Abstract
The endosomal Toll-like receptors (TLR3, TLR7 and TLR9) have been implicated in the pathogenesis of autoimmune diseases. Their signaling pathways show remarkable similarities and yet the outcomes following activation of each of these TLRs lead to clinically distinct autoimmune disease phenotypes. This review discusses how differences may arise at a molecular and cellular level to account for this diversity of responses. Understanding the roles of individual TLR pathways and the relationships between them and non-TLR innate immune pathways in the pathogenesis of diseases such as systemic lupus erythematosis highlights potential treatment targets for this spectrum of autoimmune diseases.
Keywords: dendritic cells, innate immunity, interferon regulatory factors, kidney, lupus, Toll-like receptors
The innate immune system, as opposed to adaptive immune B and T cells, uses genetically preprogrammed pattern recognition receptors (PRRs) to recognize ‘danger signals’ that emerge when a potential pathogen is present. Innate immune receptors, including Toll-like receptors (TLRs), RIG-like receptors (RLRs), Nod-like receptors and others, are typically expressed on macrophages, dendritic cells, epithelial cells and endothelial cells, where they provide rapid early responses to microbial danger signals, including the induction of proinflammatory cytokine secretion that recruits and activates additional immune responses. Unlike other TLRs that are typically present on the surface of cells and recognize bacterial danger signals, a group of TLRs including TLR3, TLR7 and TLR9 localize to cell endosomes and recognize viral danger signals (dsRNA, ssRNA and hypomethylated dsDNA, respectively). This group of endosomal TLRs has been particularly implicated in the pathogenesis of autoimmune diseases. Human-derived RNAs and DNAs that are targets of autoimmune responses in systemic lupus erythematosus (SLE) and related conditions have been found to induce activation of these receptors [1]. Altered expression and function of these receptors has been linked to clinical manifestations of lupus-like autoimmunity in animal model [2-5]. Moreover, inhibition of activation of the endosomal TLRs has been proposed to be a mechanism of action of hydroxychloroquine and related compounds, mainstays of autoimmunity therapy [6], and pharmaceutical firms have publicized their interest in developing additional inhibitors of endosomal TLRs for this purpose. However, despite overlapping activation pathways, the endosomal TLRs have at times markedly different clinical effects on autoimmune disease.
The focus of this paper is to discuss the differences in the clinical effects of endosomal TLR activation in autoimmunity, and to offer possible explanations for these differences. The proposed explanations can broadly be divided into two categories: differences in cell type function and TLR expression, and differences in the cascade of activation signals induced by particular innate immune receptors.
Common features of endosomal TLRs
In order to appreciate the differences between the endosomal TLRs, the commonalities between TLR3, TLR7, and TLR9 must first be recognized. They are each expressed widely across mammalian species, with conserved structure recognition and functional effects from mice to humans. In each case, trafficking of these TLRs from the ER to the endosomal compartment requires the functional form of the UNC93B1 chaperone protein, without which agonists of these TLRs fail to induce activation signals [7]. The endosomal TLRs are all primarily expressed in dendritic cells [8], where their activation induces secretion of type I interferons (IFN-1), such as IFN-α and IFN-β, plus additional cytokines including IL-6, IL-12 and TNF-α [9,10]. Thus, not surprisingly, each have also been shown to lead to upregulation of IFN-inducible genes [11,12], to recruit helper T cells, and to promote B-cell activation and antibody production [13]. Plasmacytoid dendritic cells (PDCs) are frequently seen as the primary producers of IFN-1, but non-PDC subsets, which we will refer to as myeloid dendritic cells, are also capable of IFN-1 production [14].
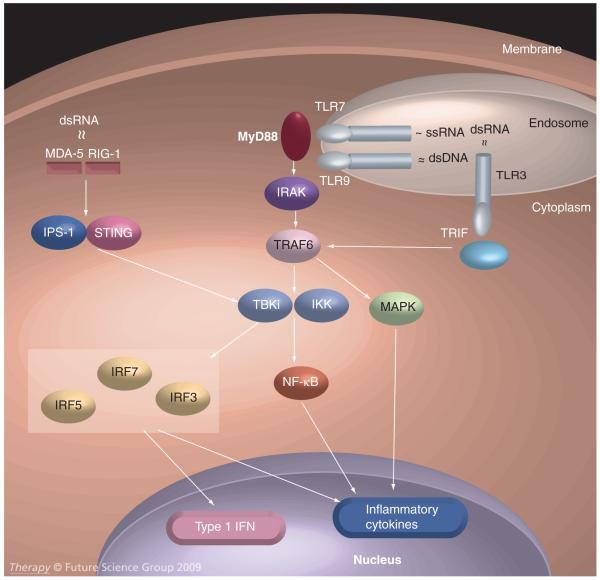
The intracellular signaling pathways of the endosomal TLRs are homologous (Figure 1). With all three receptors, agonist ligation leads to conformational changes in the receptor’s cytoplasmic tail, allowing recruitment of MyD88-family adapter molecules (TRIF in the case of TLR3 and MyD88 itself for both TLR7 and TLR9). These bind to and induce phosphorylation of IRAK-family molecules, which in turn recruit additional proteins including the TRAF6 E3 ubiquitin ligases and TANK binding kinase 1 (TBK1) or homologous proteins to a signaling complex that induces activating phosphorylations of the MAPK cascade, induces release and nuclear translocation of previously inactivated cytoplasmic NF-KB transcription factors, and phosphorylates IFN regulatory factors (IRFs), including IRF3, IRF5 and IRF7, which subsequently dimerize and translocate to the nucleus to also modulate gene expression [15-17].
