Abstract
Endothelial dysfunction has been linked to a decrease in nitric oxide (NO) bioavailability and attenuated endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation. The small (SKCa) and intermediate (IKCa) calcium-activated potassium channels play a key role in endothelium-dependent relaxation. Since the repressor element 1-silencing transcription factor (REST) negatively regulates IKCa expression, we hypothesized that augmented REST and decreased IKCa expression contributes to impaired endothelium-dependent vasodilation associated with hypertension. Acetylcholine (ACh) responses were slightly decreased in small mesenteric arteries from male stroke-prone spontaneously hypertensive rats (SHRSP) vs. arteries from Wistar Kyoto (WKY) rats. Incubation with L-NAME (100µM) and indomethacin (100µM) greatly impaired ACh responses in vessels from SHRSP. Iberiotoxin (0.1µM), selective inhibitor of large-conductance KCa (BKCa) channels, did not modify EDHF-mediated vasodilation in SHRSP or WKY. UCL-1684 (0.1µM), selective inhibitor of SKCa channels almost abolished EDHF-mediated vasodilation in WKY, and decreased relaxation in SHRSP. TRAM-34 (10µM) and charybdotoxin (0.1µM), both IKCa inhibitors, produced a small decrease of EDHF relaxation in WKY, but completely abrogated EDHF vasodilation in SHRSP. EDHF-mediated relaxant responses were completely abolished in both groups by simultaneous treatment with UCL-1684 and TRAM-34 or charybdotoxin. Relaxation to SKCa/IKCa channels agonist NS-309 was decreased in SHRSP arteries. Expression of SKCa was decreased, whereas IKCa was increased in SHRSP mesenteric arteries. REST expression was reduced in arteries from SHRSP. Vessels incubated with TRAM-34 (10µM) for 24 hours, displayed reduced REST expression, and no differences in IKCa. In conclusion, IKCa channels upregulation, via decreased REST, seems to compensate deficient activity of SKCa channels in the vasculature of spontaneously hypertensive rats.
Keywords: small calcium-activated potassium channels (SKCa), intermediate calcium-activated potassium channels (IKCa), repressor element 1-silencing transcription factor (REST)
INTRODUCTION
The endothelium plays an important role in maintaining vascular homeostasis by synthesizing and releasing vasodilator factors, such as prostacyclin (PGI2), nitric oxide (NO), and a yet unidentified endothelium-derived hyperpolarizing factor (EDHF) (1). Because EDHF appears to decline with advancing age and to be targeted in diseases such as hypertension and diabetes, knowledge of the ionic mechanisms underlying EDHF actions would be expected to improve understanding of the nature of EDHF and how vascular tone is regulated during health or disease conditions (2).
Extensive studies have been performed in order to better understand EDHF actions. Accordingly, different hypotheses try to explain the mechanisms that mediate EDHF-induced vasodilatation. One of these hypothesis suggest that EDHF leads to endothelial hyperpolarization via activation of Ca2+-activated K+ channels (KCa). Hyperpolarization of the vascular smooth muscle cells can be elicited via myo-endothelial gap junctions to result in hyperpolarization of the smooth muscle cells (3–6), and reduced Ca2+ influx via voltage-operated Ca2+ channels (7–9).
Ca2+-activated K+ (KCa) channels are especially important in EDHF-mediated relaxation and hyperpolarization in resistance-sized arteries but the role of these channels during hypertension is far from clear (10–14). Changes in expression or activity of KCa channels may therefore be a fundamental event contributing to the progression of arterial dysfunction during hypertension. It was previously shown that small-mesenteric arteries from angiotensin II hypertensive rats maintain EDHF-like responses, despite reduced expression of intermediate KCa (IKCa) and small KCa (SKCa), (15). Whether these changes in KCa channel function and expression during hypertension occur at the level of resistance-sized arteries, and whether they are needed to regulate vascular tone and hence local blood flow, is not completely understood and requires further studies (14).
Repressor element 1-silencing transcriptional factor (REST, also known as NRSF) is a transcription factor that regulates IKCa channel gene expression (16–18). The IKCa channel gene sequence is encoded by the KCNN4 sequence and REST has been shown to regulate this sequence. Cheong and colleagues (2005) showed that this transcription factor modulates potassium channel expression in vascular smooth muscle cells (17). Therefore, alterations in REST regulation can have an important impact in the development of vascular disease by KCa channels alterations. Therefore, we hypothesized that increased REST expression during hypertension negatively modulates IKCa channel expression, contributing to reduced endothelium-dependent vasodilation in hypertension.
METHODS
Animals
Five month-old male stroke-prone spontaneously hypertensive rats (SHRSP) were obtained from the breeding colony at the Medical College of Georgia. Age-matched male Wistar-Kyoto (WKY) rats were purchased from Harlan (Indianapolis IN). Rats were maintained on a 12-hour light dark cycle, housed two per cage and allowed access to normal chow and water ad libitum. Systolic blood pressure (SBP) was measured in non-anesthetized animals by tail cuff using a RTBP1001 blood pressure system (Kent Scientific Corporation, Connecticut, MA, USA). All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education.
Preparation and study of mesenteric arteries
After euthanasia, the mesentery was rapidly excised and placed in an ice-cold physiological salt solution (PSS) containing: 130 mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4.7H2O, 1.6 mM CaCl2.2H2O, and 0.026 mM EDTA. Second-order mesenteric arteries were carefully dissected and mounted as ring preparations on two stainless steel wires (≅200 mm in length with internal diameter ≅ 100 to 150 µm) in an isometric Mulvany-Halpern small-vessel myograph (Danish MyoTech, Aarhus, Denmark). One wire was attached to a force transducer and the other to a micrometer. Both dissection and mounting of the vessels were carried out in cold (4°C) PSS. The segments were adjusted to maintain a passive force of 3.5 mN. Vessels were equilibrated for 45 min in PSS at 37°C. Arterial integrity was assessed first by stimulation of vessels with KCl (120 mM) and, after washing and a new stabilization period, by contracting the segments with phenylephrine (10µM) followed by stimulation with acetylcholine (ACh; 10µM).
After being washed, the arterial rings were contracted with the thromboxane analog 9,11-dideoxy-9α,11α-methanoepoxyprostaglandin F2α (U-46619, 0.3µM). Cumulative concentration-response curves to ACh were performed under control conditions and in the combined presence of indomethacin (inhibitor of cyclooxygenase, 10 µM) and Nω-nitro-l-arginine methyl ester [L-NAME, inhibitor of NO synthase, 100µM]. To study the role of KCa channels in EDHF-mediated relaxations, ACh-induced vasorelaxation was determined in the presence of L-NAME and indomethacin. This procedure rules out any potential interference of NO and prostaglandins on EDHF-mediated responses (19). The effects of IKCa inhibitors (TRAM-34, 10µM and charybdotoxin, 0.1µM), a SKCa inhibitor (UCL-1684, 0.1µM) and a BKCa inhibitor (Iberiotoxin, 0.1µM) on ACh-induced relaxations were determined. These concentrations were chosen based on previous studies from our laboratory (14).
Western blotting
Mesenteric arteries from hypertensive and control rats were isolated, cleaned from fat, dissected and frozen in liquid nitrogen. Proteins (40 µg) extracted from the mesenteric bed were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline solution with Tween (0.1%) for 1 hour at 24°C. Membranes were then incubated with antibodies overnight at 4°C. Antibodies were as follows: anti-potassium channel KCa2.3 (also known as SK3) and KCa3.1 (also known as IK1); (1:200, Sigma-Aldrich, MO, USA); REST/NRSF (1:500, Abcam) and β-actin (1:1000, Sigma-Aldrich, MO, USA). After incubation with secondary antibodies, signals were revealed with chemiluminescence, visualized by autoradiography, and quantified densitometrically. Results were normalized by β-actin expression and expressed as percentage of control.
