Abstract
Background
Microneedles have previously been used to deliver insulin to animal models, but not in human subjects. This study tested the hypothesis that hollow microneedles can deliver insulin to modulate blood glucose levels in subjects with type 1 diabetes in a minimally invasive manner.
Methods
This study was carried out in two adults with type 1 diabetes and evaluated bolus delivery of lispro insulin using a hollow microneedle compared to a catheter infusion set (9 mm). The study first determined the minimum insulin delivery depth by administering insulin from microneedles inserted 1, 3.5, and 5 mm into the skin of fasting subjects and then assessed the efficacy of insulin delivery from microneedles inserted 1 mm into the skin to reduce postprandial glucose levels. Blood samples were periodically assayed for plasma free insulin and plasma glucose levels for up to 3.5 h.
Results
The first phase of the study indicated that microneedles inserted at the shallowest depth of 1 mm within the skin led to rapid insulin absorption and reduction in glucose levels. Bolus insulin delivery followed by consumption of a standardized meal in the second phase revealed that microneedles were effective in reducing postprandial glucose levels. Subjects reported no pain from microneedle treatments, and there were no adverse events.
Conclusions
This study provides the first proof of concept that hollow microneedles can effectively deliver bolus insulin to type 1 diabetes subjects in a minimally invasive manner.
Introduction
Insulin was first administered in 1922 by intramuscular injection. However, it was rapidly determined that subcutaneous insulin delivery had similar efficacy to intramuscular injections but was significantly less traumatic.1 Subcutaneous administration was subsequently established as the new delivery standard. Insulin is currently delivered through the subcutaneous route by means of hypodermic needles, insulin pens, and catheters connected to insulin pumps. However, these treatment methods are inconvenient and painful and often lead to poor patient compliance, especially among children and adolescents with type 1 diabetes. These patients have a tendency to omit their insulin injections because of fear, pain, anxiety, and inconvenience associated with subcutaneous needles and catheters,2–5 leading to poor diabetes management.
It has been 87 years since insulin was first given to individuals with diabetes, yet subcutaneous administration continues to remain the prevalent means of insulin delivery. In order to improve patient compliance, there is a need for new and improved delivery systems.
Intradermal insulin delivery is an attractive administration route because it is less invasive than subcutaneous delivery. Additionally, the presence of a rich capillary bed in the dermis may allow for rapid uptake of drugs delivered intradermally. Previous studies have shown that intradermal delivery can accelerate the pharmacokinetics of proteins.6
Inhaled insulin is another alternative to subcutaneous insulin delivery, which is noninvasive and can eliminate the pain and apprehension associated with injections. However, introduction of the first Food and Drug Administration-approved inhalable insulin, Exubera® (Pfizer, New York, NY), failed to achieve patient and physician acceptance for various reasons such as low bioavailability, high cost, cumbersome device design, side effects, and unknown health risks.7,8 Moreover, it requires continued delivery of basal insulin through traditional subcutaneous methods.
Several other novel and minimally invasive delivery methods such as oral, buccal, transdermal, and nasal systems are being investigated to determine effectiveness and increased patient compliance; however, these technologies are mostly still in preclinical development.
