Abstract
Background
Diarrheagenic E. coli are being recognized as important pediatric enteropathogens worldwide. However, it is unclear whether there are differences in age-related susceptibility to specific agents, especially among infants.
Methods
We conducted a passive surveillance diarrhea cohort study of 1034 children from 2 to 12 months of age in Lima, Perú. Control stool samples were collected from randomly selected children without diarrhea. All samples were analyzed for common enteric pathogens and for the diarrheagenic E. coli by a multiplex real-time PCR.
Results
The most commonly isolated pathogens from 1065 diarrheal episodes were the diarrheagenic E. coli (31%), including enteroaggregative (15.1%) and enteropathogenic E. coli (EPEC) (7.6%). Diarrheagenic E. coli, Campylobacter and rotavirus were more frequently isolated from infants ≥ 6m. Diffusely adherent E. coli and enterotoxigenic E. coli (ETEC) were more frequently isolated in diarrheal samples than in controls in older infants (p<0.05). Children ≥ 6m infected with ETEC had a 4.56-fold increased risk for diarrhea (95% CI, 1.20 to 17.28). Persistent diarrhea was more frequent in infants < 6m (13.5% vs. 3.6%, p<0.001). Among diarrheagenic E. coli positive samples, co-infections with other pathogens were more common in diarrhea than in controls (40.1% vs. 15.6%, p<0.001).
Conclusions
Diarrheagenic E. coli were more frequently isolated in older infants. In this setting with high frequency of pathogen exposure and high frequency of breastfeeding, we hypothesize that the major age-related differences result from decreased exposure to milk protective factors and with increased exposure to contaminated food and water.
Keywords: diarrheagenic E. coli, age-related susceptibility, diarrhea, infants, Peru
Introduction
Diarrhea is the third most common cause of death in children less than 5 years of age in the world, accounting for 1.87 million deaths/yr [1]. The diarrheagenic E. coli as a group are responsible for 30-40% of acute diarrhea in children [2] and are being recognized as important pathogens even in the developed world [3, 4]. In a recent review made for the World Health Organization, Enteropathogenic (EPEC) and Enterotoxigenic E. coli (ETEC) were listed as the highest priority for vaccine development after rotavirus because of their high morbidity and mortality rates [5].
E. coli associated with diarrhea have been classified into 6 groups based on clinical, epidemiological and molecular criteria: (1) EPEC; (2) ETEC; (3) Shigatoxin producing E. coli (STEC), also called enterohemorrhagic E. coli (EHEC) or Verotoxin producing E. coli (VTEC); (4) enteroinvasive E. coli (EIEC); (5) enteroaggregative E. coli (EAEC or EAggEC); and (6) diffusely adherent E. coli (DAEC) [6]. Each group has specific virulence factors that are used for identification and classification, as well as specific serotypes. However, with the exception of STEC (especially E. coli 0157:H7), the diarrheagenic E. coli are not routinely sought in most clinical laboratories.
The major disease burden [7] and the highest mortality rates occur in the first year of life [8]. However, given the limited number of studies conducted in this population [9-12] it is still unclear whether there are differences in age-related susceptibility to infection with diarrheagenic E. coli during the first year of life. The aims of this study were: 1) to determine if isolation of diarrheagenic E. coli and other common pathogens is associated with diarrhea in Peruvian infants <12mo, 2) to determine whether there are important age-related differences, and 3) to describe the pathogen-specific clinical courses.
Patients and Methods
Study Design
This prospective diarrhea cohort study was conducted in low socio-economic communities of periurban Lima (Districts of Chorrillos, Villa El Salvador, Villa Maria de Triunfo and San Juan de Miraflores).
Patients
After a house-by-house census by trained field workers, children were identified and enrolled in the study clinic in Chorrillos at 2 months of age after written informed consent was obtained from their parents. Enrollment was at 2mo instead of birth because that is the age that children were being enrolled for a coincident vaccine clinical trial (new formulation of a pediatric vaccine).
Definitions
Diarrhea was defined as ≥ 3 liquid or semi-liquid stools passed in a 24 hour period or ≥ 1 loose stool with blood [13]. A diarrheal episode was considered to have started the day the diarrhea definition was met and ended the last day of diarrhea followed by 2 or more consecutive days during which the child did not meet the diarrhea definition [13]. Persistent diarrhea was defined as diarrhea that lasted >14 days.
Diarrhea surveillance
This was a passive surveillance study. Parents were asked to bring the child to the study clinic every time the child developed a diarrhea that needed medical attention. At the clinic, a study physician obtained a detailed history and performed a complete physical exam to evaluate the diarrheal episode characteristics. A modified Vesikari score [14] was used to determine the severity of episodes. The score included duration of diarrhea (0-3 points), maximum number of stool/day (1-3), number of days with vomiting (0-3), maximum number of emesis episodes/day (0-3), presence of fever (0-1), dehydration (0-3), and treatment (0-2). A mild score was 0-8 points, moderate 9-14 points, and severe 15-18 points. We attempted to obtain a stool sample from all diarrheal episodes at the study clinic and episodes that required hospitalization or an outpatient visit outside our study clinic. Treatment including oral rehydration solution (ORS) and antibiotics was provided if needed, as well as advice regarding dietary management. For all detected diarrheal episodes a study form was filled at the clinic and the parents received a form to fill to record daily symptoms. A field worker visited the child at home at least once a week until the episode ended.