Data analyses and statistics
Experimental values were calculated relative to the maximal changes from the contraction produced by U-46619 in each segment. Agonist concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 3.0; GraphPad Software Inc., San Diego, CA). Agonist potencies and maximum response are expressed as negative logarithm of the molar concentration of agonist producing 50% of the maximum response (pD2) and maximum effect elicited by the agonist (Emax), respectively. Data are expressed as means ± SEM (n), where n is the number of experiments performed. Statistical analysis of the concentration-response curves was performed by using two-way analysis of variance (ANOVA) for comparison between the groups. Western blot data were analyzed by one-sample t test. Values of P<0.05 were considered a statistically significant difference.
Drugs
L-Phenylephrine hydrochloride, ACh, l-NAME, iberiotoxin, indomethacin, sodium nitroprusside and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) were all purchased from Sigma-Aldrich. 9,11-Dideoxy-9α,11α-methanoepoxyprostaglandin F2α (U-46619) was purchased from Calbiochem (San Diego, CA) and 6,12,19,20,25,26-Hexahydro-5,27:13,18:21,24- trietheno-11,7-metheno-7H-dibenzo [b,n] [1,5,12,16] tetraazacyclotricosine-5,13-diium ditrifluoroacetate (UCL-1684) was from Tocris (Ellisville, MI). Indomethacin was dissolved in ethanol, and TRAM-34 and UCL-1684 were dissolved in DMSO. All other stock solutions were prepared by using PSS. Control solutions containing vehicle levels of ethanol and DMSO were also used through the experimental protocol.
RESULTS
Systolic blood pressure and body weight of the rats
At 24 weeks, SHRSP displayed higher systolic blood pressure (mmHg), in comparison with WKY rats (211±7.6, n=15 vs. 119±1.8, n=15; respectively). Body weight of SHRSP was significantly decreased (296 ±6.1g; n=15) when compared with WKY rats (345±5.2g; n=15),
Endothelium-dependent and EDHF-mediated relaxation in small-mesenteric arteries
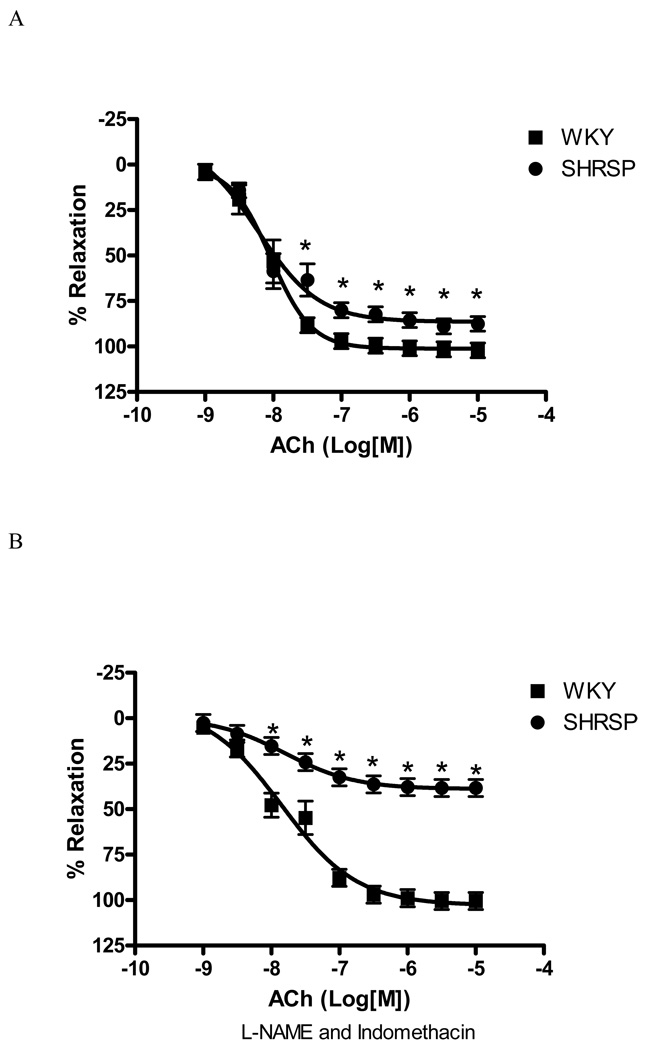
Concentration-response curves to ACh were performed in vessels contracted with U-46619 (0.3µM) to evaluate endothelium-dependent relaxation. ACh-induced relaxation was slightly impaired in small mesenteric vessels from SHRSP vs. those from normotensive rats [Emax 87.7±4% vs. 102.2±1.4%, respectively, (p<0.05)], as shown at Figure 1A.
Figure 1. EDHF-mediated relaxation is impaired in SHRSP small mesenteric arteries.
Concentration-response curves to ACh (1nM to 100µM) in U-46619-contracted (0.3µM) second-order mesenteric arteries from (■) WKY and (●) SHRSP, in the absence (A) or in the presence (B) of L-NAME (100µM) and indomethacin (10µM). Experimental values of the relaxation induced by ACh were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue, which was taken as 100%. Results are presented as mean ± SEM of n=8 in each experimental group. *, P<0.05 compared with WKY.
EDHF-mediated relaxation is particularly apparent when NO and prostaglandins production are blocked. Therefore, simultaneous inhibition of prostaglandins and NO production (by indomethacin; 10µM and L-NAME; 100µM, respectively) were performed in order to assess EDHF-mediated relaxation. At this condition, ACh-induced relaxation was greatly diminished in SHRSP, but not in WKY vessels [Emax 38.45±1.7% vs. 100.6±0.6%, respectively, (p<0.05), Figure 1B]. These results suggest impairment of EDHF production in vessels from hypertensive animals.
It has been shown that relaxations observed in the presence of NO-synthase and cyclooxygenase inhibitors are not necessarily attributed to EDHF-mediated responses, but may result of residual NO release (20, 21). To rule out this hypothesis, ACh-concentration response curve were performed in arteries from WKY and SHRSP, incubated with L-NAME (100µM) and indomethacin (10µM), in the presence or absence of Carboxy-PTIO (100µM), a NO scavenger. Addition of Carboxy-PTIO did not result in greater inhibition of ACh-induced relaxation in WKY [Emax 96.9±7.5% vs 89.1±4.1% (without Carboxy_PTIO)] or SHRSP [Emax 42.5±5.5% vs. 38.4±4.7% (without Carboxy_PTIO)]. These results show that residual NO does not seem to contribute to the differences observed in ACh responses in small-mesenteric arteries from WKY and SHRSP rats in the presence of L-NAME and indomethacin, reinforcing that relaxation is attributed to EDHF-mediated effects.
Contribution of KCa channels to EDHF-mediated relaxation
As previously discussed, KCa channels are especially important for EDHF-mediated relaxation and hyperpolarization in resistance arteries. Accordingly, the effects of KCa channel blockade on EDHF-mediated actions were always studied on arteries treated with both indomethacin (10µM) and L-NAME (100µM). The contribution of different subtypes of KCa channels, BKCa, SKCa and IKCa to ACh-induced relaxation was determined by using selective inhibitors. The effects of these inhibitors on pD2 and Emax values for ACh are summarized at Table 1.
Table 1. pD2 and Emax values for ACh-induced dilation in arteries from WKY and SHRSP.
pD2 values are −log EC50 and Emax values represent the percentage of relaxation of U-46419 Experimental values of the relaxation induced by ACh were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue, which was taken as 100%. Results are presented as mean ± SEM of n=8 in each experimental group.
| WKY | SHRSP | |||
|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | |
| Vehicle | 8.0±0.01 | 102.2±1.4 | 8.2±0.11* | 87.7±5.8* |
| L-NAME + Indomethacin | 7.8±0.08* | 100.6±0.6 | 7.8±0.22 | 38.4±1.6*† |
|
Iberiotoxin L-NAME + Indomethacin |
7.6±0.01 | 94.2±1.8 | 6.4±0.02*‡ | 50.7±3.6*‡ |
|
UCL-1684 L-NAME + Indomethacin |
6.7±0.03‡ | 17.3±2.4‡ | 8.1±0.15*‡ | 14.6±2.4‡ |
|
TRAM-34 L-NAME + Indomethacin |
7.5±0.07 | 86.0±2.7‡ | 7.4±0.12*‡ | 7.5±0.5*‡ |
|
Charybdotoxin L-NAME + Indomethacin |
7.4±0.04 | 97.4±0.9‡ | 7.8±0.31*‡ | 7.4±0.01‡ |
|
UCL-1684 + TRAM-34 L-NAME + Indomethacin |
7.1±0.02‡ | 8.6±1.5‡ | 6.6±0.15*‡ | 7.3±1.1‡ |
P<0.05 vs. WKY
P<0.05 vs. respective vehicle-treated
P<0.05 vs. respective L-NAME and indomethacin-treated.