Figure 1. Endosomal TLR and non-TLR signaling pathways.
Binding of endosomal or cytoplasmic nucleic acid ligands leads to the activation of TLR7 or TLR9 (via MyD88, IRAK and TRAF6), TLR3 (via TRIF and TRAF6) and MDA5 or RIG-I (via IPS-1 and STING). The pathways converge at the level of TBK1/IKK leading to activation of the IRF family of transcription factors, the MAPK cascade, and the NF-κB family of transcription factors, cumulatively inducing IFN-1 and inflammatory cytokine production.
Distinct clinical features reported with endosomal TLRs
The upregulation of IFN-1 and IFN-inducible genes is well recognized in systemic autoimmune disease, where it is believed to play an important pathogenic role [18]. Although initially observed in SLE [19], IFN-1 secretion and upregulation of IFN-inducible genes have also been found in other autoimmune conditions including mixed connective-tissue disease, dermatomyositis, Sjögren’s syndrome and rheumatoid arthritis [20]. Since a number of these conditions can share autoantigenic determinants with SLE but differ in their clinical tissue targeting, studies have begun to investigate whether differences in innate immune responses could lead to differences in clinical disease manifestations.
Cases in which identical antigenic stimulation has led to differing patterns of clinical disease expression have been reported. Mice with induced anti-RNP autoimmunity after stimulation with the TLR3 and TLR7 agonist U1-RNA were found to develop lung disease in TLR3-intact mice but renal disease in TLR3-null animals [4]. Likewise, mice treated with Y RNAs had different outcomes with regard to induction of renal disease and sialoadenitis based on the presence or absence of TLR3 expression of the test mice and on the endosomal TLR stimulatory patterns of the individual Y RNAs [21]. In a spontaneous lupus model, differing effects of TLR7 and TLR9 have been observed on tissue-specific disease manifestations: TLR7 knockouts had less severe nephritis than wild-type mice, while TLR9 knockouts had more severe nephritis and skin disease than wild-type mice (also supported by the finding of higher serum IFN-α levels and increased PDC activation) [22].
TLR7 has frequently been associated with the development of SLE in animal models. TLR7 knockout mice are likely to develop a milder form of SLE [22], whereas overexpression of TLR7 renders them more susceptible [23]. Antagonism of TLR7 can also prevent autoimmune lung and kidney disease [1,22]. By contrast, the effects on TLR9 knockout mice are not as clear cut. Christensen et al. reported a protective effect of TLR9 in MRL/lpr lupus mice [22], but in the ‘chronic graft versus host’ disease model, TLR9 knockout resulted in mice showing less severe nephritis [24]. In a recent study by Pawar and colleagues, combined TLR7 and 9 did not have additive or opposing effects on autoimmune lung and kidney injury [25]. In the presence of TLR7 activation, TLR9, when stimulated, loses its protective ability but does not exacerbate disease.
From an evolutionary point of view, it is highly plausible that selection pressure would exist on endosomal TLRs that would lead to distinguishable immunologic and tissue-specific effects. The primary function of these PRRs is (presumably) to recognize microbial hazards and to orchestrate antimicrobial responses that optimize fitness of the host. To the extent that endosomal TLRs recognize different microbial pathogens with different life cycles and different tissue tropisms, the optimal responses generated against those pathogens would be expected to differ. For example, TLR7 can be activated by and induces protection against influenza virus [26]. The protective response against influenza mediated by TLR7 appears to include activating antiviral immune responses systemically (including induction of protective antibody responses from activated B cells [27], while avoiding excess inflammation in the lungs that could cause life-threatening hypoxia. By contrast, TLR3 knockout mice survive influenza infection better than TLR3-intact mice, due to the propensity of TLR3 activation to induce pneumonitis [28]. However, with a different pathogen, respiratory syncyctial virus, the ability of TLR3 to mount a more efficient immune response in the lungs leads to a less severe histological presentation [29], despite the fact that respiratory syncyctial virus isolates are able to inhibit TLR7 and TLR9-induced responses [30]. Note that in both influenza virus and respiratory syncyctial virus, TLR3 stimulation leads to more aggressive immune responses, though the clinical outcomes of these aggressive TLR3 responses are opposite. The association of TLR3 with proinflammatory effects in the lung has also been observed with rhinovirus [31] and in asthma [32].
