Abstract
Growing evidence supports an active role for dysregulated macroautophagy (autophagic stress) in neuronal cell death and neurodegeneration. Alterations in mitochondrial function and dynamics are also strongly implicated in neurodegenerative diseases. Interestingly, whereas the core autophagy machinery is evolutionarily conserved and shared among constitutive and induced or selective autophagy, recent studies implicate distinct mechanisms regulating mitochondrial autophagy (mitophagy) in response to general autophagic stimuli. Little is known about pathways regulating selective, damage-induced mitophagy. We found that the parkinsonian neurotoxin MPP+ induces autophagy and mitochondrial degradation that is inhibited by siRNA knockdown of autophagy proteins Atg5, Atg7 and Atg8, but occurs independently of Beclin 1, a component of the class III (PIK3C3/Vps34) phosphoinositide 3-kinase (PI3K) complex. Instead, MPP+-induced mitophagy is dependent upon MAPK signaling. Interestingly, all treatments that inhibited autophagy also conferred protection from MPP+-induced cell death. A prior human tissue study further supports a role for ERK/MAPK-regulated autophagy in Parkinson’s and Lewy body diseases. As competition for limiting amounts of Beclin 1 may serve to prevent harmful overactivation of autophagy, understanding mechanisms that bypass or complement a requirement for PI3K-Beclin 1 activity could lead to strategies to modulate autophagic stress in injured or degenerating neurons.
Keywords: autophagy, mitochondria, Parkinson’s disease, mitogen activated protein kinases, neuronal cell death, oxidative stress
Mitochondria play critical roles in metabolism and cell survival/death decisions. Neurons are particularly dependent upon proper mitochondrial function to support high-energy demands of synaptic and axonal maintenance. Not surprisingly, alterations in mitochondrial function and dynamics are centrally implicated in many neurodegenerative diseases, including Parkinson’s disease (PD). The discovery that 1-methyl-4-phenylpyridinium (MPP+), the active metabolite of the parkinsonian neurotoxin MPTP, is a mitochondrial complex I inhibitor, stimulated intense interest in the role of mitochondrial dysfunction in PD,1 particularly as complex I dysfunction is observed in sporadic and familial PD patients.2 Mitochondrial oxidative stress has also been implicated in models related to catecholamine overload3 and pesticide exposures,4 which have been epidemiologically linked to PD.5 Kinases such as PTEN-induced kinase 1 (PINK1) and leucine-rich repeat kinase 2/dardarin (LRRK2) that are mutated in familial PD are at least partially associated with mitochondria.6,7 These recent findings highlight the potential importance of altered mitochondrial kinase signaling in parkinsonian neurodegeneration.8
MAPKs in regulation of autophagy and neuronal cell death
The extracellular signal-regulated kinase (ERK) branch of the mitogen-activated protein kinase (MAPK) family has been shown to play a dual role in neuronal survival and death.9,10 These contrary effects appear to be regulated by alterations in the kinetics and subcellular localization of ERK activation.11 We found that ERK contributes to neuronal cell injury and death in the 6-hydroxydopamine and MPP+ models of PD.12,13 In contrast to transient, nuclear signaling observed with trophic factors, 6-hydroxydopamine induces sustained, cytoplasmic ERK phosphorylation with significant mitochondrial localization.14
What are the potential downstream consequences of cytoplasmic, and particularly mitochondrial, ERK activation? Our recent study of MPP+ toxicity using SH-SY5Y neuroblastoma cells and primary dopaminergic midbrain neurons suggests a role in regulating mitophagy. MPP+ induces a robust increase in autophagic vacuoles (AVs) accompanied by loss of mitochondria proteins.13 Inhibitors of MEK/ERK signaling effectively block MPP+-induced autophagic vacuoles (AVs) and mitochondrial degradation, but do not prevent decreases in mitochondrial membrane potential or ultrastructural damage. Thus, ERK signaling appears to act downstream of mitochondrial injury in stimulating mitophagy. While it is currently unclear whether ERK promotes generalized or mitochondria-selective autophagy, or whether mitochondrial localization of activated ERK is essential, these studies indicate that identifying mitochondrial targets of ERK may offer clues into cargo recognition of damaged mitochondria.
