Abstract
Background
Though the association between diabetes mellitus (DM) and pancreatic cancer is well described, temporal patterns of changes in fasting blood glucose (FBG) and body mass index (BMI) before pancreatic cancer diagnosis are not known.
Methods
We reviewed medical records of pancreatic cancer cases seen at Mayo Clinic from 1/15/1981 through 7/9/2004 and selected those residing within 120 miles of Rochester, Minnesota and seen at Mayo Clinic within 30 days from the date of cancer diagnosis (index date). We identified ~2 matched controls per case residing locally and seen at Mayo in the year of their case’s index date. For the 736 cases and 1,875 controls with at least one outpatient FBG measurement, we abstracted all FBG values and corresponding heights and weights up to 60 months before index and grouped them into 12-month intervals preceding index. We compared FBG and BMI in each interval between cases and controls.
Results
Mean FBG values were similar between cases, compared to controls, in the −60 to −48 (102 vs. 100 mg/dl, p=0.34), and −48 to −36 month intervals (106 vs. 102 mg/dl, p=0.09); but progressively increased in the −36 to −24 (105 vs. 100 mg/dl, p=0.01), −24 to −12 (114 vs. 102 mg/dl, p=0.001), and −12 to +1 (123 vs. 102 mg/dl, p<.0001) month intervals. Though mean BMI values were generally similar in cases and controls up to 12 months before index, they were significantly lower in cases vs controls in the −12 to +1 (p<.001) month intervals.
Conclusions
Pancreatic cancer is characterized by progressive hyperglycemia beginning up to 24 months before cancer diagnosis in the setting of decreasing BMI. Pancreatic cancer can potentially be diagnosed early if biomarkers are identified that can distinguish pancreatic cancer-induced DM from type 2 DM.
Keywords: Pancreatic cancer, diabetes, screening
INTRODUCTION
Pancreatic cancer has a dismal 5-year survival and is the fourth leading cause of cancer death in the United States1. At present, there is no accepted screening strategy for sporadic pancreatic cancer, and the disease is usually diagnosed at an advanced stage. Diabetes mellitus (DM) or hyperglycemia is common in pancreatic cancer patients2, 3; further understanding of the temporal association between these two clinical conditions may provide opportunities for early detection of pancreatic cancer.
The association between DM and pancreatic cancer is intriguing. On the one hand, there appears to be a modestly elevated risk of pancreatic cancer among individuals with long-standing DM; a recent meta-analysis reported a combined age- and sex-adjusted odds ratio (OR) of 1.8 (95% confidence interval (95% CI, 1.7–1.9)4. In contrast, there is increasing evidence suggesting that new-onset DM (<2 years in duration) can be induced by pancreatic cancer. Approximately 40 to 65% of pancreatic cancer patients meet criteria for DM at cancer diagnosis when evaluated by measurement of fasting blood glucose (FBG) or by oral glucose tolerance tests2, 3, 5, 6. In addition, there is a close temporal association between the onset of DM and the diagnosis of pancreatic cancer 2, 3, 7 with DM being new-onset in the majority of cases. In our nested case-control study of 736 pancreatic cancer patients (diagnosed from 1981 through 2004) and 1,875 age-and gender-matched controls, we found that the overall prevalence of DM in cases was significantly higher than in controls (40% vs 19%, p<0.001) and that DM was more often new-onset among cases vs. controls (52% vs 24%, p<0.001). When DM prevalence was evaluated in 12 month time intervals up to 60 months before date of cancer diagnosis (or corresponding date among controls), the proportion of cases with DM, compared to controls, was higher during the −36 to −24 month, −24 to −12 month, and −12 to +1 month time intervals3. It has also been observed that new-onset DM usually resolves following tumor resection2, 8, 9.