So the question arises: if differences between these TLRs really exist, how are these mechanistic differences defined?
Differences in cell expression of endosomal TLRs
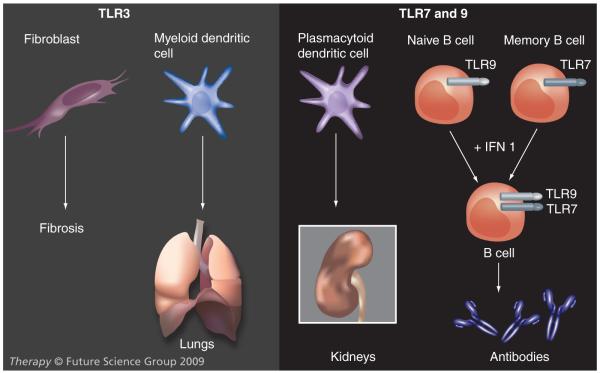
While the endosomal TLRs can be frequently found on cells expressing other nonendosomal TLRs, TLR7 and 9 are seldom found in the same cells as TLR3. Expression of endosomal TLRs on different cell types may lead to different functional effects (Figure 2). The endosomal TLRs are prominently expressed on dendritic cells, but they are not all present on the same dendritic cells. TLR3 is expressed on myeloid dendritic cells (MDCs) whereas TLR7 and 9 are coexpressed on PDCs. Thus, to the extent that activated MDCs preferentially traffic to the lung and induce autoimmune interstitial lung disease [33], these effects are consistent with the biology of a TLR3-restricted cell type [34,35]. Likewise, trafficking of different inflammatory cell subsets to the kidney to induce lupus-like lesions is more consistent with the biology of TLR7/9 expressing cells [36].
Figure 2. Variation in the expression of endosomal TLRs depending on cell type.
TLR3 expression on fibroblasts leading to fibrosis, and myeloid dendritic cells preferentially targeting the lung, while TLR7 and TLR9 expression on plasmacytoid dendritic cells preferentially targeting the kidney and on B cells with or without IFN-1 exposure leading to antibody production.
TLR: Toll-like receptor.
TLR7 and TLR9 but not TLR3 are also expressed on B cells [37,38]. Thus, TLR7 or 9 stimulation could be more likely to support conditions characterized by B cell activation and diversification, such as SLE. Differential activation of B cells between TLR7 or TLR9-driven lupus and TLR3-driven MCTD could help explain the tendency for lupus patients to develop a broader spectrum of autoantibodies than MCTD patients [39]. The ability of Fc receptor coligation to amplify TLR7 and TLR9 signals could lead to a further shift of dendritic cell phenotype [16], which may be distinct from the phenotype induced by interactions between TLR3 signals and Fc receptor-associated signals [40].
Unlike their typically coordinated expression in PDCs, in B cells TLR7 and TLR9 differ with regard to the subsets in which they are expressed and signal. Naive B cells express TLR9 but not TLR7, unless first stimulated with IFN-1 [41]. By contrast, memory B cells respond to TLR7 ligands in the absence of IFN-1 [41]. In addition, induction of a mature monoclonal rheumatoid factor antibody response in a murine system has been found to have partially nonoverlapping dependence on both TLR7 and 9 [42].
Conversely to the situation with B cells, TLR3 expression has been identified on fibroblasts, which do not express TLR 7 or 9 [43]. This could potentially account for more prominent clinical manifestations of fibrosis seen in putatively TLR3-associated conditions, such as MCTD, as opposed to TLR7/9-associated conditions such as lupus.
Within the kidney, TLR3 is constitutively expressed on tubular epithelial cells, glomerular mesangial cells and vascular smooth muscle cells [44]. IFN-γ (which is not a type 1 IFN) induces TLR3 in mesangial cells but downregulates all TLR mRNA in macrophages [44]. In lupus-prone mice, viral dsRNA induces mesangial lysis without affecting dsDNA autoantibody production, consistent with the expression of TLR3 on mesangial cells but not on B cells [45]. TLR7 and TLR9 ligands are not taken up by intrinsic renal cells but do activate antibody production consistent with effects on B cells [46]. TLR7 and TLR9 expression are observed in active glomerulonephritis, where they (but not TLR3) are observed on infiltrating immune cells [46]. TLR9 is also strongly expressed in proximal tubular cells in murine models of lupus nephritis [47].