We have developed micron-dimension needles called microneedles as a minimally invasive intradermal insulin delivery system to increase patient compliance. These microneedles have the potential to reduce the pain, apprehension, and inconvenience associated with insulin delivery because they are long enough to breach the skin's barrier and allow for transport of large molecules such as insulin, yet short enough to avoid stimulating nerve endings.9,10 Previous studies in human subjects have demonstrated that microneedles are relatively painless when compared to hypodermic needles.11
Microneedles can be fabricated as solid or hollow, single-needle or multi-needle arrays and as biodegradable microneedles encapsulating drug compounds.9,10 Previous studies have shown that solid, hollow, and biodegradable microneedles can be used to deliver insulin and lower blood glucose levels in animal models of diabetes.12–17 Microneedles have also been used to deliver drugs such as desmopressin, plasmid DNA, and oligonucleotides,9,10 as well as vaccines against hepatitis B and anthrax in animals.18
Microneedles have received attention in human subjects too. The first published study used hollow microneedles to deliver methyl nicotinate, but this device was only able to inject approximately 1 μL into the skin.19 A subsequent study used solid microneedles as a pretreatment to permeabilize the skin, which enabled delivery of naltrexone at therapeutic levels from a transdermal patch.20 Hollow microneedles have also advanced through clinical trials to administer influenza vaccine21, and solid microneedles have been coated with parathyroid hormone for delivery in Phase 2 studies.18 Additionally, microneedles have been used in human subjects to withdraw interstitial fluid for glucose level measurement.22
In this paper, we present the first proof-of-principle study showing delivery of insulin through a microneedle device in human subjects with type 1 diabetes. To first determine ideal microneedle insertion depth and then evaluate the effect of insulin delivery through a microneedle, the study was carried out in two phases. In the first phase, the minimum microneedle insertion depth was determined based on the pharmacodynamic response to insulin delivery at different microneedle insertion depths within the skin. In the second phase, the minimum depth was used to determine the efficacy of hollow microneedle insulin delivery in reducing postprandial glucose levels. This study also compared the pharmacokinetics, pharmacodynamics, and pain of microneedle-based insulin delivery to conventional insulin catheter-based delivery.
Research Design and Methods
Study design
This study was performed in the Diabetes Clinic at the Emory Children's Center at Emory University (Atlanta, GA). The study was approved by the Institutional Review Board at Emory University. All subjects provided informed consent to participate in the study.
The study was an open-label, within-subjects, controlled design. The study involved two subjects (one male and one female, 38 and 43 years old, respectively) with type 1 diabetes, who were managed with an insulin pump, were in good glycemic control, and met the inclusion/exclusion criteria. In order to be included in the study, subjects were required to have type 1 diabetes for at least 2 years, be using a conventional Food and Drug Administration-approved insulin pump with lispro insulin for the past year, and have mean hemoglobin A1c levels ≤8% for the past year and a body mass index within the 85th percentile for their age. Subjects were excluded if they had type 2 diabetes, acanthosis nigricans, a clinically significant major organ system disease, were on glucocorticoid therapy, had an insulin requirement of ≥150 U/day or an illness on the day of the study, or were pregnant or breastfeeding. The demographics of the two study subjects are shown in Table 1.
Table 1.
Demographics of Study Subjects
| Parameter | Subject 1 | Subject 2 |
|---|---|---|
| Age (years) | 43 | 38 |
| Race | Caucasian | Caucasian |
| Gender | Female | Male |
| Mean HbA1ca | 6.5 | 6.2 |
| Weight (kg) | 63.5 | 78.0 |
| BMI (kg/m2) | 23.3 | 25.5 |
| Time since diagnosis (years) | 30.0 | 28.5 |
| Duration of pump use (years) | 12.2 | 7.5 |
| Length of pump catheter (mm) | 9.0 | 9.0 |
| Mean insulin per day (units) | 40.0 | 45.0 |
| ICR (units/g) | 1:7.5 | 1:10 |
BMI, body mass index; ICR, insulin to carbohydrate ratio.
Mean hemoglobin A1c over the past year.