For the current study, we analyzed our nested case-control study data to investigate important additional questions regarding the role of two clinical correlates of DM in the time preceding diagnosis of pancreatic cancer, namely FBG levels and body mass index (BMI). We have clinically observed that even subjects with long standing diabetes exhibit worsening of glucose control before pancreatic cancer diagnosis. We therefore hypothesized that the pattern of change in FBG differs among pancreatic cancer patients based on their diabetic status (i.e., whether or not they met glycemic criteria for DM) and duration of DM (new-onset vs. long-standing).
Up to 80% of pancreatic cancer patients develop either DM or hyperglycemia2. Understanding the differences in clinical profile of patients who do and do not develop DM may provide clues to the pathogenesis of pancreatic cancer-induced DM. In a recent case-control study of a different sample of 512 pancreatic cancer patients and 933 matched controls, we noted that pancreatic cancer patients with DM at diagnosis, compared to those without DM, were more likely to be older, have a greater frequency of positive family history of DM, and a higher self-reported usual adult BMI2. We also examined these clinical correlates of DM in the present study.
Obesity is recognized as a modest risk factor for pancreatic cancer10. It is also a common clinical observation that pancreatic cancer patients lose weight prior to diagnosis of their cancer. In this study, we determined mean BMI at 12 month intervals for up to 60 months prior to the diagnosis of cancer in a pancreatic cancer cohort and compared it to BMI over the same time period in a cohort of controls.
MATERIALS AND METHODS
This study was approved by the Mayo Clinic Foundation Institutional Review Board.
Ascertainment of cases and controls
As described previously3, we searched the Mayo Diagnostic Index using the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) codes for pancreatic cancer (157.0–157.9, excluding 157.4 [malignant neoplasm of islets of Langerhans])11 and retrieved cases seen at Mayo Clinic, Rochester, Minnesota, from January 15, 1981 through July 9, 2004 and residing within a 120 mile radius of Rochester, the county seat of Olmsted County, Minnesota. We further restricted this search to individuals who were either first diagnosed at Mayo Clinic or seen at Mayo Clinic within 30 days of initial pancreatic cancer diagnosis. We reviewed the medical records of 1,172 cases thus identified to confirm the diagnosis of pancreatic adenocarcinoma (histologic confirmation present in 988 [84%] or compatible clinical presentation when histologic evidence was not available in 184 [16%]). For each case, we selected two Olmsted County residents who were seen at Mayo Clinic in the same calendar year as the matched case’s date of pancreatic cancer diagnosis (defined as the index date for each case and their control) and were matched to the case’s gender (same) and age (±1 year). Controls whose first Mayo Clinic encounter was less than 30 days from the index date were excluded.
Data collection and definitions
We electronically retrieved all outpatient FBG values in the Mayo records for 60 months before the index date for 1,172 cases and 2,344 controls. Of these individuals, the 736 cases and 1,875 controls who had at least one outpatient FBG value in the preceding 60 months or within one month following the index date (provided they received no treatment for pancreatic cancer) constituted the study population; further analyses were restricted to this subset of cases and controls. Inpatient and outpatient medical records were reviewed manually to abstract demographic and clinical data, including age, sex, parental history of DM, smoking status, and height (ht) and weight (wt) for calculating BMI (wt in kg/ht in m2) at each FBG throughout the period from 60 months before and through one month after the index date. The average BMI value >2 years before index was defined as usual adult BMI. Subjects were categorized as having DM if they had any FBG ≥126 mg/dl12. DM duration was classified as long-standing if subjects met criteria for DM for more than 24 months before the index date and as new-onset if the first date that subjects met criteria for DM was within 24 months before the index date and they had one or more previous FBG values <126 mg/dl.
Data analysis
Both outpatient FBG and BMI values in cases and controls were grouped in 12-month intervals from 60 months before the index date until the index date. For subjects without a FBG within the above specified 60 month interval, we included FBG values up to 30 days after the index date providing no surgical intervention had been performed before the FBG measurements. Therefore, the time intervals (windows) into which we categorized FBG and BMI values were −60 to −48 months, −48 to −36 months, −36 to −24 months, −24 to −12 months, and −12 to +1 months relative to index date.