Thus, differences exist in the cell expression profiles of the individual endosomal TLRs. In animal models, such differences have been identified even in the kidney itself. It is thus plausible to argue that different outcomes may be expected between stimulation of TLR3, 7 and 9 owing to differences in the target cells activated, even if the cellular activation program initiated by these receptors were otherwise identical. Differential TLR-induced tissue trafficking is also possible. Studies are needed to assess whether such differences in cell expression profiles account for any of the variation in clinical phenotypes proposed to occur after selective activation of sets of these TLRs.
Anti-inflammatory effects of endosomal TLRs
Each of the endosomal TLRs have also been observed in some circumstances to induce anti-inflammatory effects, such as prevention of renal disease with TLR3 and TLR9 [21,22] and prevention of sialadenitis with TLR7 [21]. Potentially anti-inflammatory pathways reported to be induced by these TLRs under some circumstances include induction of suppressor of cytokine signaling proteins [48,49] and enhancement of proteosome destruction of intracellular proinflammatory mediators [50]. In order to selectively eliminate only the proinflammatory sequelae of endosomal TLR activation, a more optimal strategy might involve specific inhibition of only the proinflammatory pathways downstream of TLR activation, or cell-targeting therapies that selectively eliminate TLR-expressing cell types particularly implicated in the pathogenesis of tissue-specific immunophenotypes. Thus, B-cell targeting may make sense for TLR7-associated lupus nephritis, while anti-MDC therapy might be more helpful for (putatively) TLR3-associated autoimmune pneumonitis.
miRNAs in cell differentiation
The fact that various cell types respond differently to particular innate immune signals leading to distinct patterns of immunopathology begs an additional question: what is it about the differentiation of one cell type as opposed to another that accounts for such differences? In this context, it is relevant to consider cellular pathways that are implicated in cell differentiation. miRNA are short noncoding RNA molecules that inhibit gene expression [51]. RNA binding proteins have high affinity for the AU-rich elements (ARE) usually present in the 3′-UTR of the mRNA [52]. This decreases mRNA stability or inhibits translation [40]. Originally studied in the pathogenesis of various cancers, their role as regulators of cellular differentiation programs has been highlighted [53]. Recent studies have introduced the possibility that regulation of cellular differentiation at this level can also be associated with the clinical expression of autoimmune disease. Autoantibodies generated in autoimmune syndromes may reflect differences in the biology of target tissues themselves or differences in the inflammatory infiltrates that traffic to different tissues. Regarding the latter possibility, Skriner and colleagues have observed that different epitopes are targeted on the hnRNP A2/B1 antigen, a known ARE-binding protein, between patients with SLE and those with MCTD [54]. Likewise, Jimenez-Boi recently reported the presence in autoimmune and inflammatory conditions of antibodies to two additional proteins involved in miRNA/ARE regulation of mRNA transcripts: T cell intracytoplasmic antigen 1 (TIA-1) and TIA-1-related protein (TIAR) [55]. Interestingly, anti-TIAR antibodies were associated with lupus nephritis whereas in systemic sclerosis anti-TIA-1 was associated with lung involvement. Anti-TIA-1 antibodies could also be found in SLE patients and were generally associated with more severe disease activity compared with patients negative for anti-TIA-1. This raises the question whether differences in miRNA/ARE biology form the underpinnings of clinical differences in autoimmune targeting between renal-targeted and lung-targeted syndromes of systemic disease. In addition, there have been reports of miRNA 146a/b (miR-146a/b) targeting TRAF6 and IRAK1, and thereby potentially interacting with TLR signaling [56].