Microneedle device: fabrication and insertion
Hollow microneedles (Fig. 1) with a bevel angle of 30° and effective tip radii between 60 and 80 μm were fabricated by pulling fire-polished borosilicate glass pipettes (BF150-86-15, Sutter Instrument, Novato, CA) with a micropipette puller (P-97, Sutter Instrument) and beveler (BV-10, Sutter Instrument). The microneedles were then cleaned in an ultrasonic deionized water bath (SW-34, Sonicwise Ultrasonics, San Diego, CA) for 2 min and dried for 2 h in an oven at 180°C (VC-300, Grieve Corp., Round Lake, IL) followed by steam sterilization in an autoclave (Scientific Series 3021-S, AMSCO, Erie, PA). The microneedles were inserted at a 90° angle into the abdominal skin at various depths ranging from 1 mm to 5 mm using a custom-made rotary drilling device.23 The device was calibrated such that each 360° turn of the device moved the microneedle tip 800 μm in its axial direction. Graded markings on the device allowed for controlled insertion (with ± 10 μm accuracy) into the skin at the desired depths. Drilling was used to precisely control insertion depth for this study. Our preliminary results assessing infusion through microneedles inserted without rotation have been similarly effective (data not shown). Thus, while rotation facilitated this study that varied insertion depth, a final device design may not require rotation. The microneedle was connected to a 3-mL syringe (Becton Dickinson) by means of a flexible intravenous extension set tubing (2C5685, Baxter, Deerfield, IL). The syringe was further connected to a syringe pump (NE-1000, New Era Systems, Farmingdale, NY) that controlled the insulin delivery flow rate. The microneedles were removed from the skin immediately after insulin delivery was completed.
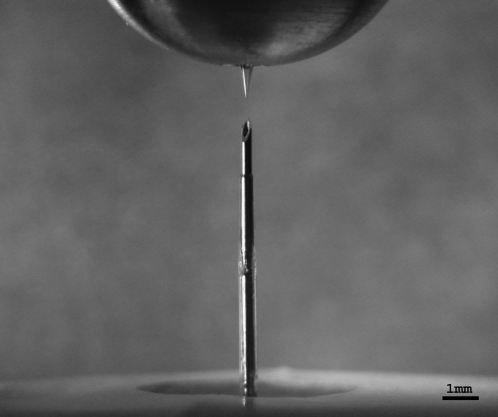
FIG. 1.
(Top) A 1-mm hollow microneedle in a holder compared to (bottom) a 9-mm infusion catheter.
Insulin
Humalog insulin (lispro, Eli Lilly, Indianapolis, IN) was used in this study. The control treatments used a 100-U insulin formulation, whereas the microneedle treatments used a 50-U insulin, which was prepared by diluting 100-U insulin with sterile diluent for Humalog (Eli Lilly).
Study protocol
Phase 1: effect of microneedle insertion depth
This phase was conducted to determine the minimum microneedle insertion depth for effective bolus insulin delivery through a microneedle device in fasting subjects. The study was carried out in Subject 1 and included four study visits. The first visit served as the control visit (subcutaneous insulin catheter: 9 mm) and the subsequent three visits served as the study visits (microneedle: 5 mm, 3.5 mm, and 1 mm insertion depths, respectively).
Prior to each visit, the subject underwent overnight fasting for a minimum of 10 h. In an effort to minimize the amount of insulin on board, subjects turned their pumps off 2 h prior to insulin delivery. Capillary glucose measurements were then taken every 30 min using a glucose meter (FreeStyle Lite™, Abbott Laboratories, Abbott Park, IL) until blood glucose levels remained stable or were at the onset of rising. An intravenous catheter was then placed in the subject's antecubital fossa, and a 10-mL blood sample was drawn. The specimen was collected in a sterile vacuum blood collection tube (BD Vacutainer Plus plastic serum tube 367820, Becton Dickinson) followed by centrifugation at 1,300 g (Vanguard V6500, Hamilton Bell, Montvale, NJ), separation, and immediate freezing at −4°C. A capillary glucose measurement was also taken at this time.
The abdominal insulin administration site was then wiped with 70% isopropyl alcohol (Becton Dickinson). This was followed by administration of an insulin bolus into the subject's abdomen either by inserting a subcutaneous insulin catheter (9 mm, Paradigm® Quick-set®, Medtronic MiniMed, Northridge, CA) connected to a conventional insulin pump (Paradigm, Medtronic MiniMed) in the case of the control treatment or through a microneedle connected to a programmable syringe pump at the rate of 1 mL/min for the study treatment. The insulin dose was determined based on the subject's blood glucose level, individual insulin requirement, and type of delivery device. After insulin delivery, the pumps were immediately shut off, and subjects received no basal insulin during the experiment. Capillary glucose level measurements and blood draws were collected periodically at 30 min, 45 min, 60 min, 75 min, 90 min, and every 30 min thereafter until blood glucose levels returned to normal. All samples were assayed for plasma glucose and free insulin concentrations (Esoterix, Calabasas Hills, CA).