All statistical analyses were performed using SAS 9.1.3 (SAS Institute Inc, Cary, NC). We compared mean FBG and BMI values between cases and controls in each time window and used linear regression analyses to assess the observed and predicted mean FBG and BMI in each time interval between cases and controls. We also compared the median FBG in each time window between cases and controls by overall DM status (diabetic vs. non-diabetic) and duration (new-onset vs. long-standing). Among pancreatic cancer cases (n=736), we compared the prevalence of conventional risk factors for DM (age, sex, parental history of DM, and usual adult BMI) between diabetic (n=296) and non-diabetic cases (n=440).
RESULTS
The demographic and clinical characteristics of the 736 cases and 1,875 controls included in the study are presented in Table 1. Overall, in the time period −60 to +1 month relative to index, mean FBG was higher among cases (126.5±50.5 mg/dl) compared to controls (104.7±31.1 mg/dl, p<0.001), and mean BMI was lower among cases compared to controls (26.7±5.4 vs. 27.3±5.3 kg/m2, p=0.01). As reported previously3, in the analysis of DM preceding index date, we found that a greater proportion of cases (296 of 736, 40.2%), compared to controls (360 of 1,875, 19.2%, p<.0001) met criteria for DM at any time in the 60 months before index.
Table 1.
Demographic and clinical characteristics of cases and controls over the time period of −60 to +1 months relative to index date.
± values represent standard deviation.
Date of pancreatic cancer diagnosis defined as index date for each case and their control. FBG, fasting blood glucose; BMI, body mass index (weight in kg/height in m2); To convert FBG values from mg/dl to mmol/L, multiply by 0.055.
| Cases (n=736) | Controls (n=1,875) | p-value | |
|---|---|---|---|
| Age (yr), mean ± std dev | 68.6 ± 11.3 | 68.7 ± 11.4 | 0.81 |
| Sex, male n (%) | 404 (54.9) | 992 (52.9) | .36 |
| FBG (mg/dl), mean ± std dev | 126.5 ± 50.5 | 104.7 ± 31.1 | <.001 |
| BMI, mean ± std dev | 26.7 ± 5.4 | 27.3 ± 5.3 | .01 |
| Parental history of DM, n (%) | 117 (17.4) | 311 (17.5) | .96 |
| Ever smokers, n (%) | 467 (65.9) | 1025 (56.2) | <.001 |
Temporal pattern of FBG in cases and controls