ERK has been implicated in slowing AV maturation in response to the carcinogen lindane, which induces autophagic vacuolation through an mTOR-independent mechanism.15 In our system, however, there are several lines of evidence indicating active induction of autophagy by MPP+.13 These include data showing that maturation and degradation are preserved, and that AV increases are dependent upon Atg5, Atg7 and Atg8 levels. The possibility of concurrent lysosomal effects of ERK activity remains to be investigated.
Besides a role in regulating autophagy/mitophagy, mitochondrial ERK signaling causes reduced mitochondrial respiration,16 and cytoplasmic ERK phosphorylates and activates m-calpain.17 Interestingly, recent Autophagy addenda highlight papers showing stimulation of autophagy by decreased ATP/AMP ratio and by calpains.18,19 In addition to ERK, c-Jun N-terminal kinase (JNK) contributes to LC3 lipidation and loss of mitochondrial proteins during MPP+ injury,13 although the effects of the JNK inhibitor SP600125 were not as robust as the effects of MEK inhibitors. Thus, each of the major branches of the MAPK family, ERK,13,20 JNK,13,21 and p38 MAPK,22 has been implicated in regulating autophagy in different cell types and contexts.
Novel regulatory mechanisms of mitochondria-targeted autophagy?
Mitochondria could be targets of autophagic digestion in at least three scenarios: 1) basal, constitutive turnover, 2) starvation-induced turnover, and 3) enhanced degradation of damaged mitochondria. It is interesting to note that the yeast protein Uth1 is required for mitophagy in the context of bulk phase starvation- or rapamycin-induced autophagy.23 Moreover, loss of mitochondrial potential24 and increased lipid oxidation25 correlate with induction of mitophagy in hepatocytes and yeast, respectively. MPP+ does cause a progressive depletion of mitochondrial membrane potential and increased phospholipid oxidation (authors’ unpublished data), suggesting the possibility of oxidized macromolecular signals, including redox activation of ERK,14 in autophagic cargo recognition. The involvement of unknown mitochondrial phosphorylation targets is further supported by the observation that a yeast mitochondrial protein phosphatase related to mammalian PP2C was recently shown to be essential for mitophagy.26
One particularly interesting observation is the discovery that MPP+ induces autophagy through a mechanism independent of the class III phosphatidylinositol 3 kinase (PI3K/Vps34)-Beclin 1 pathway,13 which is essential for development- and deprivation-related autophagy. Neither wortmannin nor 3-methyladenine, two PI3K inhibitors used extensively to study physiological autophagy, were able to decrease MPP+-elicited AVs in either SH-SY5Y cells or primary dopaminergic neurons.13 Given opposing roles of class I and class III PI3Ks on autophagy induction,27 the effects of PI3K inhibitors may vary depending upon relative activation of the two classes. 3-Methyladenine also inhibits phosphorylation of Akt, JNK and p38 MAPK.28 Each of these pathways plays a prominent role in cell fate decisions, complicating interpretation of 3-methyladenine studies in the context of autophagy elicited during cell injury. We also investigated the effects of siRNA-mediated knockdown of Beclin 1, and found no effects on MPP+ elicited autophagy.13 Knockdown of core autophagy proteins that are essential for membrane extension, Atg5, Atg7, and Atg8, each effectively inhibited MPP+-induced autophagy,13 supporting the existence of a Beclin 1-PI3K-independent pathway of autophagy induction.