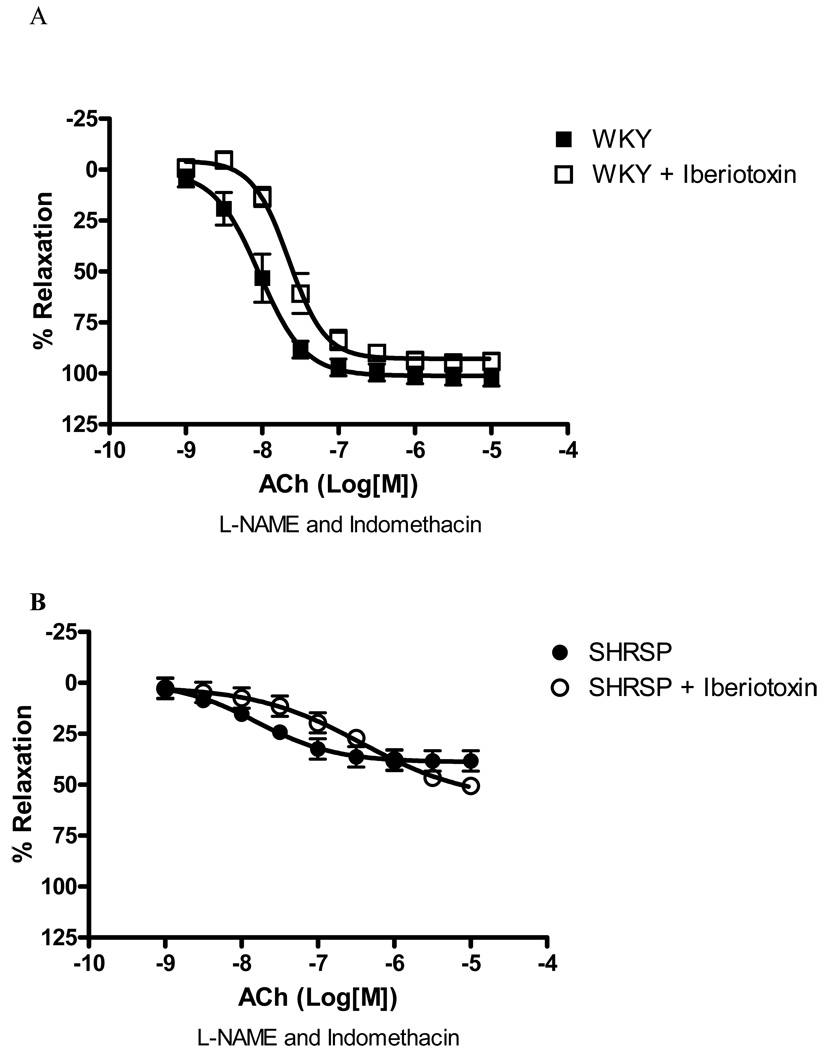
Iberiotoxin (0.1µM), a BKCa channel inhibitor, did not significantly change EDHF-mediated relaxation either in arteries from SHRSP or WKY rats (Figure 2), suggesting a minor contribution of BKCa channel to EDHF-mediated relaxation.
Figure 2. Inhibition of BKCa does not affect EDHF-induced relaxation in SHRSP or WKY small mesenteric arteries.
Concentration-response curves to ACh in U-46619-contracted (0.3µM) second-order mesenteric arteries from (A) WKY and (B) SHRSP in the absence (closed symbols) or presence (open symbols) of iberiotoxin (0.1µM). All the curves were performed in the presence of L-NAME (100µM) and indomethacin (10µM). Experimental values of the relaxation induced by ACh were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue. Results are presented as mean ± SEM of n=5 in each experimental group.
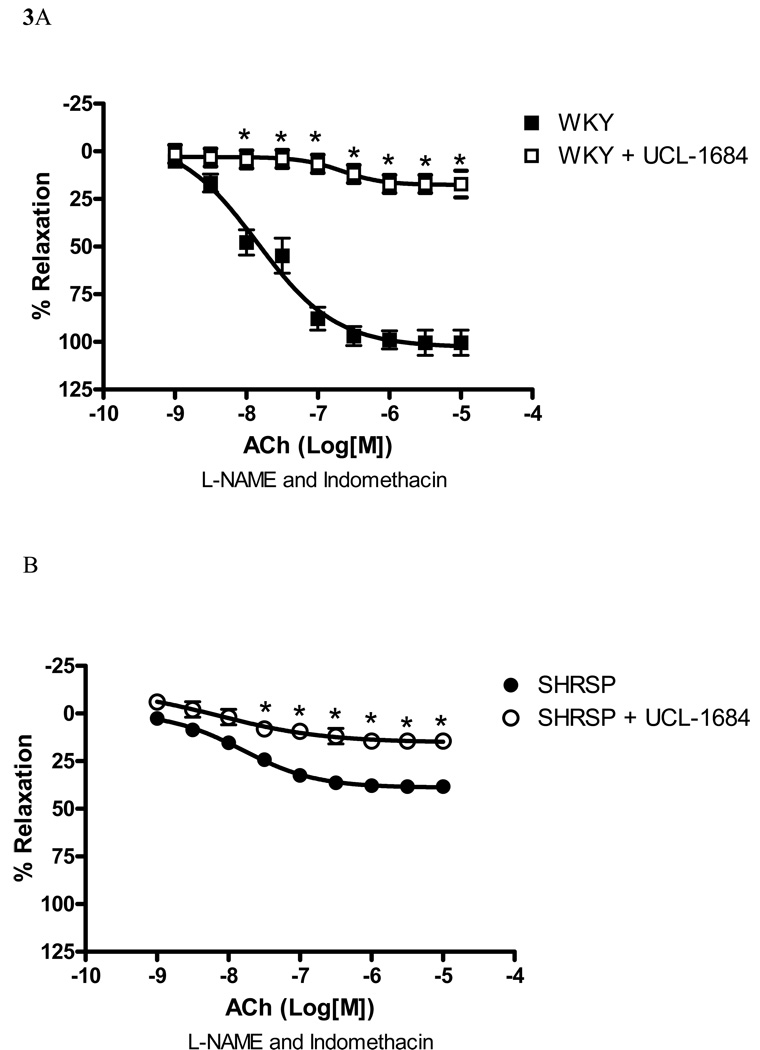
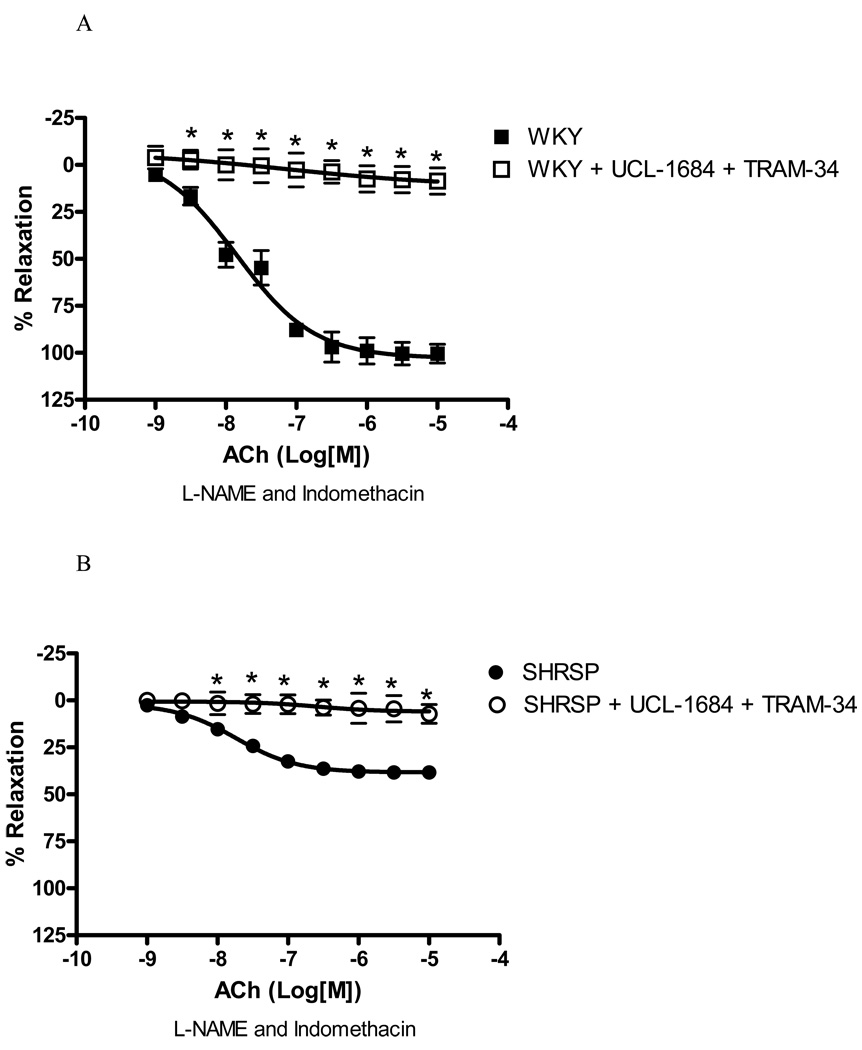
UCL-1684 (0.1µM), a SKCa channel inhibitor, greatly impaired EDHF-induced relaxation in WKY small arteries [82.8% of inhibition of Emax, (p<0.05), Figure 3A], as well as reduced the relaxation in SHRSP arteries [61.9% inhibition of Emax, (p<0.05), Figure 3B]. These data suggest that the SKCa channels mediate EDHF-induced relaxation in arteries from both normotensive and hypertensive rats.