Signaling differences between endosomal TLRs
TLR3 signaling can be immediately appreciated to be different from that of TLR7 and 9, since TLR3 uses TRIF rather than MyD88 as its primary signaling adapter molecule. However, TRIF is highly homologous to MyD88, and the downstream events in both signaling systems, including NF-kB activation, MAP kinase activation and induction of IFN-1 inducible genes can be difficult to distinguish. In a study of MRL/lpr mice susceptible to developing SLE, deletion of the MyD88 adaptor protein resulted in amelioration of disease, but treatment of these mice with poly-I:C, a TLR3/TRIF agonist that can also activate RLR [57], reconstituted SLE-like autoimmunity with nephritis as if MyD88 signaling had been intact [58]. This suggests that in mice developing in the absence of MyD88-mediated signals, activation of a combination of RLR and TLR3 signals can substitute for a MyD88-mediated response, and suggests that additional (potentially developmental) conditions must exist for TLR3-induced responses to support protection against, as opposed to induction of, lupus nephritis. For example, the absence of MyD88 pathways might lead SLE-inducing PDCs to respond to a TLR3/RLR agonist instead of the usual TLR7 or 9. Given that TLR7 and TLR9 are often expressed on the same cell types and have only been shown to signal through the identical MyD88 pathway, one might speculate that no signaling differences could exist between these two TLRs. However, chimeric TLR receptors created with the extracellular region of TLR4 fused with the transmembrane and cytoplasmic regions of TLR3, 7 and 9 showed these TLRs to localize to the endosomal compartment, and to exhibit at least subtle differences in the levels of expression of a panel of inflammatory cytokines [59]. The functional differences observed with TLR7 versus TLR9 stimulation and knockouts suggests that differential signaling may be possible. It thus appears that the cytoplasmic domains of the endosomal TLRs define distinctive signaling properties, and that additional studies with chimeric TLRs (such as swapping the extracellular and intracellular domains of TLR7 and 9) may provide for characterization of differences in the signaling pathways of endosomal TLRs in future work. Moreover, a schema can be proposed whereby even different stimuli of the same TLR could potentially induce distinct immune responses (Figure 3).
Figure 3. Stimulus-specific tuning of innate immune signaling.
Individual innate immune stimuli ligate specific TLRs. The specific TLR(s) ligated directly interact with the TBK1 phosphorylation complex to help dictate the specific IRFs that are activated, leading to a stimulus-specific response. Cross-reactivity of innate immune stimuli with other innate immune receptors such as RLRs or DAI can also impact the specificity of the IRFs activated by TBK1. Expression of regulatory molecules including miRNAs may occur within stimulus-specific responses, further feeding back on the pathway.
DAI: DNA-dependent activator of IFN regulatory factors; IRF: Interferon regulatory factor; RLR: RIG-like receptor; TBK: TANK binding kinase 1; TLR: Toll-like receptor.
Signaling differences between non-TLR immune pathways
Non-TLR innate immune pathways, including some that have overlapping danger signal recognition with the endosomal TLRs, also induce similar patterns of IFN-1 activation, as well as induction of clinical autoimmunity. Cytoplasmic DNA can stimulate receptors including DNA-dependent activator of IFN regulatory factors (DAI) [60]. In mice lacking DNase II, DNA from engulfed apoptotic cells and erythroid precursor cell nuclei accumulates in macrophages, leading to TLR-independent, IFN-mediated autoimmune pathology [61]. Depending upon their specific physiochemical properties, cytoplasmic RNAs could activate the cytoplasmic RLRs RIG-I or MDA5 [62]. RIG-I and MDA5 each also induce IFN-1 expression, with RIG-I and cytoplasmic DNA recognition signals mediated by the TLR-independent STING pathway [57], and both RIG-I and MDA5 signaling through IPS-1 [63].
Downstream signaling events inducing IRFs
A key common feature in all of these IFN-1-activating pathways, both TLR-dependent and non-TLR dependent exists, which may also account for receptor-to-receptor signaling diversity: these pathways converge downstream at TBK1 or the homologous IKK-i, which phosphorylate IκB to induce NF-κB activation, and also phosphorylate IRFs to activate IFN-1-associated and other proinflammatory responses after either cytoplasmic innate immune receptor or TLR-associated activation. Notably, phosphorylation of specific IRFs by TBK1 appears to depend on the formation of a complex including upstream elements of the innate immune receptor pathway in addition to TBK1 - the entire complex appears to dictate which IRFs are allowed into proximity of activated TBK1 to receive their activating phosphorylation. Thus, signal transduction by specific innate immune receptors is linked to induction of specific IRFs: RLR signaling depends on IRF7 [64], while DAI signaling depends on IRF3 and is IRF7 independent [60]. Likewise, TLR9 and TLR7 activation lead to a complex including IRF7 (which is constitutively expressed in PDCs) [65]. TLR7 and TLR9 in PDCs stimulate IRF7 to a far greater extent than IRF3 [64]. By contrast, the activated TLR3 complex is able to bind and activate both IRF3 and IRF7 relatively equally [66]. IRF3 is expressed in all cell types whereas IRF7 (as well as IRF5) are primarily expressed in B cells and dendritic cells. Glucocorticoid drugs, which have demonstrated effectiveness for lupus-like autoimmune diseases irrespective of specific immunophenotype, are able to inhibit the activated form of TBK1 itself and the activation of IRF3 [67,68]. More potent inhibitors of TBK1-like activities would thus be anticipated to have potent but potentially nonspecific anti-inflammatory effects, and could have other serious side effects, since TBK1 has also been implicated in oncogenesis and angiogenesis [69,70]. Therefore, it appears that IRFs are involved in TLR and non-TLR signaling, with differences observed in different cell types.
