvD2 as 7S, 16R, 17S-trihydroxy-4Z, 8E, 10Z, 12E, 14E, 19Z-docosahexaenoic acid.
Figure 1. Stereochemical assignment, biosynthesis and total organic synthesis of RvD2.
(a) Rv biosynthesis illustrating potential PMN, eosinophil transcellular biosynthesis. Ion chromatograms (m/z 375) depicting human leukocyte-derived RvD2 with UV spectrum and related isomers with the ω-2 OH metabolites of PD1 and 7,17 diHDHA. (b). RvD2 prepared by total organic synthesis (c) and (d) co-injection with leukocyte-derived RvD2. MS/MS of leukocyte-derived RvD2 (e) and synthetic RvD2 (f) with prominent ions at m/z 357 [M-H-H2O], 339 [M-H-2H2O], 313 [M-H-CO2-H2O], 295 [M-H-CO2-2H2O], 277, 259 [277-H2O], 247, 233 [277-CO2], 141 and 113. (g) Leukocyte-derived RvD2-d5. Representative of n=3–5.
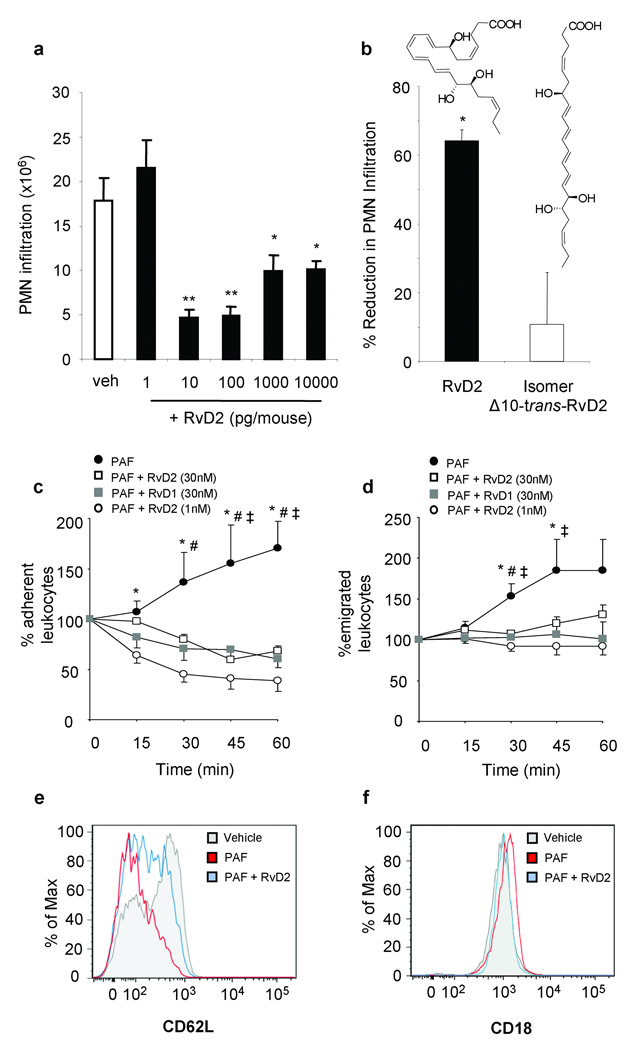
RvD2 displayed potent actions in microbial peritonitis, with a drastic ~70% reduction in zymosan-stimulated PMN infiltration at doses as low as 10 pg (Fig. 2). Importantly, Δ10-trans-RvD2 isomer was essentially inactive, indicating that specific geometry of endogenous RvD2 is required for bioactivity. To determine whether RvD2 decreases leukocyte-endothelial interactions, cremasteric microcirculation was analyzed. Platelet activating factor (PAF; 100 nM)6,9 superfusion caused increased leukocyte adherence and emigration that was markedly reduced by 1 nM RvD2 (Fig. 2c & d). Representative microcirculation before and after RvD2 superfusion are in supplementary movies. These potent RvD2 actions were recapitulated with human cells to identify potential cellular directed actions (i.e. endothelial cells and PMN). RvD2 potently reduced PAF-stimulated capture and adhesion of PMN by HUVECs under flow (Fig. S4)10. Noteworthy, RvD2 also reduced complement-mediated (C5a) PMN:endothelial interactions (Fig. S5a–c), a key mediator in sepsis11. Consistent with the impact of RvD2 on leukocyte:endothelial interactions, RvD2 diminished PAF-stimulated CD62L shedding on isolated human PMN, and CD18 surface expression (Fig. 2e & f). RvD2 alone did not alter PMN adhesion molecule expression (n=3 not shown). To obtain further evidence for direct actions of RvD2 on human leukocytes, we monitored reactive oxygen species (ROS). Importantly, RvD2 did not stimulate extracellular superoxide, while it potently reduced C5a-stimulated extracellular superoxide generation (Fig. S5e & f).