Figure 3. SKCa inhibition affects EDHF-induced relaxation in SHRSP and WKY small mesenteric arteries.
Concentration-response curves to ACh in U-46619-contracted (0.3µM) second-order mesenteric arteries from ((A) WKY and (B) SHRSP in the absence (closed symbols) or presence (open symbols) of UCL-1684 (0.1µM). All the curves were performed in the presence of L-NAME (100µM) and indomethacin (10µM). Experimental values of the relaxation induced by ACh were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue. Results are presented as mean ± SEM of n=5 in each experimental group. *, P<0.05 compared with respective control (vehicle).
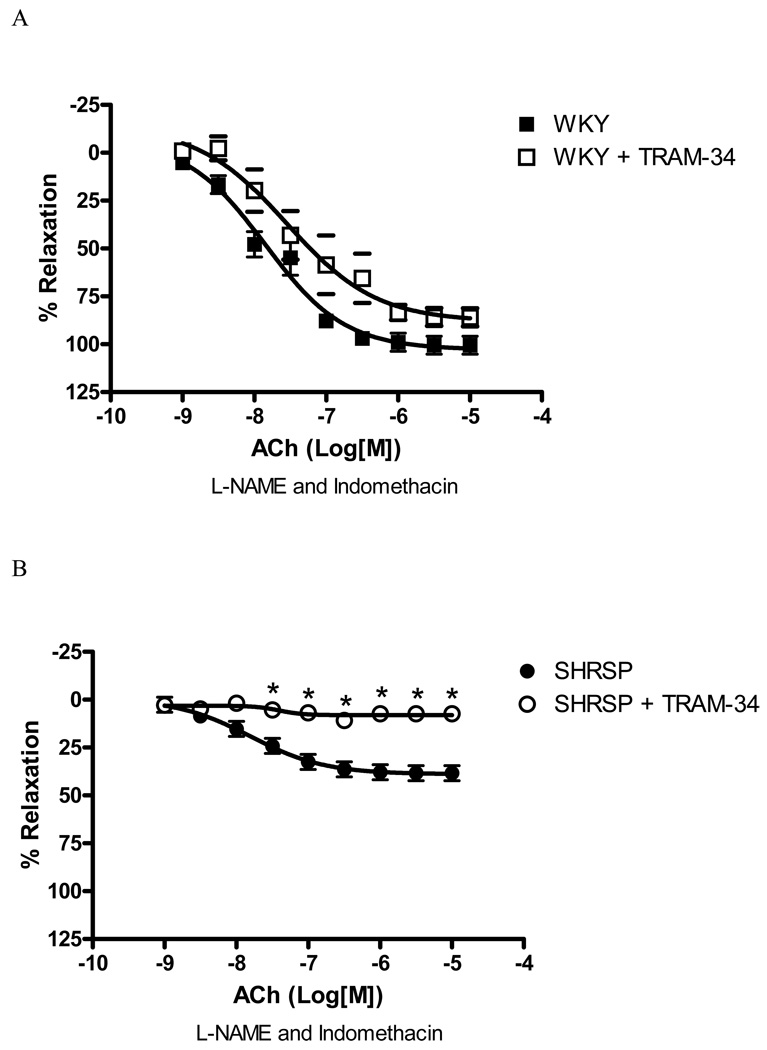
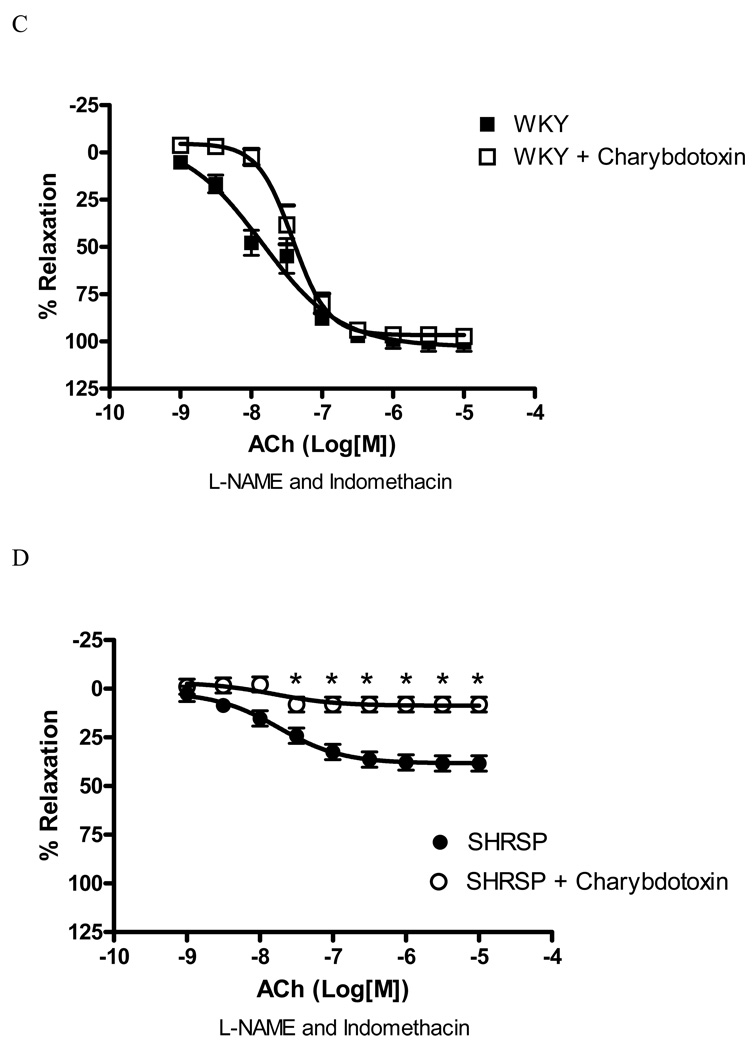
The blockade of IKCa channel with TRAM-34 (10µM) significantly inhibited EDHF-mediated relaxation in arteries from SHRSP [80.5% inhibition of Emax, (p<0.05), Figure 4B], but not in WKY (18.9% inhibition of Emax – Figure 4A). Inhibition of IKCa channel with charybdotoxin (0.1µM) produced similar results. Charybdotoxin significantly inhibited EDHF-mediated relaxation in arteries from SHRSP [78.9% inhibition of Emax, (p<0.05), Figure 4D], but not in WKY (5.3% inhibition of Emax – Figure 4C). These findings suggest that the IKCa channels greatly contribute to EDHF-mediated relaxation in small arteries from SHRSP, but play a minor role in arteries from WKY.