IRFs & autoimmune disease
While IRF3 and 7 have been most consistently implicated in proinflammatory signaling, to date polymorphisms in the IRF5 gene have been most strongly linked to susceptibility for lupus-like autoimmunity [71]. Like IRF3 and IRF7, IRF5 is activated by phosphorylation, whereupon it forms a homodimer, translocates to the nucleus, and binds to regulatory motifs in DNA that modulate gene expression, notably in the promoter regions of cytokine genes [72]. Interestingly, IRF5 shows variation in its actions depending on very fine-grained differences in cell specificity. IRF5-deficient PDCs have normal IFN-1 secretion while IRF5-deficient MDCs show impaired cytokine production [73]. In mice, IRF5 deficiency prevents dendritic cell induction of TNF-α by TLR3, 4 or 9, but the same deficiency prevents dendritic cell induction of IFN-1 only by TLR9, without affecting TLR3 or TLR4-induced IFN-1 production [74]. As with many inflammatory pathway genes, such as TNF-β, the IRF5 gene encodes an mRNA 3′-UTR with an ARE element [75]. Thus, cell-type-specific differences in inflammatory responses could also be mediated by effects at the level of IRF5 mRNA regulation. Future therapeutic agents the modulate regulation of the IRF5 transcript could thus also have relevance for treatment of lupus-like autoimmune syndromes.
Conclusion
The systemic rheumatic diseases have long been characterized as conditions with the potential to affect many different target tissues, but clinicians have had few tools available to either predict the likelihood of specific end-organ involvement or to therapeutically influence the expression of end-organ involvement. Recent data suggest that innate immune signals may participate in determining the tissue targets of immune responses, and hence contribute to the clinical disease patterning in individual patients. Additional study of this area may lead to improved tools for the assessment of risk of target organ involvement, new therapeutic modalities to modify this risk, and new appreciation for mechanisms by which current therapies may impact on tissue targeting.
Future perspective
Innate immune regulation is capable of dramatically influencing the phenotype of systemic autoimmune diseases with regard to organ involvement and severity. Measurement and modification of these innate immune pathways provides new opportunities to screen patients at risk of particular organ involvement, to tailor therapy toward the organ systems specifically at risk in each individual patient.
Executive summary.
Innate immune activation leading to IFN-1 production
-
■
Following stimulation by nucleic acid ligands, innate immune system proteins (endosomal Toll-like receptors [TLRs] and cytoplasmic RIG-like receptors) signal through generally homologous pathways involving a series of mediators that ultimately result in the production of IFN-1.
Clinical diversity in autoimmune disease
-
■
A diverse set of systemic autoimmune syndromes including lupus are characterized by IFN-1 activation.
-
■
Phenotypic variability in the autoimmune diseases sharing IFN-1 activation may be mediated in part by which specific innate immune pathway(s) become activated.
Differences in endosomal TLR signaling
-
■
Differences in innate immune pathways that may contribute to different clinical outcomes exist at the cellular, transcriptional and translational levels.
Therapeutic goals
-
■
Putative therapeutic targets exist that may target specific differences in innate immune activation pathways, and have relevance to particular clinical subsets of autoimmune disease.
Acknowledgments
Dr Greidinger’s work was supported by the US Department of Veterans Affairs (Merit Review grant), the NIH (grants AI-1842 and AR-48805) and the Lupus Research Institute.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savarese E, Steinberg C, Pawar RD, et al. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 2008;58:1107–1115. doi: 10.1002/art.23407. [DOI] [PubMed] [Google Scholar]
- 3.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greidinger EL, Zang Y, Jaimes K, et al. A murine model of mixed connective tissue disease induced with U1 small nuclear RNP autoantigen. Arthritis Rheum. 2006;54:661–669. doi: 10.1002/art.21566.■ Shows a murine model of autoimmune disease in which changes in innate immune signaling lead to either interstitial lung disease resembling mixed connective tissue disease or systemic lupus erythematosus (SLE)-like nephritis.
- 5.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978.■ Supports the importance of Toll-like receptor (TLR)7 in SLE, showing that the Yaa mutation in mice leads to duplication of the TLR7 genes leading to enhanced anti-snRNP autoantibody production and a heightened susceptibility to lupus-like disease.