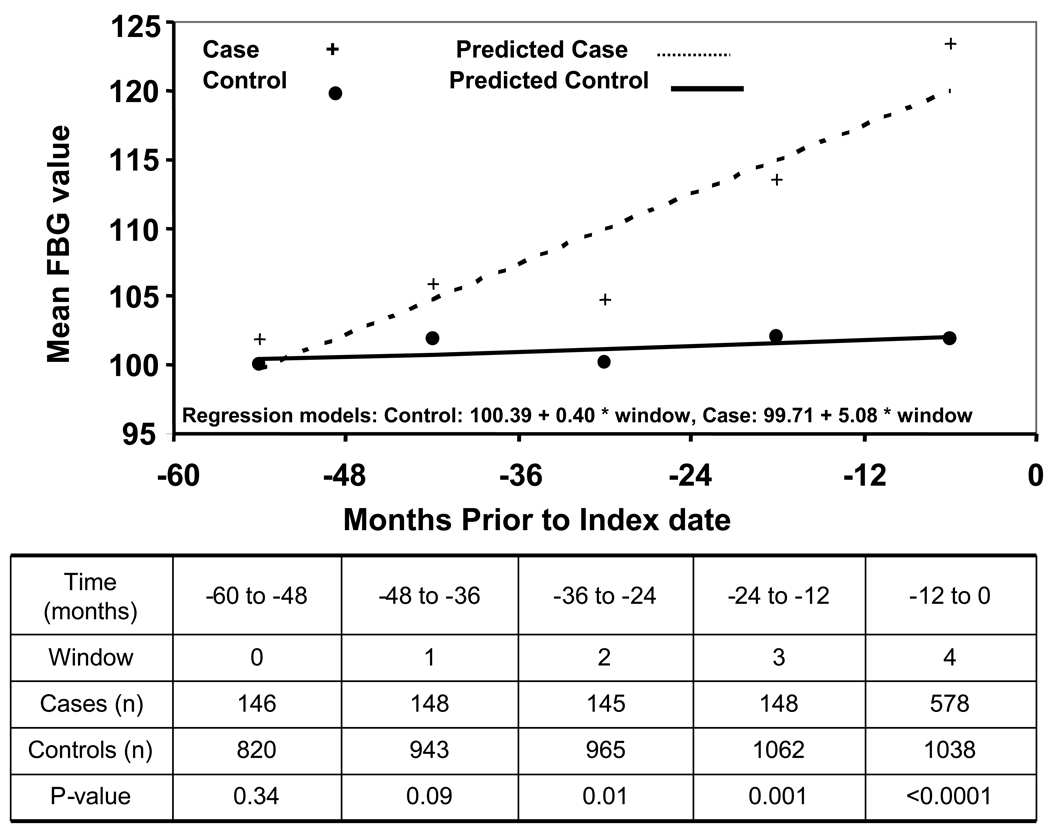
Mean FBG values were similar between cases and controls in the −60 to −48 (102 vs. 100 mg/dl, p=0.34) and −48 to −36 month intervals (106 vs. 102 mg/dl, p=0.09) but mean FBG values were progressively higher for cases compared to controls in the −36 to −24 (105 vs. 100 mg/dl, p=0.01), −24 to −12 (114 vs. 102 mg/dl, p=0.001), and −12 to +1 (123 vs. 102 mg/dl, p<.0001) month intervals (Figure 1). Median FBG was similar among non-diabetic cases and non-diabetic controls in the −60 to −48 (p=0.72), −48 to −36 (p=0.79), −36 to −24 (p=0.81), and −24 to −12 (p=0.1) month intervals but was higher among nondiabetic cases (104 mg/dl) vs. nondiabetic controls (96 mg/dl, p<0.001) in the −12 to +1 month time interval (Figure 2). Among diabetic subjects with new-onset DM (<2 yr duration), cases had a significantly lower median FBG at −60 to −48 month interval compared to controls (96 vs. 109 mg/dl, p=0.005). Cases with new-onset DM also had a generally lower median FBG than controls with new-onset DM in the −48 to −36 (p=0.63), −36 to −24 (p=0.08), and −24 to −12 (p=0.03) month intervals. However, median FBG was significantly higher among cases with new-onset DM vs. controls with new-onset DM in the −12 to +1 months relative to index date (134 vs. 124 mg/dl, p=0.003).
Figure 1.
Observed and predicted fasting blood glucose at 12 month intervals up to 60 months before index date among pancreatic cancer cases and controls Date of pancreatic cancer diagnosis defined as index date for each case and their control. FBG, fasting blood glucose; to convert FBG values from mg/dl to mmol/L, multiply by 0.055.
Figure 2.
Median fasting blood glucose by DM status and duration at 12 month intervals up to 60 months before index date among pancreatic cancer cases and controls Date of pancreatic cancer diagnosis defined as index date for each case and their control. DM diabetes mellitus, FBG fasting blood glucose, new-onset DM < 2 yr in duration, long standing DM ≥2 yr in duration
Among diabetic subjects with long-standing DM, cases and controls had similar median FBG in all time intervals except for −12 to +1 months, where cases had a significantly higher median FBG (178 vs. 146 mg/dl, p=0.002).