Figure 2. RvD2 potently reduces leukocyte-endothelial interactions to reduce microbial peritonitis.
(a,b) Leukocyte infiltration in peritonitis. (b) Equidose comparison (100 pg) of RvD2 and Δ10-trans-RvD2. (c-d) Leukocyte trafficking in vivo. PAF(100 nM)-stimulated leukocyte adherence (c) and emigration (d) ± RvD2 or RvD1. (e-f) Adhesion receptor surface expression. Results (n=3–6) are mean ± s.e.m. *P<0.05, **P<0.01 (a, b), *P<0.01 (PAF vs. PAF + RvD2 1nM), #P<0.05 (PAF vs. PAF + RvD1 30nM) and ‡P<0.05 (PAF vs. PAF + RvD2 30nM) (c-d) ANOVA.
Next, we assessed the contribution of nitric oxide (NO), an established anti-adhesive mediator12,13 in RvD2-reduced leukocyte adherence in post-capillary venules. The non-selective nitric oxide synthase inhibitor, L-NAME, before addition of RvD2 partially reversed the decreased leukocyte adherence and emigration (Fig. 3a & b). To obtain additional evidence for NO generation by RvD2 in vivo, vascular fluorescence was monitored (see Methods). Topical administration of RvD2 (100 pg/ear) increased fluorescence intensity, whereas lower doses (1 and 10 pg) were ineffective (Fig. S6a). L-NAME given before topical RvD2 application abolished this response (Fig. S6b), indicating that RvD2-stimulated vascular responses at this dose were NO-dependent. Of note, i.v. injection of RvD2 at doses that inhibited PMN infiltration in peritonitis (10 pg) did not increase fluorescence intensity indicating that only local elevated doses of RvD2 stimulated vascular responses (not shown). Additionally, RvD2 superfusion (1 nM) did not cause an increase in vascular permeability (Fig. S6e). Topical RvD2 (10 or 100pg) did not induce leukocyte infiltration into ear skin compared to chemoattractant leukotriene B4 (Fig. S6f). These results demonstrate that high focal delivery of RvD2 stimulates rapid NO production consistent with its anti-adhesive effects but not to a level that is pro-inflammatory. Corroboratory results were obtained with HUVECs whereby RvD2 dose-dependently stimulated NO generation (Fig. 3c), suggesting topical actions were likely mediated via endothelial nitric oxide synthase (eNOS). To test this, peritonitis was evaluated in eNOS−/− mice. In concurrence with an earlier report14, no changes were observed with respect to leukocyte infiltration between wild type and eNOS−/− mice. RvD2 reduction in leukocytes was eliminated in eNOS−/− mice (Fig. 3d), an effect that has also been reported for aspirin and local aspirin-triggered lipoxins15. Notably, RvD2 also stimulated vasoprotective prostacyclin (6-keto-PGF1α; Fig. S7a), this dose-response proved bell-shaped like other lipid mediators1,2,6. RvD2-stimulated prostacyclin and NO were pertussis-toxin sensitive implicating a role for G-protein coupled receptor(s) (Fig. S7b & c). Thus, RvD2 regulates leukocyte adherence via both direct actions on PMN (vide supra) and endothelial vasoactive substances.
Figure 3. Modulation of leukocyte trafficking by RvD2 is nitric oxide dependent.
(a,b) Leukocyte trafficking in vivo. Cremasters were superfused with PAF (100 nM), L-NAME (100 µM) and RvD2 (1 nM) and leukocyte adhesion (a) and emigration (b) was quantified (n=3–5). (c) Nitric oxide (NOx; nitrate/nitrite) generation in primary HUVECs incubated with RvD2 (n=4–6). (d) Zymosan-stimulated PMN infiltration after administration of RvD2 (100 ng; i.v.) in wild-type and eNOS−/− mice (n=3–5). Results are mean ± s.e.m., *P<0.05, ***P<0.001 two-way ANOVA (a,b), one-way ANOVA (c), two-tailed unpaired Student’s t-test (d).