- 6.Rutz M, Metzger J, Gellert T, et al. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- 7.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 8.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubert FX, Voisine C, Louvet C, et al. Differential pattern recognition receptor expression but stereotyped responsiveness in rat spleen dendritic cell subsets. J. Immunol. 2006;177:1007–1016. doi: 10.4049/jimmunol.177.2.1007. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson AA, Sturfelt G, Truedsson L, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 11.Coccia EM, Severa M, Giacomini E, et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and λ interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 12.Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 13.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diebold SS, Montoya M, Unger H, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZJ. Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Sato S, Ishii KJ, et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 18.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baechler EC, Batliwalla FM, Reed AM, et al. Gene expression profiling in human autoimmunity. Immunol. Rev. 2006;210:120–137. doi: 10.1111/j.0105-2896.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 21.Greidinger EL, Zang Y, Martinez L, et al. Differential tissue targeting of autoimmunity manifestations by autoantigen-associated Y RNAs. Arthritis Rheum. 2007;56:1589–1597. doi: 10.1002/art.22601. [DOI] [PubMed] [Google Scholar]
- 22.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013.■■ Highlights the different clinical manifestations occurring in two similar TLR pathways following knockout of either pathway, showing that in a lupus-susceptible mouse model, TLR9 deficiency led to worse disease but TLR7 deficiency led to less severe clinical disease.
- 23.Deane JA, Pisitkun P, Barrett RS, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Z, Chen F, Madaio MP, Cohen PL, Eisenberg RA. Modulation of autoimmunity by TLR9 in the chronic graft-vs-host model of systemic lupus erythematosus. J. Immunol. 2006;177:7444–7450. doi: 10.4049/jimmunol.177.10.7444. [DOI] [PubMed] [Google Scholar]
- 25.Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 26.Hammerbeck DM, Burleson GR, Schuller CJ, et al. Administration of a dual toll-like receptor 7 and toll-like receptor 8 agonist protects against influenza in rats. Antiviral Res. 2007;73:1–11. doi: 10.1016/j.antiviral.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Heer AK, Shamshiev A, Donda A, et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 28.Le Goffic R, Balloy V, Lagranderie M, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:E53. doi: 10.1371/journal.ppat.0020053.■ Demonstrates TLR3 deficient mice to have a protective advantage against influenza-induced pneumonia.
- 29.Rudd BD, Smit JJ, Flavell RA, et al. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 30.Schlender J, Hornung V, Finke S, et al. Inhibition of toll-like receptor 7- and 9-mediated α/β interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sajjan US, Jia Y, Newcomb DC, et al. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J. 2006;20:2121–2123. doi: 10.1096/fj.06-5806fje. [DOI] [PubMed] [Google Scholar]
- 32.Bachar O, Adner M, Uddman R, Cardell LO. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, an effect mediated by JNK and NF-κB signaling pathways. Eur. J. Immunol. 2004;34:1196–1207. doi: 10.1002/eji.200324569. [DOI] [PubMed] [Google Scholar]
- 33.Greidinger EL, Zang Y, Fernandez I, et al. Tissue targeting of Anti-RNP autoimmunity; effects of T cells and myeloid dendritic cells. Arthritis Rheum. 2009;60(2):534–542. doi: 10.1002/art.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters W, Cyster JG, Mack M, et al. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J. Immunol. 2004;172:7647–7653. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- 35.Demedts IK, Bracke KR, Maes T, Joos GF, Brusselle GG. Different roles for human lung dendritic cell subsets in pulmonary immune defense mechanisms. Am. J. Respir. Cell Mol. Biol. 2006;35:387–393. doi: 10.1165/rcmb.2005-0382OC. [DOI] [PubMed] [Google Scholar]
- 36.Pawar RD, Patole PS, Ellwart A, et al. Ligands to nucleic acid-specific Toll-like receptors and the onset of lupus nephritis. J. Am. Soc. Nephrol. 2006;17:3365–3373. doi: 10.1681/ASN.2006030263. [DOI] [PubMed] [Google Scholar]
- 37.Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell Immunol. 2000;203:55–65. doi: 10.1006/cimm.2000.1673. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 39.Burdt MA, Hoffman RW, Deutscher SL, Wang GS, Johnson JC, Sharp GC. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum. 1999;42:899–909. doi: 10.1002/1529-0131(199905)42:5<899::AID-ANR8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Anderson P, Phillips K, Stoecklin G, Kedersha N. Post-transcriptional regulation of proinflammatory proteins. J. Leukoc. Biol. 2004;76:42–47. doi: 10.1189/jlb.1103536. [DOI] [PubMed] [Google Scholar]
- 41.Bekeredjian-Ding IB, Wagner M, Hornung V, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 42.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–2665. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- 44.Patole PS, Pawar RD, Lech M, et al. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol. Dial. Transplant. 2006;21:3062–3073. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- 45.Patole PS, Grone HJ, Segerer S, et al. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J. Am. Soc. Nephrol. 2005;16:1326–1338. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- 46.Pawar RD, Patole PS, Zecher D, et al. Toll-like receptor-7 modulates immune complex glomerulonephritis. J. Am. Soc. Nephrol. 2006;17:141–149. doi: 10.1681/ASN.2005070714. [DOI] [PubMed] [Google Scholar]
- 47.Benigni A, Caroli C, Longaretti L, et al. Involvement of renal tubular Toll-like receptor 9 in the development of tubulointerstitial injury in systemic lupus. Arthritis Rheum. 2007;56:1569–1578. doi: 10.1002/art.22524. [DOI] [PubMed] [Google Scholar]
- 48.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034.■ Demonstrates that TLR induction upregulates the TAM system, which in turn activates the immune suppressor SOCS genes, suggesting that therapeutic upregulation of SOCS genes could have value in lupus-like autoimmunity.