Prevalence of conventional DM risk factors in pancreatic cancer-associated DM
Pancreatic cancer patients with DM, compared to those without DM, were older (mean age 70±10 vs. 68±12, p=0.02), had a higher usual adult BMI (28±6 vs. 26±5 kg/m2, p<.001), and a greater frequency of positive parental history of DM (24 vs. 14%, p<.001) (Table 2).
Table 2.
Prevalence of conventional diabetes risk factors among pancreatic cancer patients with and without diabetes mellitus.
DM diabetes mellitus, BMI body mass index (kg/m2), usual adult BMI defined as BMI ≥2 years prior to index date
| Pancreatic cancer | |||
|---|---|---|---|
| Variable | DM (n=296) | No DM (n=440) | p-value |
| Age (yr), mean±std dev | 70±10 | 68±12 | 0.02 |
| Sex (% male) | 56 | 54 | 0.7 |
| Parental history of DM (% positive) | 24 | 14 | 0.001 |
| Usual adult BMI (kg/m2), mean±std dev | 28±6 | 26±5 | <.001 |
Temporal pattern of BMI in cases and controls
Compared to controls, cases had either similar (−60 to −48 month interval, p=0.07 and −36 to −24 month interval, p=0.17) or higher mean BMI (−48 to −36 month interval, p=0.01 and −36 to −24 month interval, p=0.04) (Figure 3). However, mean BMI was lower among cases in the −12 to +1 months (p<.001) before the index date.
Figure 3.
Observed and predicted body mass index at 12 month intervals up to 60 months before index date among pancreatic cancer cases and controls Date of pancreatic cancer diagnosis defined as index date for each case and their control. BMI body mass index (kg/m2)
DISCUSSION
The temporal association between changes in FBG and the diagnosis of pancreatic cancer has not been previously studied. In the present study, we evaluated the temporal pattern of FBG and BMI up to 60 months before the index date among 736 pancreatic cancer cases and 1,875 controls. FBG values were comparable in cases and controls in the −60 to −48 and −48 to −36 month time intervals. A progressive increase in FBG was seen among pancreatic cancer cases beginning 36 months before the diagnosis of cancer. This likely reflects the development of new-onset diabetes in over 50% of pancreatic cancer cases, as was seen in our previous studies2, 3, 13. Further, we and others have reported that new-onset DM that develops proximal to a diagnosis of pancreatic cancer improves following resection of cancer2, 8, 9. Together, these data provide increasing evidence that new-onset DM associated with pancreatic cancer is induced by the cancer.
A significant increase in FBG among all subsets (non-diabetic, new-onset, and long-standing DM) of pancreatic cancer cases, compared to controls, in the −12 to +1 month time interval suggests that pancreatic cancer is a profoundly diabetogenic state, which affects glucose metabolism in most, if not all patients. Why then do some cancer patients develop DM and others do not? We propose that development of diabetes in pancreatic cancer is the result of interaction between the powerful diabetogenic milieu induced by the cancer and the susceptibility of the host to develop DM14. Data from the present study suggest that those with increased risk of developing DM, i.e., those who have conventional risk factors for DM, such as older age, obesity, and parental history of DM, are more likely to develop DM when pancreatic cancer develops compared to those who do not have any risk factors for DM. These observations are similar to our recent report in a different study population of pancreatic cancer cases with (n=240) and without (n=269) DM where we found that the prevalence of conventional DM risk factors was significantly higher among pancreatic cancer cases with DM compared to those without DM2. The above observations also suggest that a distinction between type 2 DM and pancreatic cancer induced DM based solely on these clinical profiles is likely to be difficult.