Figure 4. IKCa inhibition affects EDHF-induced relaxation in SHRSP, but not WKY small mesenteric arteries.
Concentration-response curves to ACh in U-46619-contracted (0.3µM) second-order mesenteric arteries from (A, C) WKY and (B, D) SHRSP in the absence (closed symbols) or presence (open symbols) of (A, B) TRAM-34 (10µM) or (C, D) charybdotoxin (0.1µM). All the curves were performed in the presence of L-NAME (100µM) and indomethacin (10µM). Experimental values of the relaxation induced by ACh were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue. Results are presented as mean ± SEM of n=5 in each experimental group. *, P<0.05 compared with respective control (vehicle).
Simultaneous inhibition of IKCa and SKCa completely abolished EDHF-induced relaxation in WKY [91.9% inhibition of Emax, (p<0.05), Figure 5A], and SHRSP [88% inhibition Emax, (p<0.05), Figure 5B].
Figure 5. EDHF-induced relaxation is completely abolished after simultaneous inhibition of IKCa and SKCa.
Concentration-response curves to ACh in U-46619-contracted (0.3µM) second-order mesenteric arteries from (A) WKY and (B) SHRSP in the absence (closed symbols) or presence (open symbols) of UCL-1684 (0.1µM) and TRAM-34 (10µM). All the curves were performed in the presence of L-NAME (100µM) and indomethacin (10µM). Experimental values of the relaxation induced by ACh were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue. Results are presented as mean ± SEM of n=5 in each experimental group. *, P<0.05 compared with respective control (vehicle).
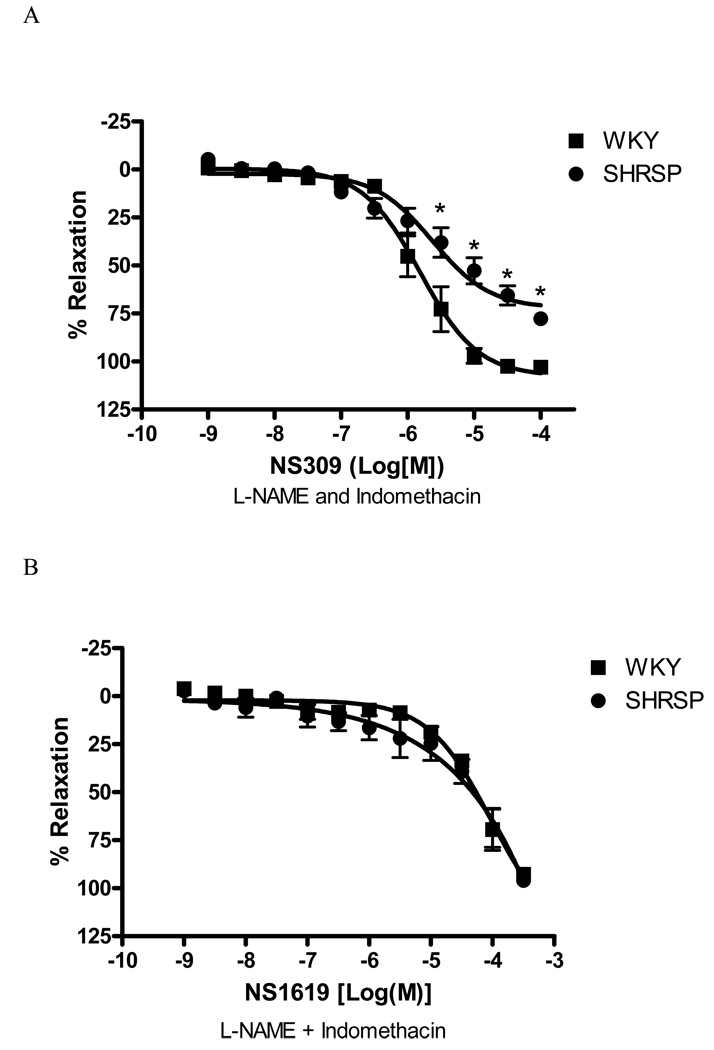
Concentration-response curves to NS309, a SKCa and IKCa channels opener, were also performed. The response to NS309 was decreased in arteries from SHRSP, when compared to those in control arteries [Emax 77.9±3.2 vs. 103.1±3.1%, respectively, (p<0.05), Figure 6]. NS309-induced relaxation was abolished with concomitant incubation of UCL-1684 (0.1µM) and charybdotoxin (0.1µM) – (data not shown).
Figure 6. Relaxation-response to IKCa and SKCa, but not BKCa, channels opener is impaired in SHRSP small mesenteric arteries.
Concentration-response curves to (A) NS309 (1nM to 100µM) and (B) NS1619 in U-46619-contracted (0.3µM) second-order mesenteric arteries from WKY (■) and SHRSP (●). All the curves were performed in the presence of L-NAME (100µM) and indomethacin (10µM). Experimental values of the relaxation induced by NS-309 were calculated relative to the maximal changes from the contraction produced by U-46619 in each tissue. Results are presented as mean ± SEM of n=5 in each experimental group. * P<0.05 vs. WKY.
Responses to NS1619 (a BKCa channel opener) were similar in vessels from SHRSP and WKY rats (Emax 96.1±2.1, n=5 and 92.8±1.4, n=5; respectively) and were fully blocked by incubation with iberiotoxin (0.1µM).
Expression of KCa channel isoforms in small- mesenteric arteries
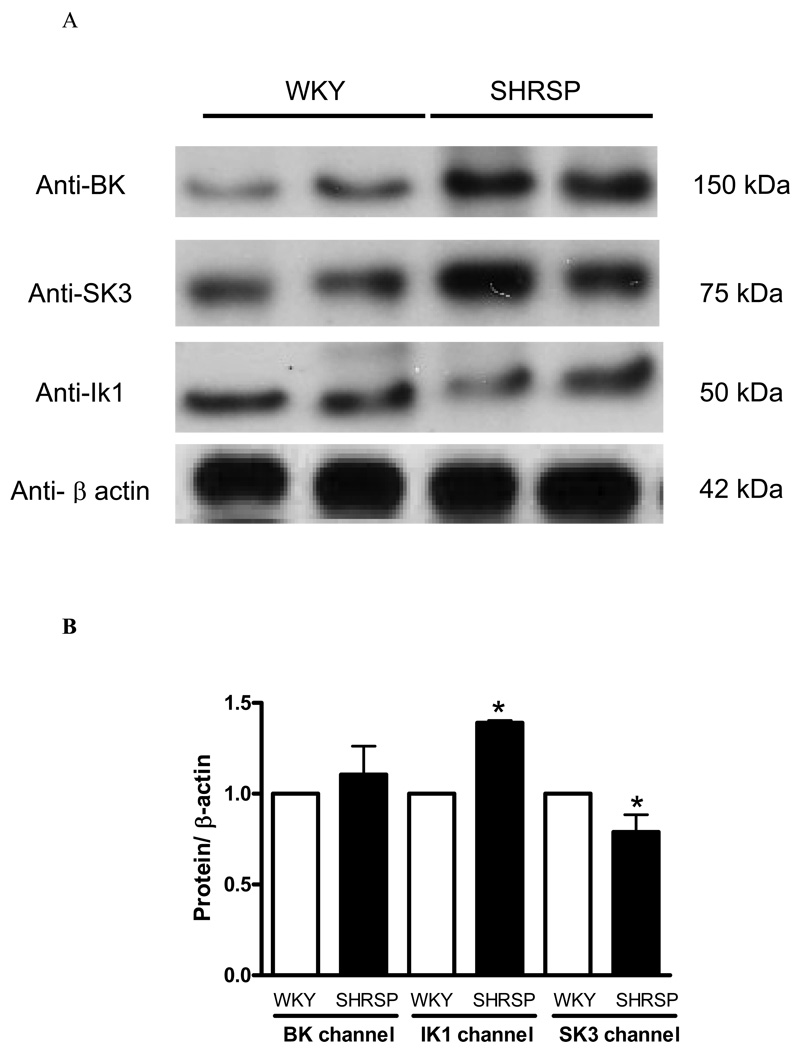
The functional studies performed in small-mesenteric arteries from normotensive and hypertensive animals indicated that EDHF-mediated relaxation has different profiles and is mediated by different subtypes of KCa channel. Analysis of protein expression of BK, SK3 (a SKCa isoform) and IK1 (also known as IKCa) channels were performed to better characterize possible differences in the expression of the KCa channels isoforms, between WKY and SHRSP arteries. SK3 isoform was chosen because this isoform is present in the vascular endothelium (22).