The first phase of the study did not involve the consumption of any food after insulin delivery, and the subject fasted throughout the study. If at any time the subject developed severe hypoglycemia (capillary glucose <70 mg/dL) or severe hyperglycemia (capillary glucose >300 mg/dL), felt ill, or desired to withdraw from testing, the study was discontinued. The subject was asked to qualitatively describe and compare the pain associated for each of the four delivery procedures.
Phase 2: effect of microneedle-based insulin delivery on postprandial glucose levels
This phase was carried out in Subject 2 and involved three study visits: the first served as the control visit (subcutaneous insulin catheter: 9 mm) and the last two as the study visits (microneedle: 1 mm). The protocol for this study was identical to that in phase 1; however, the subject consumed a standardized mixed meal comprising 75 g of carbohydrates, 12 g of protein, and 14 g of fat immediately following the insulin bolus. Capillary glucose levels and blood draws were collected according to the same schedule as phase 1. The subject was asked to qualitatively describe and compare the pain associated with both the delivery procedures. The insulin delivery sites were imaged (PowerShot SD400, Canon, Tokyo, Japan) periodically before and after the insulin bolus for the control and first microneedle visit.
Results
Phase 1
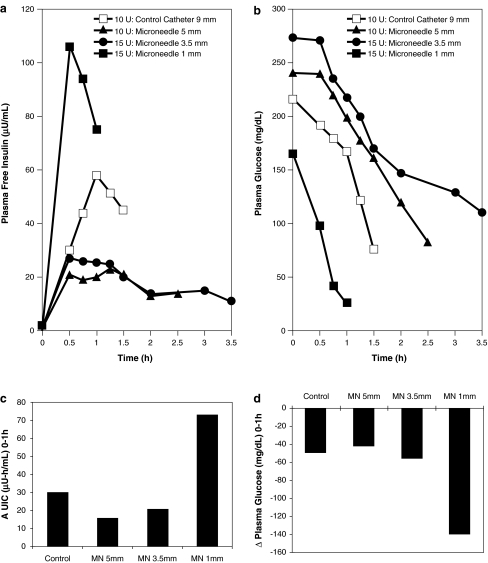
To determine the minimum transdermal insertion depth of microneedles for effective insulin delivery, we initially administered 10 units of 100-U Humalog insulin to Subject 1 using a conventional catheter. As expected, plasma free insulin levels rose, reaching a peak at tmax = 1 h (Fig. 2a), and plasma glucose levels decreased correspondingly (Fig. 2b). At the next two study visits, the subject received a bolus of 10 and 15 units of 50-U humalog at microneedle insertion depths of 5 mm and 3.5 mm, respectively. Although at both of these depths, tmax occurred at 30 min after bolus delivery, both plasma insulin concentration values were lower than that of the catheter. The plasma glucose levels decreased correspondingly, however, at slower rates than in the case of the standard catheter. We then used the microneedle to deliver 15 units of 50-U insulin intradermally at a shallow depth of 1 mm. This led to a significant increase in insulin levels with a peak at tmax = 30 min. Plasma glucose levels declined very rapidly, and the test was discontinued at 1 h because of hypoglycemia.
FIG. 2.
Microneedle-based insulin delivery at 1, 3.5, and 5 mm insertion depths in comparison to 9-mm catheter control (Phase 1). (a) Plasma free insulin level and (b) corresponding plasma glucose level response. Microneedle-based insulin delivery at 1 mm led to high insulin absorption and rapid glucose level reduction. (c) AUIC for 0–1 h and (d) change in plasma glucose levels from 0 to 1 h. Within 1 h of insulin bolus, the 1-mm microneedle (MN) delivery case led to an AUIC more than twice that of the catheter control and produced a higher change in plasma glucose levels.