- 49.Dai X, Sayama K, Yamasaki K, et al. SOCS1-negative feedback of STAT1 activation is a key pathway in the dsRNA-induced innate immune response of human keratinocytes. J. Invest. Dermatol. 2006;126:1574–1581. doi: 10.1038/sj.jid.5700294. [DOI] [PubMed] [Google Scholar]
- 50.Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- 51.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 52.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 53.Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- 54.Skriner K, Sommergruber WH, Tremmel V, et al. Anti-A2/RA33 autoantibodies are directed to the RNA binding region of the A2 protein of the heterogeneous nuclear ribonucleoprotein complex. Differential epitope recognition in rheumatoid arthritis, systemic lupus erythematosus, and mixed connective tissue disease. J. Clin. Invest. 1997;100:127–135. doi: 10.1172/JCI119504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jimenez-Boj E, Kedersha N, Tohidast-Akrad M, et al. Autoantibodies to the translational suppressors T cell intracytoplasmic antigen 1 and T cell intracytoplasmic antigen 1-related protein in patients with rheumatic diseases: increased prevalence in systemic lupus erythematosus and systemic sclerosis and correlation with clinical features. Arthritis Rheum. 2008;58:1226–1236. doi: 10.1002/art.23435. [DOI] [PubMed] [Google Scholar]
- 56.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl Acad. Sci. USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317.■ Describes a novel mediator of innate immune signaling leading to IFN-1 expression that may be a relevant target for inhibition as an anti-inflammatory treatment strategy.
- 58.Sadanaga A, Nakashima H, Akahoshi M, et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 59.Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J. Biol. Chem. 2004;279:19008–19017. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- 60.Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 61.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 63.Kawai T, Takahashi K, Sato S, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 64.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 65.Honda K, Yanai H, Mizutani T, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl Acad. Sci. USA. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoenemeyer A, Barnes BJ, Mancl ME, et al. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 2005;280:17005–17012. doi: 10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- 67.Reily MM, Pantoja C, Hu X, Chinenov Y, Rogatsky I. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. EMBO J. 2006;25:108–117. doi: 10.1038/sj.emboj.7600919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCoy CE, Carpenter S, Palsson-McDermott EM, Gearing LJ, O’Neill LA. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and −4 by targeting TBK1 activation. J. Biol. Chem. 2008;283:14277–14285. doi: 10.1074/jbc.M709731200. [DOI] [PubMed] [Google Scholar]
- 69.Korherr C, Gille H, Schafer R, et al. Identification of proangiogenic genes and pathways by high-throughput functional genomics: TBK1 and the IRF3 pathway. Proc. Natl Acad. Sci. USA. 2006;103:4240–4245. doi: 10.1073/pnas.0511319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chien Y, White MA. Characterization of RalB-Sec5-TBK1 function in human oncogenesis. Methods Enzymol. 2008;438:321–329. doi: 10.1016/S0076-6879(07)38022-1. [DOI] [PubMed] [Google Scholar]
- 71.Sigurdsson S, Nordmark G, Goring HH, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am. J. Hum. Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. J. Interferon Cytokine Res. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- 73.Takaoka A, Yanai H, Kondo S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 74.Paun A, Reinert JT, Jiang Z, et al. Functional characterization of murine interferon regulatory factor 5 (IR-5) and its role in the innate antiviral response. J. Biol. Chem. 2008;283:14295–14308. doi: 10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham RR, Kyogoku C, Sigurdsson S, et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc. Natl Acad. Sci. USA. 2007;104:6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]