No differences were found in BK protein levels between the groups (Figure 7B). IK1 protein expression was increased in mesenteric arteries from SHRSP, when compared to WKY arteries (Figure 7B). On the other hand, protein expression levels of SK3 in mesenteric arteries from SHRSP were reduced compared with those in WKY values (Figure 7B). Although IKCa channel was increased in arteries of SHRSP, it seems that this up-regulation is not sufficient to restore impaired EDHF-mediated relaxation in SHRSP.
Figure 7. IKCa and SKCa channel expression is altered in mesenteric arteries from SHRSP.
On the top, (A) representative immunoblots for BK, SK3, IK1 and β-actin expression in WKY and SHRSP mesenteric arteries. On the bottom, corresponding bar graphs demonstrating the (B) BK, (C) IK1 and (D) SK3 channel expression. Values, expressed in arbitrary units, are mean ± SEM of n=5 experiments and were normalized by β-actin protein expression. * P<0.05 vs. WKY.
Modulation of IKCa expression by REST
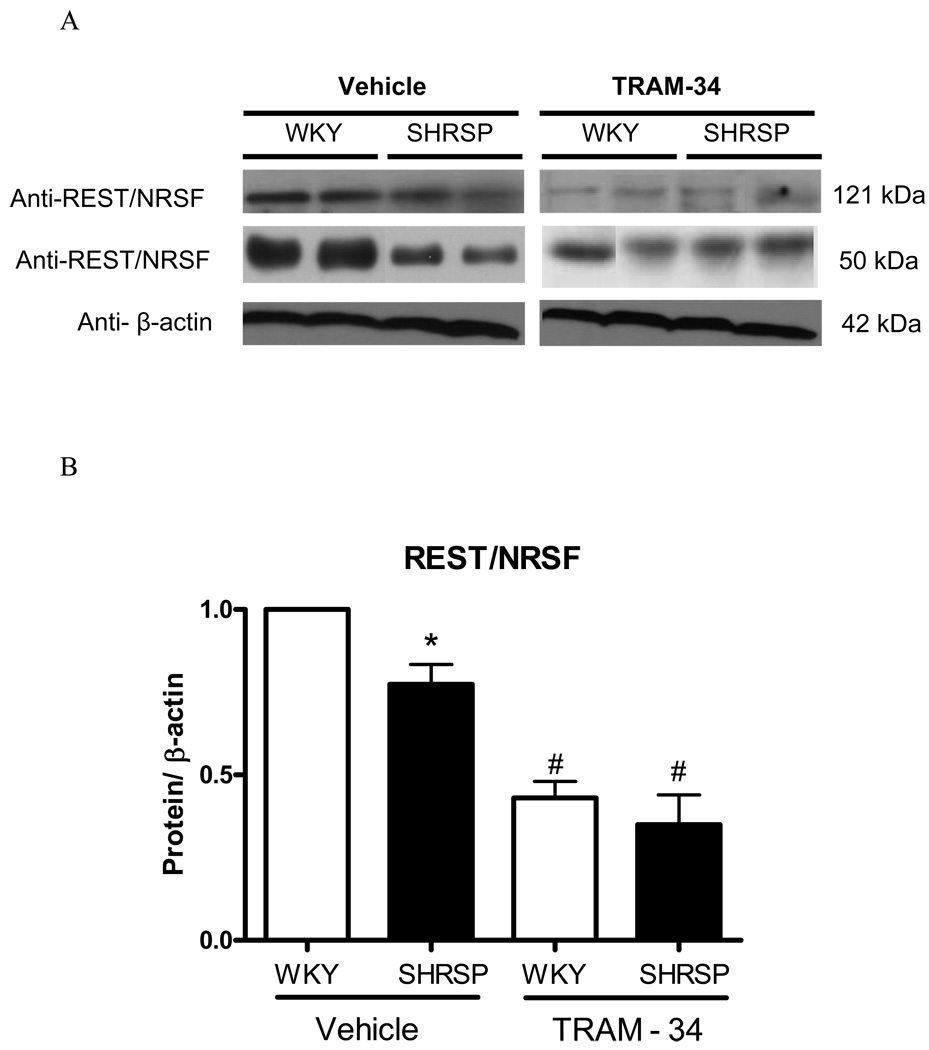
Because REST was shown to negatively regulate IKCa channel expression (17), we evaluated protein levels for this transcription factor. Anti-REST antibody resulted in a predicted band size of 121kDa and an additional band was observed at 50kDa position. Mesenteric arteries from SHRSP displayed reduced REST expression, when compared with arteries from WKY (Figure 8A–B).
Figure 8. REST expression in mesenteric arteries from SHRSP and WKY.