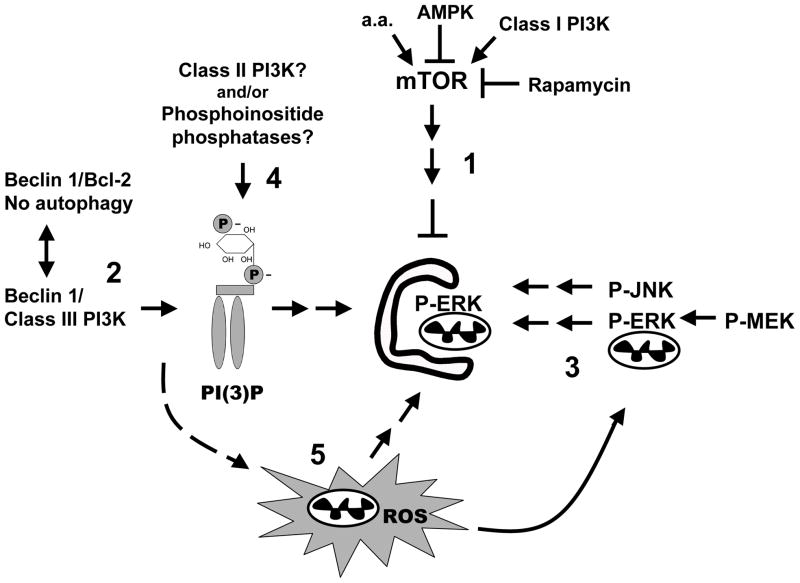
Potential mechanisms of Beclin 1-PI3K-independent autophagy include alternative pathways that increase phosphatidylinositol-3 phosphate (PI(3)P) levels (Fig. 1). For example, activation of phosphatases that remove the D4 phosphate from PI(3,4)P229 and/or inhibition of phosphatases involved in removing the D3 phosphate from PI(3)P30 could contribute to sufficient levels of PI(3)P to trigger autophagy. Additionally, class II PI3Ks that are resistant to wortmannin can produce both PI(3)P and PI(3,4)P2.31 Hypothetically, PI(3)P-independent mechanisms of vesicle nucleation/trafficking, or of FYVE/PX domain protein recruitment, are also possible. Intracellular ROS appear to be necessary for starvation-32 or rapamycin-induced25 autophagy, acting downstream of Beclin 1.32 While it is unknown whether or not mitochondrial ROS are sufficient to induce autophagy, it is possible that MPP+ activates autophagy downstream of Beclin 1 by directly contributing to elevations in mitochondrial ROS (Fig. 1).
Fig. 1. Schematic diagram of potential regulatory pathways for autophagy/mitophagy.
The most studied pathways of autophagy regulation, including mTOR-mediated suppression of autophagy, which is reversed by amino acid deprivation, rapamycin, or AMP-kinase (1), and the Beclin 1-class III PI3K pathways (2) are shown. In addition, there is cell type-dependent stimulation of autophagy by ERK and JNK signaling (3), although kinase targets remain unidentified. Alternative mechanisms for generating PI(3)P exist in mammalian cells, including wortmannin-resistant class II PI3Ks and phosphoinositide phosphatases (4, see text). Recent studies also suggest a role for ROS acting downstream of Beclin 1 to reduce cleavage of LC3 from preautophagosomal membranes (5). The signals involved in generating mitochondrial ROS during starvation remain undefined, but ROS are involved in mitochondrial activation of ERK. It is unknown if ERK acts directly to phosphorylate mitochondrial targets or if its effects on autophagy reflect other cytoplasmic sites of action.
Role of autophagy in neuronal injury and degeneration
Evidence has been accumulating that either too little or too much autophagy can be detrimental to cells. Harmful imbalances in autophagic regulation are conceptualized as a state of autophagic stress.33 Neurons may be particularly vulnerable to developing autophagic stress,33 in part due to the need for transport along neurites for long distances.34 Under physiological conditions, axons and dendrites are almost devoid of AVs and lysosomes. However, neurological disease states lead to robust increases in AVs and lysosomes.35–37 Impaired AV clearance might lead to autophagic stress in lysosomal storage diseases.38 Alternatively, autophagic stress could be caused by excessive autophagic flux that exceeds the capacity for regenerative protein and organelle synthesis.
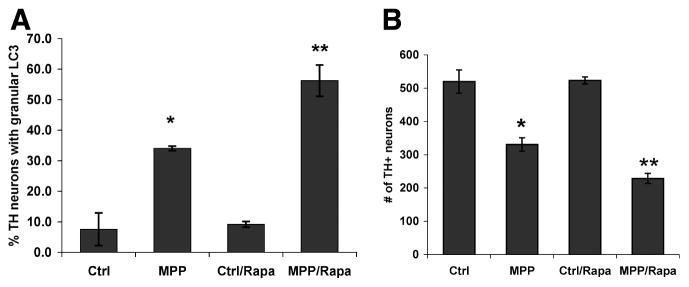
It has been proposed that competition between Bcl-2 and the autophagy machinery for limiting amounts of Beclin 1 may serve as a regulatory control to prevent harmful overactivation of autophagy.39 Thus, Beclin 1-independent autophagy/mitophagy may reflect an escape from homeostatic regulatory mechanisms in pathologic systems. Indeed, siRNA-mediated inhibition of damage-induced autophagy significantly reduced MPP+-mediated toxicity in SH-SY5Y cells.13 Additionally, our unpublished data indicate the reciprocal finding, that co-treatment with rapamycin enhanced AV content and cell death in MPP+-treated primary dopaminergic midbrain neurons (Fig. 2).