A comparison of the area under the insulin curves (AUICs) for the first hour after delivery (Fig. 2c) indicated that the 1-mm microneedle led to higher insulin absorption than the other study and control treatments. Further, comparison of the change in plasma glucose levels over the first hour after insulin delivery (Fig. 2d) revealed that the shallow 1-mm microneedle delivery was most effective in reducing glucose levels. We made these comparisons only during the first hour since data for all four delivery conditions were available only for the first hour.
Overall, this phase of the study led to establishment of the 1-mm microneedle depth as not only the minimum, but also the optimum, transdermal depth for effective insulin delivery among the three microneedle depths considered. Microneedle-based delivery at the 1 mm depth was at least as effective as subcutaneous catheter delivery. Based on these findings, this depth was used in subsequent experiments on the second subject to determine effect of microneedle insulin delivery on postprandial glucose levels.
Phase 2
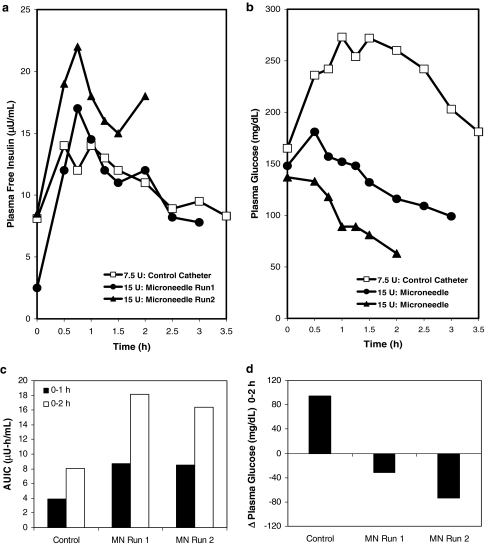
Insulin delivery was assessed with a bolus infusion immediately before a 75-g carbohydrate meal. Based on the second subject's insulin-to-carbohydrate ratio of 1 unit for every 10 g of carbohydrate ingested, insulin delivery through the subcutaneous catheter was assessed with a bolus of 7.5 units of 100-U Humalog. Plasma free insulin levels rose over the course of the first hour and then steadily declined (Fig. 3a). Plasma glucose levels initially increased and eventually started to decrease after 1.5 h. Plasma glucose level approximately returned to the pre-meal glucose value at 3.5 h (Fig. 3b).
FIG. 3.
Microneedle-based insulin delivery at 1 mm insertion depth in comparison to 9-mm catheter control following consumption of a standardized meal immediately after insulin bolus (Phase 2). (a) Postprandial plasma free insulin level profile and (b) corresponding plasma glucose level response. Microneedle-based delivery led to rapid decline in postprandial plasma glucose levels. (c) AUIC for 0–1 h and 0–2 h and (d) change in plasma glucose levels from 0 to 2 h. Microneedle (MN)-based insulin delivery led to an AUIC slightly more than double that of the catheter control for both 0–1 h and 0–2 h periods and larger reduction in plasma glucose levels. Similarity of results from the two microneedle runs demonstrates reproducibility.
The next two visits involved delivery of 15 units of 50-U Humalog at 1 mm through the microneedle device, followed by the consumption of an identical standardized meal within 5 min of bolus infusion. In both microneedle experiments, the tmax occurred at 45 min after infusion. Moreover, plasma glucose levels decreased throughout the duration of both experiments. An evaluation of the AUIC over a 1-h and a 2-h period immediately after delivery revealed that the pharmacokinetics of the insulin delivery were faster for the microneedle as compared to the subcutaneous catheter (Fig. 3c). Similarly, measurement of plasma glucose levels showed that microneedles were more effective than the catheter (Fig. 3d).