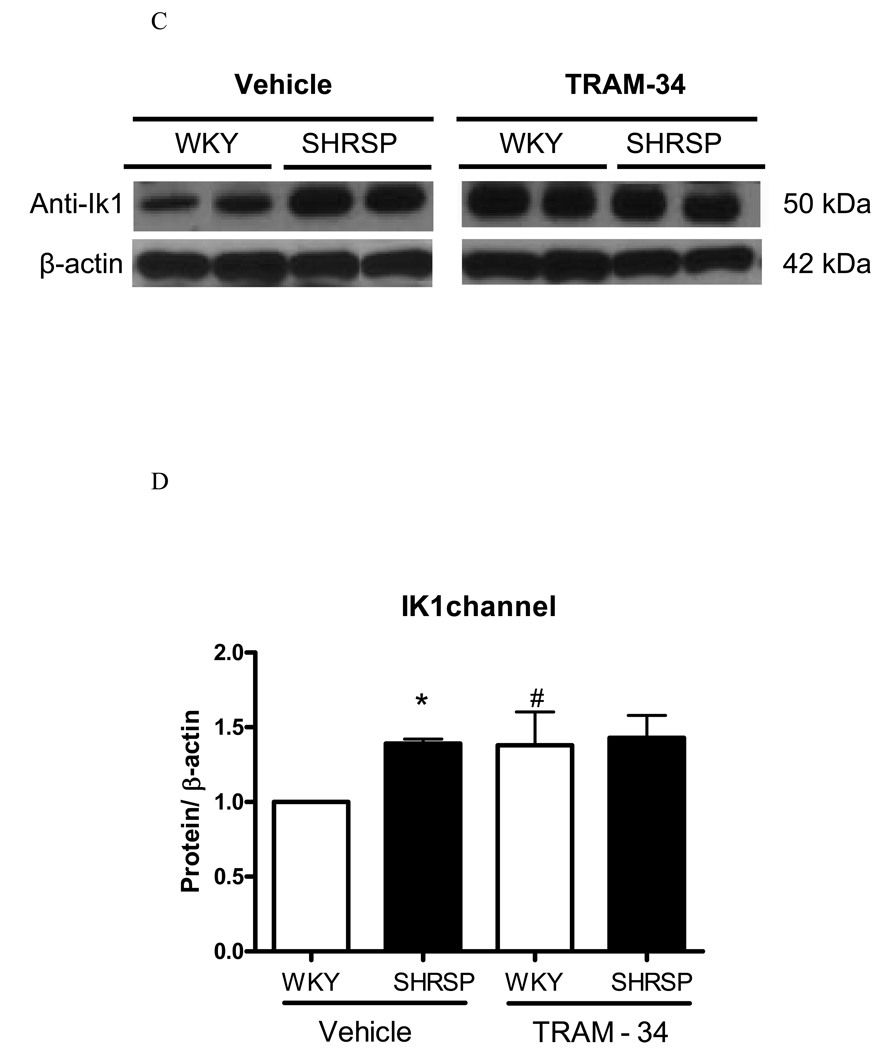
(A) Representative immunoblots for REST, and β-actin expression in WKY and SHRSP mesenteric arteries, after vehicle or TRAM-34 (10µM, 24 hours) incubation and (B) corresponding bar graphs demonstrating REST expression. (C) Representative immunoblots for IK1 and β-actin expression in WKY and SHRSP mesenteric arteries, after vehicle or TRAM-34 (10µM, 24 hours) incubation and (D) corresponding bar graphs demonstrating IK1 expression. Values, expressed in arbitrary units, are mean ± SEM of n=5 experiments and were normalized by β-actin protein expression. * P<0.05 vs. WKY, # P<0.05 vs. vehicle SHRSP.
We took advantage from a pharmacological approach to inhibit IKCa channel, and determined whether blockade of these channels could lead to changes in REST or IK1 expression. After 24 hours of incubation with TRAM-34 (10µM) or vehicle (DMSO), proteins were extracted from mesenteric vessels from normotensive and hypertensive rats and expression of both IK1 and REST was determined. REST expression was drastically reduced after TRAM-34 incubation, both in arteries from WKY and SHRSP (Figure 8A–B). The difference in IK1 expression between arteries from normotensive and hypertensive animals was abolished after 24 hours of IKCa channel blockade. In order to control for changes in protein expression due to the 24 hour-incubation period, we compared IK1 and REST expression in freshly harvested mesenteric arteries and in arteries incubated for 24 hours with DMSO. No significant differences were found in IK1 and REST expression between freshly isolated and incubated arteries from control or hypertensive animals, indicating that changes in protein expression between WKY and SHRSP vessels are not due to the incubation procedure. Collectively, these results demonstrate that modifications in the activity of IKCa channel resulted in changes of REST and IKCa channel expression, reinforcing that REST negatively modulates IK Ca channel in vascular cells.
DISCUSSION
The endothelium is a major regulator of vascular tone, releasing vasoactive substances such as EDHF, NO, cyclooxygenase metabolites, endothelin-1 and other endothelium-derived constrictor factors (23). In most models of experimental hypertension, the endothelium-dependent relaxation is impaired. However, this endothelial dysfunction presents different characteristics depending on the experimental model studied (24). Konishi and Su first showed that endothelium-dependent relaxation is impaired in arteries from SHR (23). Similar results have been reported in the mesenteric artery from SHRSP (25, 26). Although ACh-induced relaxation was slightly impaired in small mesenteric vessels from SHRSP animals, in the present study we observed pronounced impairment in EDHF-mediated responses.
The IKCa and SKCa channels occur in abundance in endothelial cells and their activation results in EDHF-like hyperpolarization of these cells (10–15, 27, 28). The resulting endothelial hyperpolarization spreads via myoendothelial gap junctions to result in the EDHF-induced hyperpolarization and relaxation of the smooth muscle (2). EDHFs-mediated responses exhibit exquisite sensitivity to the combination of charybdotoxin and apamin (29)? showing the importance of both SKCa and IKCa, respectively, to EDHFs-mediated relaxation. Accordingly, experiments conducted in a transgenic mouse (SK3T/T), in which SK3 expression levels are suppressed with dietary doxycycline, demonstrated that decreased SK3 expression results in a pronounced and reversible elevation of blood pressure, around 30mmHg (30). In addition, K(Ca)3.1(−/−) [or IK1(−/−)] mice display reduced endothelial and smooth muscle hyperpolarization in response to acetylcholine and significant increase in arterial blood pressure, around 30 mmHg (31). Furthermore, IK1(−/−)/SK(T/T) mice treated with dietary doxycycline, which results in combined IK1/SK3 deficiency, display impaired acetylcholine-induced EDHF-mediated relaxation dilation in conduit arteries and in resistance arteries as well as elevated arterial blood pressure (32). These results indicate that the endothelial SKCa and IKCa are fundamental regulators of blood pressure and it seems likely that EDHF, through vascular dependent mechanisms, can influence blood pressure levels.
We have examined the relative contributions of SKCa, IKCa and BKCa to EDHF-mediated response, in SHRSP small mesenteric arteries. Our results demonstrate that the mechanisms underlying EDHF-mediated responses of small mesenteric arteries from SHRSP and WKY rats are distinct. EDHF-mediated relaxation in small mesenteric arteries from WKY rats was prevented by the blockade of SKCa channels, with UCL-1684.
In SHRSP, although SKCa contributes to EDHF-meditate relaxation, this was mainly mediated by IKCa channels. These results are in agreement with a previous report showing that EDHF-mediated relaxation is impaired in SHRSP and that KCa channels have a differential contribution in hypertensive and normotensive animals (26). In addition, the response to NS309 was decreased in arteries from SHRSP, further reinforcing that SKCa and IKCa function is impaired in mesenteric arteries from SHRSP. Unfortunately, no selective agonist for these channels, SKCa and IKCa, are available and although NS309-induced relaxation seems to be abolished in conditions where endothelium is removed (33, 34), it is important to consider that NS309 has been shown also to inhibit L-type voltage-dependent Ca2+-channel (35).
In agreement with our functional data, a differential expression of SKCa and IKCa channels was observed between the groups. Small arteries from SHRSP, when compared with WKY arteries, showed decreased levels of SKCa channel, whereas IKCa channel expression was increased.
Induction of IKCa plays an important role in the vasculature. The gene encoding IKCa, KCNN4, contains a functional REST binding site that is repressed by REST (17). REST has been considered a dynamic factor in smooth muscle cells. Cheong et al (2006) showed that REST gene expression declines when the cells proliferate in culture. Furthermore, they showed that loss of REST is a switch enabling KCNN4 expression (16). Indeed, some reports speculate that REST may be a common factor helping to suppress vascular diseases (16). Here, we showed for the first time that REST expression is closely associated with IKCa expression in arteries from hypertensive animals
Given that IKCa channels are important modulators of EDHF-induced relaxation in small mesenteric vessels from SHRSP, we have hypothesized that REST modulation of IKCa channel expression is decreased in hypertension, as a compensatory mechanism in response to impaired EDHF-mediated relaxation. Our main finding was that REST is down-regulated whereas IKCa channels are over expressed in arteries from SHRSP, when compared with WKY. Furthermore, we showed that the blockade of IKCa channels, by using TRAM-34 for 24 hours, induces vascular down-regulation of REST expression, and also abolishes the differences in IKCa channels expression between arteries from SHRSP and WKY. It is possible that continued inhibition of IKCa channels with TRAM-34 resulted in a compensatory tentative of the vascular tissue to restore this channel function. We speculate that this phenomenon, decreased REST expression and consequently, augmented IKCa channels expression, is a compensatory mechanism in the vasculature, to counter balance impaired EDHF-mediated relaxation.