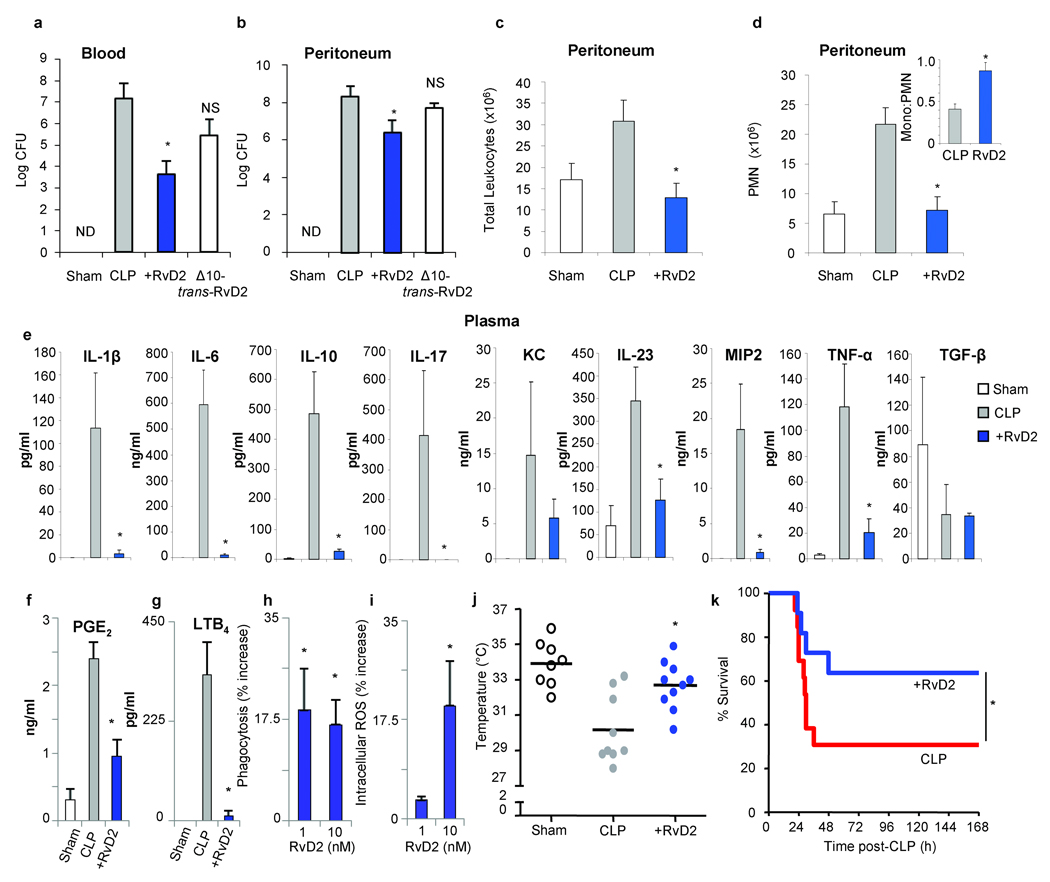
Next, anti-inflammatory and pro-resolving actions of RvD2 were evaluated in cecal ligation and puncture (CLP), an established murine microbial sepsis that closely resembles human pathology16,17. Omega-3 fatty acids are beneficial in some inflammatory conditions, including sepsis18–20, although the mechanistic basis underlying protection is still emerging. To this end, RvD2 significantly reduced the amount of live aerobic bacteria in both blood and peritoneum at 12 h post-CLP, while Δ10-trans-RvD2 was essentially not active (Fig. 4a & b). This was associated with a significant reduction in total leukocytes and specifically, PMN infiltration into the peritoneum (Fig. 4c & d). Interestingly, the ratio of mononuclear cells to PMN was increased with RvD2 (Fig. 4d inset), similar to results obtained with sterile zymosan-stimulated peritonitis (Fig. S9a). Intraperitoneal delivery of RvD2 at 1h post-CLP also reduced both blood and peritoneal bacteria (Fig. S8). Given the role of macrophages in clearance of bacteria, cellular debris and apoptotic PMNs to facilitate inflammation-resolution1,2, RvD2 treatment promoted phagocyte-dependent bacterial clearance observed in inguinal lymph nodes (Fig. S10d). Evidence for direct macrophage actions were obtained in vitro, where RvD2 potently enhanced macrophage phagocytosis of opsonized-zymosan (Fig. S9b). To obtain additional evidence for this pro-resolution role of RvD2, cytokines were monitored during CLP both locally (peritoneum) and systemically (plasma). RvD2 drastically reduced levels of pro-inflammatory cytokines associated with poor outcomes in sepsis17, namely IL-6, IL-1β, IL-23 and TNF-α (Fig. 4e & S10a). RvD2 reduced cytokine levels while enhancing bacterial clearance, a response also observed for macrophage scavenger receptor A21. Hence, it’s plausible that RvD2 prevents persistent amplification signals downstream of pattern-recognition receptors, dampening responses of classically activated macrophages22,23. RvD2 also drastically decreased IL-17, as well as IL-10, which is of interest in light of its detrimental impact on survival in sepsis24. Thus, RvD2 differs in action than lipoxin A4 which stimulates IL-1025. High levels of both pro- and anti-inflammatory cytokines, including IL-10, are predictive of early mortality in sepsis and diminishing IL-10 levels proved beneficial in sepsis26,27. Pro-inflammatory mediators, including prostaglandin E2 (PGE2) and LTB4 were also decreased in peritoneum by RvD2 (Fig. 4f, g and Fig. S10b & c). Of interest, in addition to macrophage-directed actions, RvD2 directly enhanced PMN E.coli phagocytosis that was accompanied by an increase in intracellular ROS (Fig. 4h & i). RvD2 does not possess direct antibacterial activity compared to ampicillin (Fig. S11). RvD2-treated CLP mice also showed protection at 12h post-CLP from hypothermia (Fig. 4j). Accordingly, RvD2 dramatically increased survival rates among CLP-operated mice (Fig. 4k) and activity levels12h post-CLP were resumed (Supplementary movie 3).