Assessment of dermal delivery
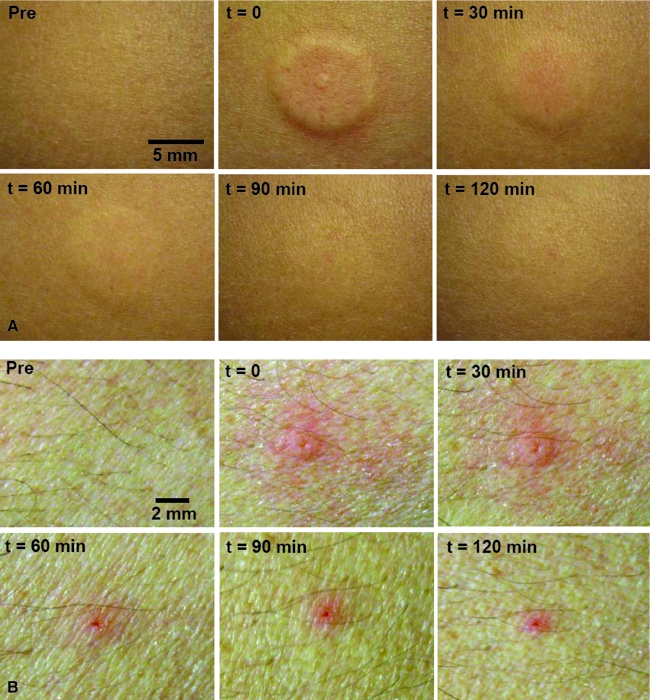
Because skin thickness is approximately 3 mm,24 microneedle insertion to 1 mm depth is expected to be intradermal. To determine if the 1 mm insertion depth led to intradermal insulin delivery, the skin at the microneedle insertion site was visually observed immediately after bolus infusion. Upon observing the skin, a raised wheal extending approximately 5 mm from the point of insertion was seen, consistent with the appearance of an intradermal injection (Fig. 4A). Over time, the skin wheal became less apparent and ultimately disappeared after 2 h. Mild erythema was seen at the infusion site; however, this effect was short-lived and disappeared after 30 min. In comparison, visual observation of the subcutaneous catheter infusion site did not show any skin wheal (Fig. 4B); however, moderate erythema lasting for over 2 h was observed in this case. There were no adverse events in either subject.
FIG. 4.
Surface view of the abdominal infusion site before, immediately after, and at 30-min intervals after insulin delivery in Subject 2. (A) Insulin infusion site for microneedle-based delivery. A raised skin wheal was seen immediately after delivery. Over time, the wheal subsided, and skin returned to normal. (B) Insulin infusion site for catheter-based delivery. Moderate erythema was seen at the point of catheter insertion and slight erythema in the vicinity of the insertion site. The erythema decreased over time but still remained mild at 2 h. Color images available online at www.liebertonline.com/dia.
Pain and comfort assessment
Lastly, we asked the subjects to qualitatively assess the pain they experienced with microneedle-based insulin delivery and compare the assessment with that of catheter-based delivery. Both subjects indicated that all microneedle insulin deliveries were less painful than catheter-based deliveries. Subjects indicated a mild tingling sensation during microneedle delivery, which we attribute to the relatively fast delivery flow rate of 1 mL/min. Other studies have shown that slower flow rates (<0.5 mL/min) do not cause this tingling sensation (data not shown). Both subjects expressed a preference for microneedle-based delivery over their conventional catheter-based pumps.
Discussion
This is the first study assessing the application of microneedles for insulin delivery in human subjects. We demonstrated in two subjects with type 1 diabetes that microneedles can be used to effectively reduce glucose levels in a less painful manner as compared to traditional catheter infusion sets. In this initial study, we delivered insulin doses that were 1.5 to two times higher than the subject's usual dose to ensure that insulin delivered via microneedles reached the systemic circulation. However, the rapid decline in postprandial glucose levels and the high AUIC values indicate that the doses administered in this study were higher than required. Future studies with a larger patient population are needed to compare insulin delivery using microneedles and subcutaneous catheters at the same dose.