It has been previously shown that TRAM-34 treatment modulates IK1 and REST expression. Tharp and colleagues (2008) demonstrated that coronary balloon injury robustly increased IK1 and decreased REST expression 2 hours postangioplasty. Interestingly, they showed that TRAM-34 delivered via balloon catheter blocked not only IK1 upregulation, but also the suppression of REST, which is in close opposition to the results presented here. However, one needs to consider that a different experimental model (an in vivo model for coronary injury), different TRAM-34 concentration and treatment duration may partially explain the differential results. In addition, our results point to the important observation that IK1 activity is able to modulate REST expression. REST expression probably is modulated by various factors, but the lack of information on REST expression regulation precludes further speculations.
Although KCa channels seem to play a major role in EDHFs-mediated relaxation (29), endothelial cell hyperpolarization can also result from endothelial to vascular smooth muscle cells interactions through myo-endothelial gap junctions (36, 37), as well as activation of inwardly K+ channels and Na+-K+-ATPase (38, 39). Other important point is that alterations in the vessel structure and vascular remodeliling, as a consequence of prolonged hypertension, could contribute to impaired EDHF-response in small-mesenteric arteries from SHRSP. It seems likely that increases in the number of SMC in the media may have an impact on EDHF ability to hyperpolarize the smooth muscle (40). These are important points that also need to be further evaluated in order to completely understand EDHF-dependent mechanisms involved in vascular relaxation during physiological and pathophysiological conditions.
In conclusion, we have shown that small-mesenteric arteries from SHRSP display significant impairment in EDHF-mediated relaxation. In addition, SKCa channels importantly contribute to this phenomenon both in normotensive and hypertensive animals, but IKCa channel plays a greater and important role in the EDHF-mediated relaxation in arteries from SHRSP. Protein levels of SKCa and IKCa channels were altered in arteries from hypertensive and REST seems to be a negative modulatory mechanism, controlling the augmented levels of IKCa channels in the SHRSP vasculature.
Acknowledgment
This study was supported by grants from the National Institutes of Health (HL-71138 and HL-74167). F.R.C.G. and F.S.C. are supported by doctoral fellowships from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - São Paulo, Brazil). The authors declared no disclosures.
LIST OF ABBREVIATIONS
- ACh
acetylcholine
- BKCa
large-conductance calcium-activated potassium channels
- EDHF
endothelium-derived hyperpolarizing factor
- EET
epoxyeicosatrienoic acid
- Emax
maximum effect elicited by the agonist
- IKCa
intermediate calcium-activated potassium channels
- KCa
calcium-activated potassium channels
- KIR
inward rectifier K+ channels
- L-NAME
Nω-nitro-l-arginine methyl ester
- NO
nitric oxide
- pD2
negative logarithm of the molar concentration of agonist producing 50% of the maximum response
- PGI2
prostacyclin
- PSS
physiological salt solution
- REST
repressor element 1-silencing transcription factor
- SHRSP
stroke-prone spontaneously hypertensive rats
- SKCa
small calcium-activated potassium channels
- U-46619
9,11-dideoxy-9α, 11α-methanoepoxyprostaglandin F2α
- WKY
Wistar Kyoto
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kozlowska H, Baranowska M, Gromotowicz A, Malinowska B. [Endothelium-derived hyperpolarizing factor (EDHF): potential involvement in the physiology and pathology of blood vessels] Postepy Hig Med Dosw (Online) 2007;61:555–564. [PubMed] [Google Scholar]
- 2.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31(9):641–649. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 3.Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol Heart Circ Physio. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- 4.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998 Nov 19;396(6708):269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 6.Edwards G, Félétou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, et al. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002 Dec;137(8):1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy ME, Brayden JE. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J Physiol. 1995 Dec 15;489(Pt 3):723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinton JM, Langton PD. Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol. 2003 Mar;138(6):1031–1035. doi: 10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002 Mar;135(5):1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003 Feb;138(4):594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgers RH, Todd J, Jr, Webb RC. Regional heterogeneity in acetylcholine-induced relaxation in rat vascular bed: role of calcium-activated K+ channels. Am J Physiol Heart Circ Physiol. 2006 Jul;291(1):H216–H222. doi: 10.1152/ajpheart.01383.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2007 May;292(5):H2275–H2284. doi: 10.1152/ajpheart.00949.2006. [DOI] [PubMed] [Google Scholar]
- 16.Cheong A, Wood IC, Beech DJ. Less REST, more vascular disease? Cell Cycle. 2006;5(2):129–131. doi: 10.4161/cc.5.2.2310. [DOI] [PubMed] [Google Scholar]
- 17.Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol Cell. 2005 Oct 7;20(1):45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol. 2008 Jun;28(6):1084–1089. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 20.Cohen RA, Plane F, Najibi S, Huk I, Malinski T, Garland CJ. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan S, Rahman A, Nilsson H, Clapp L, MacAllister R, Ahluwalia A. NO contributes to EDHF-like responses in rat small arteries: a role for NO stores. Cardiovasc Res. 2003 Jan;57(1):207–216. doi: 10.1016/s0008-6363(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 22.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14(13):1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 23.Konishi M, Su C. Role of endothelium in dilator responses of spontaneously hypertensive rat arteries. Hypertension. 1983;5(6):881–886. doi: 10.1161/01.hyp.5.6.881. [DOI] [PubMed] [Google Scholar]
- 24.Boulanger CM. Secondary endothelial dysfunction: hypertension and heart failure. J Mol Cell Cardiol. 1999;31:39–49. doi: 10.1006/jmcc.1998.0842. [DOI] [PubMed] [Google Scholar]
- 25.Diederich D, Yang ZH, Buhler FR, Luscher TF. Impaired endothelium-dependent relaxations in hypertensive resistance arteries involve cyclooxygenase pathway. Am J Physiol Heart Circ Physiol. 1990;258:H445–H451. doi: 10.1152/ajpheart.1990.258.2.H445. [DOI] [PubMed] [Google Scholar]
- 26.Sunano S, Watanabe H, Tanaka S, Sekiguchi F, Shimamura K. Endothelium-derived relaxing, contracting and hyperpolarizing factors of mesenteric arteries of hypertensive and normotensive rats. Hypertension. 1999;126(3):709–716. doi: 10.1038/sj.bjp.0702355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. Faseb J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- 28.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001 Oct;81(4):1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 29.Büssemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, et al. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42(4):562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- 30.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-donductance Ca2+activated K+(SK3) channels modulates arterial tone and blood pressure. Circ Research. 2003;93(2):124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 31.Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006 Sep 1;99(5):537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 32.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009 May 5;119(17):2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 33.Feng J, Liu Y, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, et al. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation. 2008 Sep 30;118 14 Suppl:S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009 Apr;23(4):1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morimura K, Yamamura H, Ohya S, Imaizumi Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J Pharmacol Sci. 2006 Mar;100(3):237–241. doi: 10.1254/jphs.sc0060011. [DOI] [PubMed] [Google Scholar]
- 36.Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90(10):1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 38.Weston AH, Richards GR, Burnham MP, Félétou M, Vanhoutte PM, Edwards G. K+-induced hyperpolarization in rat mesenteric artery: identification, localization and role of Na+/K+-ATPases. Br J Pharmacol. 2002;136(6):918–926. doi: 10.1038/sj.bjp.0704787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris D, Martin PE, Evans WH, Kendall DA, Griffith TM, Randall MD. Role of gap junctions in endothelium-derived hyperpolarizing factor responses and mechanisms of K(+)-relaxation. Eur J Pharmaco. 2000;402(1–2):119–128. doi: 10.1016/s0014-2999(00)00512-4. [DOI] [PubMed] [Google Scholar]
- 40.Sandow SL, Bramich NJ, Bandi HP, Rummery NM, Hill CE. Structure, function, and endothelium-derived hyperpolarizing factor in the caudal artery of the SHR and WKY rat. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):822–828. doi: 10.1161/01.ATV.0000067425.06019.D7. [DOI] [PubMed] [Google Scholar]