Figure 4. RvD2 reduces bacterial levels, systemic inflammation and enhances survival in microbial sepsis.
(a,b) Aerobic bacteria levels in blood (a) and peritoneal exudates (b) from sham or CLP-operated mice±RvD2-Me (100 ng; i.v.) at 12h (n=5–6 per group). (c,d) Peritoneal leukocyte differentials (n=3–5). (e) Plasma cytokine levels (n=3–6) (f, g) Peritoneal PGE2 and LTB4 levels. (h,i) Phagocytosis of E. coli and intracellular ROS in human PMN. (j) 12h temperatures of CLP-mice (k) Kaplan-Meier survival analysis of vehicle (n=13) and RvD2-treated (n=12) CLP mice. Results are mean±s.e.m. (a-g) *P<0.05 two-tailed unpaired Student’s t-test, (h-j) *P<0.05 by one-way ANOVA, (k) *P<0.05 one-tailed log-rank test.
The present results establish the complete stereochemistry of endogenous RvD2 and its potent stereoselective actions facilitating resolution. Local and systemic bacterial burden in microbial sepsis were controlled and significantly dampened with RvD2. This potent D-series resolvin protected from excessive leukocyte infiltration and overzealous cytokine production, as well as enhanced clearance of microbes, thus preventing sepsis-induced lethality. Sepsis remains a clinical challenge, with high mortality rates and increasing prevalence5,28. Given the uncontrolled inflammatory pathogenesis of sepsis, anti-inflammatory therapies are used for sepsis management in humans, but have ultimately failed due primarily to sustained immunosuppression5. Overall, these results indicate that RvD2 is a potent endogenous mediator which actively promotes resolution suggesting new therapeutic approaches that do not compromise host-defence.
Methods Summary
Intra-vital microscopy (IVM)
Experiments were approved by and performed under guidelines of the Ethical Committee for Use of Animals, Barts and The London School of Medicine and Home Office regulations (Guidance on the Operation of Animals, Scientific Procedures Act, 1986). IVM was used to observe actions of RvD2 on platelet activating factor (PAF; C16 form: C26H54NO7P; 100 nM; Sigma (1h prior to RvD2;Time 0)) -stimulated leukocyte responses within the cremasteric microcirculation of C57 BL/6 mice. Mice were anesthetized with xylazine (7.5 mg/kg) and ketamine (150 mg/kg), and cremaster prepared29. In some experiments, FITC-albumin (1mg) was administered i.v. to assess vascular leakage.