Phase 1 of the study revealed that the 1 mm microneedle depth had the fastest pharmacokinetics and pharmacodynamics. We hypothesize that this is due to the fact that the microneedle was inserted into the papillary dermal region, which has a rich capillary network.25 It is this heavily vascularized area that likely permits effective insulin uptake and systemic absorption. Although this result could also be attributed to the fact that the experiment at 1 mm involved 50% more insulin than the 9-mm catheter case, the increase in AUIC and change in plasma glucose levels are both more than doubled at 1 mm, which suggests that there may be increased efficacy. Moreover, subcutaneous delivery at 3.5 mm also used a 50% higher insulin dose than the control but had a lower AUIC than both the 1-mm microneedle and the 9-mm control, which also suggests increased efficacy and is consistent with our hypothesis that uptake by dermal capillaries may be the cause of faster pharmacokinetics and dynamics. Additional studies are needed to validate these findings with repeated measurements with consistent doses for each treatment in multiple subjects.
The 3.5-mm and 5-mm deliveries had lower insulin peaks and slower glucose response compared to the 9-mm catheter, which may be due to the fact that the microneedle was not deep enough in the hypodermis for effective systemic delivery. This explanation is consistent with Subject 1's observation from her daily experience that she typically has poor absorption in the upper hypodermis and generally requires long infusion sets (>9 mm) for effective delivery.
Phase 2 of the study further demonstrated that microneedles can be used to effectively reduce postprandial glucose levels. This was shown on two occasions in the same subject. The glucose levels for the microneedle infusions steadily declined after insulin infusion.
Visual examination of the skin indicated that microneedles caused intradermal delivery, as shown by the presence of a raised skin wheal at the site of microneedle delivery, which differed from the site of subcutaneous catheter delivery. Additionally, microneedles were reported to cause less pain compared to catheter infusion sets.
The potential medical significance of this study is that microneedles may reduce pain and apprehension related with insulin delivery. With pain, anxiety, and fear of needles being the main reason for noncompliance among diabetes patients, we believe microneedles may provide a means to increase patient compliance. Further, designing the microneedle device to be a miniature integrated patch-like device without any tubing, bulky pumps, or catheter may further improve patient compliance by increasing comfort and convenience. Overall improved patient compliance would ultimately lead to reduced healthcare costs for diabetes patients due to potential lower frequency of hypo- and hyperglycemic events and related hospitalizations. As indicated previously, microneedles have been used to extract interstitial fluid from human subjects to successfully detect glucose levels.22 By combining the use of microneedles for insulin delivery and glucose monitoring, we envision that microneedles may ultimately be incorporated into a fully integrated closed-loop microdevice system for continuous glucose monitoring and insulin delivery. The results of this study bring us one step closer to developing such a minimally invasive solution for diabetes therapy.
Conclusions
Overall, this study demonstrated microneedle-based insulin delivery to two subjects with type 1 diabetes for the first time. Microneedles inserted 1 mm into the skin were able to effectively reduce postprandial glucose levels. A raised wheal at the surface of the skin immediately after insulin bolus infusion confirmed delivery of insulin into the intradermal space. Subjects reported minimal pain and preferred microneedle-based insulin delivery over subcutaneous catheter delivery. While it appears from the results of this study that microneedle-based delivery may have faster onset of action than subcutaneous catheter-based delivery, further studies with a larger patient population and equal insulin doses are required to confirm this hypothesis.
Acknowledgments
We thank Maureen McGrath, Jane McCurdy, and Megan Consendine for their assistance in carrying out this study. This work was supported by the Emory Egelston Seed Grant Program and was carried out at the Emory Children's Center.