Fig. 2. Rapamycin co-treatment synergistically increases AV content and exacerbates MPP+-elicited TH neuron loss.
Primary mouse embryonic midbrain cultures were treated with MPP+ (5 micromolar) in the presence or absence of rapamycin (50 nanomolar) and analyzed at 24 h for AVs by LC3 immunohistochemistry (A) and at 48 h for TH+ neuron number (B). * p < 0.05 vs. Ctrl; ** p < 0.05 vs. MPP+ alone; ANOVA/Fisher’s LSD.
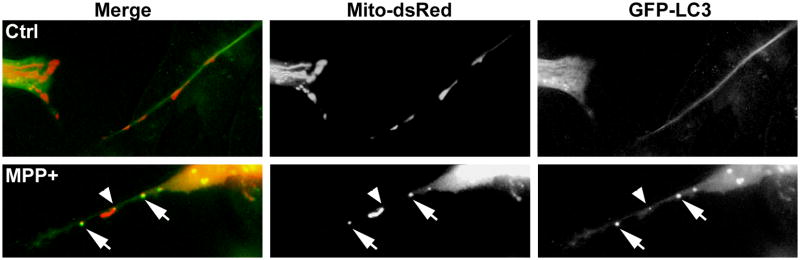
Interestingly, our unpublished studies indicate that increased AVs, some of which colocalized with mitochondria, are observed in neuronal processes of retinoic acid-differentiated neuronal cultures subjected to acute (Fig. 3), or chronic, low dose treatments of MPP+. It has been proposed that LC3-MAP1B interactions may contribute to remodeling of axons during excitotoxicity.37 Given the metabolic demands of maintaining dendrites and axons, involvement of autophagy in blunting or retraction of neurites may initially represent an adaptive response. Moreover, we propose that this neurodegenerative response comes with a cost -- loss of function and loss of target-derived trophic support, which would serve to tip the balance towards neuronal cell death. Likewise, eliminating impaired mitochondria or protein aggregates would be beneficial,40 but not if these rates exceed the regenerative capacity of the cell, or hypothetically, if proteins critical for neuronal function are nonselectively included in the AVs through a bystander mechanism.
Fig. 3. MPP+ elicits GFP-LC3 puncta that colocalize with mitochondria in neuronal processes.
SH-SY5Y cells were co-transfected with mitochondrially-targeted dsRed and GFP-LC3 for two days followed by retinoic acid-induced differentiation for an additional three days prior to treatment. In control cultures, GFP-LC3 (green) is diffuse in neurites with no overlap with mitochondrial profiles (red), and only rare, small somatic puncta, as seen in an adjacent cell (left side of panel). MPP+-treated cells develop GFP-LC3 puncta adjacent to (arrowhead) or colocalized (yellow, arrows) with mitochondrial profiles along neurites.
To summarize, distinct regulatory pathways are being discovered in yeast and mammalian systems for mitophagy and pathologically-induced autophagy on a background of conserved core Atg protein mechanisms. Our studies implicate mitochondrial ERK activation and Beclin 1-independent autophagy in neurite degeneration and cell death in response to the mitochondrial complex I inhibitor MPP+. While stimulation of autophagy holds promise for therapies to clear aggregated proteins implicated in neurodegenerative diseases,40 the prospects for future therapies may depend upon our ability to correct age- or disease-related factors that promote autophagic stress. Thus, relevant questions extend beyond debates over whether autophagy is “good” or “bad”, towards a greater emphasis on factors that contribute to pathological imbalances in the system and how this relates to disease.
Acknowledgments
The authors’ autophagy research has been supported by funding from the National Institutes of Health (NS040817, AG026389) and the University of Pittsburgh Center for the Environmental Basis of Human Disease.