Cecal ligation and puncture (CLP)
CLP was performed in male FVB mice16, in accordance with the Harvard Medical Area standing committee on animals protocol #02570. The cecum was ligated below the ileocecal valve for mid-grade sepsis16. A through and through puncture was performed with a 20 gauge needle, followed by one additional puncture in the distal tip of the cecum. Mice received saline (500 µl s.c.) followed by i.v. administration of vehicle (0.1% ethanol), or RvD2 methyl ester (100 ng) at the time of puncture. In some experiments, RvD2-Me (1 µg) was administered i.p. 1h post-CLP. At 12h, rectal temperature was measured, blood collected by cardiac puncture and peritoneal exudates obtained. Blood and peritoneal bacteria levels were determined by growth on tryptic soy agar plates. Plasma and peritoneal cytokine levels were determined by Searchlight array (Woburn, MA). Peritoneal cells were differentiated using Wright-Giemsa staining.
Statistics
Data are mean ± s.e.m. Multiple group comparisons were made using one-way or two-way ANOVA followed by Dunnett’s or Bonferroni post tests where appropriate and direct comparisons made using a two-tailed unpaired Student’s t-test. Kaplan-Meier survival curves were analyzed using a one-tailed log-rank test. In all cases, a P value <0.05 was considered significant.
Supplementary Material
Acknowledgements
The authors acknowledge support from National Institutes of Health grants GM-38765 and P50-DE016191 (C.N.S.), Welcome Trust Programme grant 086867/Z/08/Z (R.J.F and M.P.) and Project grant 085903/Z/08 (R.J.F.) and Arthritis research campaign UK fellowships 18445 and 18103 (to L.V.N. and D.C., respectively). M.S. recieved a National Research Service Award from NHLBI (HL087526). We thank Jeremy W. Winkler and Jasim Uddin for work related to RvD2 synthesis and Padmini Pillai, Kimberly Martinod, Gabby Fredman and Jesmond Dalli for technical assistance, and Mary H. Small for assistance with the manuscript. We also thank Children’s Hosptial Boston, Dept. of Pathology for tissue sectioning and Birgitta Schmidt, MD for expert histopathology.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions
M.S. and L.V.N. designed and carried out experiments, analyzed data and wrote the manuscript; L.S., R.Y. and D.C. carried out experiments and analyzed data; N.A.P. synthesized RvD2; R.J.F. and M.P. designed experiments, analyzed data and contributed to the manuscript; C.N.S. planned the project, designed experiments, analyzed data and wrote the manuscript.
Competing Interests
The resolvins are biotemplates for stable analogs. Patents on these are awarded and assigned to Brigham and Women’s Hospital with C.N. Serhan the inventor. These analog patents are licensed for clinical development.
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare competing financial interests:
References
- 1.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;(3):401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 2.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;(8):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;(196):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;(8):802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu T, Radmark O, Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci U S A. 1984;81:689–693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 10.Cooper D, Norling LV, Perretti M. Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J Leukoc Biol. 2008;83:1459–1466. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- 11.Rittirsch D, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bucci M, et al. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci U S A. 2005;102:904–908. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 18.Singer P, et al. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 19.Farolan LR, Goto M, Myers TF, Anderson CL, Zeller WP. Perinatal nutrition enriched with omega-3 polyunsaturated fatty acids attenuates endotoxic shock in newborn rats. Shock. 1996;6:263–266. doi: 10.1097/00024382-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pluess TT, et al. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med. 2007;33:789–797. doi: 10.1007/s00134-007-0591-5. [DOI] [PubMed] [Google Scholar]
- 21.Haworth R, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvak V, et al. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flierl MA, et al. Adverse functions of IL-17A in experimental sepsis. Faseb J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 25.Souza DGe, et al. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179:8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- 26.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee BE, et al. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol. 2005;78:639–646. doi: 10.1189/jlb.0405206. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez AR, Spur BW. First total synthesis of 7(S),16(R),17(S)-Resolvin D2, a potent anti-inflammatory lipid mediator. Tetrahedron Letters. 2004;45:8717–8720. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.