Author Disclosure Statement
M.P. is a consultant, advisory board member, shareholder, and/or inventor on patents licensed to a number of companies developing microneedles for drug delivery applications. None of these companies yet has a microneedle-based product, and the microneedle device presented in this study is not directly related to any microneedle devices currently under development at these companies. E.F. and J.G. have no competing financial interests.
References
- 1.Cefalu WT. Concept, strategies, and feasibility of noninvasive insulin delivery. Diabetes Care. 2004;27:239–246. doi: 10.2337/diacare.27.1.239. [DOI] [PubMed] [Google Scholar]
- 2.Mollema ED. Snoek FJ. Heine RJ. van der Ploeg HM. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med. 2001;18:671–674. doi: 10.1046/j.1464-5491.2001.00547.x. [DOI] [PubMed] [Google Scholar]
- 3.Zambanini A. Newson RB. Maisey M. Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–246. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 4.Hanas R. Ludvigsson J. Experience of pain from insulin injections and needle-phobia in young patients with IDDM. Pract Diabetes Int. 1997;14:95–100. [Google Scholar]
- 5.Hanas R. Reducing injection pain in children and adolescents with diabetes: a review of indwelling catheters. Pediatr Diabetes. 2004;5:102–111. doi: 10.1111/j.1399-543X.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 6.Guy RH. Transdermal science and technology—an update. Jpn Soc Drug Deliv System. 2007;22:442–449. [Google Scholar]
- 7.Barnett AH. The future of inhaled insulin and incretinmimetics in the management of diabetes. Primary Care Diabetes. 2008;2:59–61. doi: 10.1016/j.pcd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Bailey CJ. Barnett AH. Why is Exubera being withdrawn? BMJ. 2007;335:1156. [Google Scholar]
- 9.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Prausnitz MR. Gill HS. Park JH. Microneedles for drug delivery. In: Rathbone MJ, editor; Hadgraft J, editor; Roberts JS, editor; Lane ME, editor. Modified Release Drug Delivery. 2nd. New York: Healthcare; 2008. pp. 295–309. [Google Scholar]
- 11.Gill HS. Denson DD. Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martanto W. Davis SP. Holiday NR. Wang J. Gill HS. Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21:947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y. Hagiwara E. Saeki A. Sugioka N. Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29:82–88. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Roxhed N. Samel B. Nordquist L. Griss P. Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55:1063–1071. doi: 10.1109/TBME.2007.906492. [DOI] [PubMed] [Google Scholar]
- 15.Davis S. Martanto W. Allen MG. Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 16.Gardeniers HJGE. Luttge R. Berenschot EJW. de Boer MJ. Yeshurun SY. Hefetz M. van't Oever R. van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Systems. 2003;12:855–862. [Google Scholar]
- 17.McAllister DV. Wang PM. Davis SP. Park JH. Canatella PJ. Allen MG. Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prausnitz MR. Mikszta JA. Cormier M. Andrianov AK. Microneedle-based vaccines. In: Compans RWOW, editor. Current Topics in Microbiology and Immunology: Vaccines for Pandemic Influenza. Berlin/Heidelberg, Germany: Springer-Verlag; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivamani RK. Stoeber B. Wu GC. Zhai H. Liepmann D. Maibach H. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res Technol. 2005;11:152–156. doi: 10.1111/j.1600-0846.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 20.Wermeling DP. Banks SL. Hudson DA. Gill HS. Gupta J. Prausnitz MR. Stinchcomb AL. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105:2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland D. Booy R. De Looze F. Eizenberg P. McDonald J. Karrasch J. McKeirnan M. Salem H. Mills G. Reid J. Weber F. Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 22.Wang PM. Cornwell M. Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7:131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- 23.Wang PM. Cornwell M. Hill J. Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 24.Bronaugh RL. Stewart RF. Congdon ER. Methods for in vitro percutaneous absorption studies. II. Animal models for human skin. Toxicol Appl Pharmacol. 1982;62:481–488. doi: 10.1016/0041-008x(82)90149-1. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence PF. Bell RM. Dayton MT. Essentials of Surgical Specialties. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]