Weight loss, which is often profound, is an important and common symptom of pancreatic cancer. It is also well recognized that obesity is a modest risk factor for pancreatic cancer10. However, the temporal pattern of change in BMI prior to the diagnosis of pancreatic cancer, especially in comparison to age-and sex-matched controls, has previously not been investigated. We found that pancreatic cancer patients have a progressive decline in their BMI starting approximately 12 months before the diagnosis of cancer. We have also clinically observed that pancreatic cancer patients with severe new-onset diabetes often rapidly lose weight many months before the onset of cancer-associated symptoms of cachexia (i.e., anorexia, fatigue and weakness from muscle wasting). We have previously reported that the median time from the onset of the first cancer-related symptom to diagnosis of pancreatic cancer in our study population was 2 months (range 0.5 to 36 months)3. In comparing the temporal trends in FBG and BMI among pancreatic cancer patients, our results suggest that the marked increase in FBG is concurrent with a significant decline in BMI, particularly in the 12 months preceding the diagnosis of cancer.
The high prevalence of new-onset DM in pancreatic cancer suggests that the incidence of pancreatic cancer would be higher among individuals with new-onset DM than the general population. In our previous population based study individuals with new-onset DM had an approximately 8-fold higher likelihood of being diagnosed with pancreatic cancer within 3 years of meeting criteria for DM, compared to the general population15. However, pancreatic cancer patients do not have cancer-specific symptoms until late in the course of their disease and consequently studies that have screened individuals with new-onset DM who have cancer-specific symptoms reported mostly unresectable disease16, 17.
For DM or hyperglycemia to be useful as a screening tool, it should be an early phenomenon that is associated with early stage pancreatic cancer in asymptomatic individuals. The increase in FBG noted in the present study and the higher prevalence of DM among cases3, compared to controls, nearly 12 to 24 months before cancer diagnosis suggests that new-onset hyperglycemia or DM is an early phenomenon in pancreatic cancer. Previous studies based on retrospective review of computed tomography scans have suggested that pancreatic cancer is either not detectable or is resectable on scans performed >6 months before clinical diagnosis18, 19. We have previously reported that approximately 50% of stage I/II pancreatic cancer patients have DM2. Therefore, new-onset diabetes not only defines a high-risk group for pancreatic cancer but also appears to be a marker of early, asymptomatic cancer. Its occurrence in nearly half the patients with pancreatic cancer makes it an attractive screening criterion for early pancreatic cancer and suggests a ‘window of opportunity’ to detect pancreatic cancer earlier using hyperglycemia as a marker.
However, at the present time there remain significant challenges to the use of a hyperglycemia or DM-based strategy to screen for pancreatic cancer. Given the relatively low incidence of pancreatic cancer and the vast number of individuals with type 2 DM, screening for pancreatic cancer among asymptomatic individuals, in our opinion, is likely to require a two-step process20. New-onset DM appears to be an attractive first sieve to enrich the general population and define a subset that is at a higher risk for pancreatic cancer. However, the population of new-onset diabetics potentially linked to pancreatic cancer will need to be further enriched through a second sieve, which may be a unique clinical phenotype or biomarker for pancreatic cancer induced DM3, 20. Presently, a reliable biomarker for early pancreatic cancer or pancreatic cancer induced DM is not available. Some putative mediators such as pancreatic cancer derived S100A8 N-terminal peptide21 and connexin2622 have been proposed, but data are preliminary. An improved understanding of the pathogenesis of pancreatic cancer induced DM and the differences between this entity and type 2 DM is likely to be critical in the identification of a unique biomarker for pancreatic cancer induced DM and its further validation as a screening tool.
An important limitation of our study is that many pancreatic cancer cases were seen at Mayo Clinic only once, close to the date of diagnosis of their cancer, and therefore did not have previous FBG values in our medical records. These individuals contributed to the FBG values in the −12 to +1 month time interval. However, our results did not differ when we restricted our analyses to cases that had been seen at least 6 months before the diagnosis of the cancer, at a time when they were likely to be asymptomatic (data not shown). Also, our data on FBG and BMI is not longitudinal, i.e, patients did not have FBG and BMI values available at every time interval. Therefore, we could not calculate the rate of change in either FBG or BMI between time intervals. However, given the low incidence of pancreatic cancer, a prospective cohort study examining these temporal trends is likely to be challenging to execute.