References
- 1.Mandemakers W, Morais VA, De Strooper B. A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J Cell Sci. 2007;120:1707–16. doi: 10.1242/jcs.03443. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–71. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 3.Callio J, Oury TD, Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J Biol Chem. 2005;280:18536–42. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280:42026–35. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 5.Abbott RD, Ross GW, White LR, Sanderson WT, Burchfiel CM, Kashon M, Sharp DS, Masaki KH, Curb JD, Petrovitch H. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: recent findings from the Honolulu-Asia Aging Study. J Neurol. 2003;250(Suppl 3):III30–9. doi: 10.1007/s00415-003-1306-7. [DOI] [PubMed] [Google Scholar]
- 6.Abeliovich A, Flint Beal M. Parkinsonism genes: culprits and clues. J Neurochem. 2006;99:1062–72. doi: 10.1111/j.1471-4159.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- 7.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–69. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 8.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Current topics in medicinal chemistry. 2006;6:787–99. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- 10.Subramaniam S, Unsicker K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience. 2006;138:1055–65. doi: 10.1016/j.neuroscience.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–6. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: Implications for Parkinson’s disease. J Neurochem. 2001;77:1058–66. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulich S, Horbinski C, Patel M, Chu C. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Rad Biol Med. 2007 doi: 10.1016/j.freeradbiomed.2007.04.028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corcelle E, Nebout M, Bekri S, Gauthier N, Hofman P, Poujeol P, Fenichel P, Mograbi B. Disruption of autophagy at the maturation step by the carcinogen lindane is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Cancer Res. 2006;66:6861–70. doi: 10.1158/0008-5472.CAN-05-3557. [DOI] [PubMed] [Google Scholar]
- 16.Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–55. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glading A, Uberall F, Keyse SM, Lauffenburger DA, Wells A. Membrane-proximal ERK signaling is required for M-calpain activation downstream of EGF receptor signaling. J Biol Chem. 2001;276:23341–8. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- 18.Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy. 2007;3:238–40. doi: 10.4161/auto.3710. [DOI] [PubMed] [Google Scholar]
- 19.Demarchi F, Bertoli C, Copetti T, Eskelinen E-L, Schneider C. Calpain as a novel regulator of autophagosome formation. Autophagy. 2007;3:235–7. doi: 10.4161/auto.3661. [DOI] [PubMed] [Google Scholar]
- 20.Pattingre S, Bauvy C, Codogno P. Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem. 2003;278:16667–74. doi: 10.1074/jbc.M210998200. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy. 2006;2:39–46. doi: 10.4161/auto.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simone C. Signal-dependent control of autophagy and cell death in colorectal cancer cell: The role of the p38 pathway. Autophagy. 2007:3. doi: 10.4161/auto.4319. [DOI] [PubMed] [Google Scholar]
- 23.Kissova I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem. 2004;279:39068–74. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 24.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–7. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 25.Kissova I, Deffieu M, Samokhvalov V, Velours G, Bessoule JJ, Manon S, Camougrand N. Lipid oxidation and autophagy in yeast. Free Radic Biol Med. 2006;41:1655–61. doi: 10.1016/j.freeradbiomed.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem. 2007;282:5617–24. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 27.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–8. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 28.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–98. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 29.Ivetac I, Munday AD, Kisseleva MV, Zhang XM, Luff S, Tiganis T, Whisstock JC, Rowe T, Majerus PW, Mitchell CA. The type Ialpha inositol polyphosphate 4-phosphatase generates and terminates phosphoinositide 3-kinase signals on endosomes and the plasma membrane. Mol Biol Cell. 2005;16:2218–33. doi: 10.1091/mbc.E04-09-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nandurkar HH, Layton M, Laporte J, Selan C, Corcoran L, Caldwell KK, Mochizuki Y, Majerus PW, Mitchell CA. Identification of myotubularin as the lipid phosphatase catalytic subunit associated with the 3-phosphatase adapter protein, 3-PAP. Proc Natl Acad Sci U S A. 2003;100:8660–5. doi: 10.1073/pnas.1033097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcaro A, Zvelebil MJ, Wallasch C, Ullrich A, Waterfield MD, Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol Cell Biol. 2000;20:3817–30. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu CT. Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol. 2006;65:423–32. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993;121:305–15. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen A, Larsen KE, Behr GG, Romero N, Przedborski S, Brundin P, Sulzer D. Expanded CAG repeats in exon 1 of the Huntington’s disease gene stimulate dopamine-mediated striatal neuron autophagy and degeneration. Hum Mol Genet. 2001;10:1243–54. doi: 10.1093/hmg/10.12.1243. [DOI] [PubMed] [Google Scholar]
- 36.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 37.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–68. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shacka JJ, Klocke BJ, Young C, Shibata M, Olney JW, Uchiyama Y, Saftig P, Roth KA. Cathepsin D deficiency induces persistent neurodegeneration in the absence of Bax-dependent apoptosis. J Neurosci. 2007;27:2081–90. doi: 10.1523/JNEUROSCI.5577-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin Z-H, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]