In conclusion, we report that pancreatic cancer patients, compared to age-and sex-matched controls, have higher FBG beginning at 24 to 36 months before the diagnosis of their cancer. This increase in FBG is concurrent with a gradual decline in the BMI, especially up to 12 months before cancer diagnosis. Onset of hyperglycemia up to 24 months before the diagnosis of cancer defines a time frame in which future use of biomarkers and risk stratification may discriminate pancreatic cancer-associated diabetes from type 2 diabetes mellitus and facilitate earlier diagnosis of pancreatic cancer.
STUDY HIGHLIGHTS
WHAT IS CURRENT KNOWLEDGE?
Diabetes is common in pancreatic cancer and is predominantly new-onset (<2 years duration).
Temporal pattern of changes in fasting blood glucose and body mass index prior to diagnosis of pancreatic cancer have not been studied.
WHAT IS NEW HERE?
There is a progressive increase in fasting blood glucose in pancreatic cancer patients beginning up to 24 months before cancer diagnosis.
Pancreatic cancer patients have gradual decline in the BMI, especially up to 12 months before cancer diagnosis.
This study suggests a time frame in which future use of biomarkers and risk stratification may discriminate pancreatic cancer-associated diabetes from type 2 diabetes mellitus and facilitate earlier diagnosis of pancreatic cancer.
Acknowledgments
GRANT SUPPORT
Dr Chari’s research was funded by grants from NIH (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701).
Footnotes
Financial Disclosures: None
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, Petersen GM. Pancreatic Cancer-associated Diabetes Mellitus: Prevalence and Temporal Association with Diagnosis of Cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–493. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–107. [PubMed] [Google Scholar]
- 7.Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. Italian Pancreatic Cancer Study Group. N Engl J Med. 1994;331:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 8.Fogar P, Pasquali C, Basso D, Sperti C, Panozzo MP, Tessari G, D'Angeli F, Del Favero G, Plebani M. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res. 1994;14:2827–2830. [PubMed] [Google Scholar]
- 9.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–1050. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) 2007;Volume 2007 [Google Scholar]
- 12.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30:S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 13.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–645. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 14.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncology. 2009;10(1):88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chari ST, Leibson CL, de Andrade M, Rabe KG, Ransom JE, Petersen GM. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa Y, Tanaka M, Inoue K, Yamaguchi K, Chijiiwa K, Mizumoto K, Tsutsu N, Nakamura Y. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344–2349. doi: 10.1002/cncr.10493. [DOI] [PubMed] [Google Scholar]
- 17.Damiano J, Bordier L, Le Berre JP, Margery J, Dupuy O, Mayaudon H, Bauduceau B. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes & Metabolism. 2004;30:203–207. doi: 10.1016/s1262-3636(07)70111-8. [DOI] [PubMed] [Google Scholar]
- 18.Gangi S, Fletcher JG, Nathan MA, Christensen JA, Harmsen WS, Crownhart BS, Chari ST. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. Am J Roentgenol. 2004;182:897–903. doi: 10.2214/ajr.182.4.1820897. [DOI] [PubMed] [Google Scholar]
- 19.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–2163. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 20.Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007;34:284–294. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basso D, Valerio A, Seraglia R, Mazza S, Piva MG, Greco E, Fogar P, Gallo N, Pedrazzoli S, Tiengo A, Plebani M. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer F, Koczan D, Adam U, Benz S, von Dobschuetz E, Prall F, Nizze H, Thiesen HJ, Hopt UT, Lobler M. Expression of connexin26 in islets of Langerhans is associated with impaired glucose tolerance in patients with pancreatic adenocarcinoma. Pancreas. 2004;29:284–290. doi: 10.1097/00006676-200411000-00007. [DOI] [PubMed] [Google Scholar]