Control stool samples
A stool sample was obtained from enrolled infants when they were healthy (as defined by absence of diarrhea one week before and after the control stool sample was collected) to evaluate colonization. 516 children were randomly selected for stool sampling at 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 months. A field worker visited these children at home to collect these samples.
Laboratory studies
Stool samples were inoculated into Cary-Blair medium and clean vials, and transported in a cooling container to the laboratory in 4 hours. Samples were analyzed for the presence of enteric viruses, bacteria and parasites using conventional and molecular techniques. ELISA was used for rotavirus; direct exam for eggs of parasites; routine stool cultures were used to detect Salmonella, Shigella, Campylobacter and Vibrio sp. A multiplex real time PCR was utilized for the detection of diarrheagenic E. coli; 5 lactose fermenting colonies suggestive of E. coli from each McConkey plate were analyzed for the presence of the genes diagnostic of diarrheagenic E. coli : aggR (EAEC), st1, st2, lt (ETEC), eae (EPEC), eae, stx1, stx2 (STEC), ipaH (EIEC), and daaD (DAEC) [15]. All isolated EPEC strains were evaluated for the presence of bfpA in a separate PCR reaction [16]. Strains that were eae+/bfp+ were considered “typical” EPEC whereas eae+/bfp- strains were considered “atypical” EPEC.
Data analysis
We tested age-differences (children <6mo and ≥ 6mo) on the incidence of diarrhea calculating the individual incidence of diarrhea for each child at each age category and using the Student paired t test. To take into account within-individual clustering of stool samples and diarrheal episodes we used random-effects models. To test age-differences on the mean duration of diarrhea we used random-effects linear regression. To test age-differences in the proportion of persistent diarrhea, the proportion of episodes in which at least one pathogen was isolated, and the odds of diarrhea given the isolation of each individual pathogen we used random-effects logistic regressions. All the statistical analysis was performed using STATA version 10.1 (Stata Corp). A significance level of p < 0.05 was used.
Ethical Considerations
The study was approved by the Ethical Review Board of the Instituto de Investigación Nutricional, Universidad Peruana Cayetano Heredia, and the Naval Medical Research Center Detachment.
Results
1034 Peruvian children were followed between September-2006 and December-2007. 42 children were lost to follow up so only their observed days were analyzed. The remaining children were administratively censored when the study clinic was closed. There were 400,984 child/days of observation with 1,065 diarrheal episodes detected. 43% of episodes were in children < 6 months of age. The incidence of diarrhea that required medical attention was 0.98 episodes/child/year. The mean duration of diarrheal episodes was 5.9 ± 5.1 days. There were 494 children (47.8%) with no diarrhea episodes, 279 (27%) with one episode, 129 (12.5%) with 2 episodes, 65 (6.3%) with 3 episodes, 37 (3.6%) with 4 episodes and 30 (2.9%) with >5 episodes requiring medical attention (one child had 10 episodes). The comparison of the diarrhea surveillance data between children <6 months of age and older infants is presented on Table1. The incidence and duration of diarrhea were significantly higher in younger infants. There were 84 persistent diarrhea episodes (7.9%), with a higher proportion of persistent episodes in younger children. However, the isolation of common enteric pathogens was significantly higher in older infants. The socio-demographic characteristics of these children are described on Table 2. Of interest, 80.7% of children were exclusively breastfed at 6 months and 54.3% had some breastfeeding at 12 months of age.
Table 1.
Diarrhea surveillance data in 1034 Peruvian infants.
| Children < 6 months | Children ≥ 6 months | |
|---|---|---|
| Observed child/days | 137,654 | 263,330 |
| Observed child/days with diarrhea | 3,264 | 2,994 |
| Number of diarrheal episodes | 458 | 607 |
| Passive surveillance diarrhea incidence, episodes/child/year | 1.24 | 0.85 a |
| Diarrhea average prevalence, % | 2.37 | 1.14 |
| Duration of diarrhea episodes, mean ± SD | 7.1 ± 6.1 | 4.9 ± 3.8 |
| median (range) | 6 (1 – 43) | 4 (1 – 31) b |
| Persistent diarrhea, n (%) | 62 (13.5) | 22 (3.6) b |
| Diarrheal episode with a pathogen isolated, n (%) | 138 (34.0) | 337 (63.6) b |
p= 0.0018,
p< 0.0001
Table 2.
Socio-demographic characteristics of children in the study.
| n=1034 | |
|---|---|
| Gender, % male | 50.9 |
| Birth weight in Kg, mean ± SD | 3.4 ± 0.4 |
| Breastfeeding of any duration, % | 98.7 |
| Exclusive breastfeeding at 6 months, % | 80.7 |
| Partial breastfeeding at 12 months, % | 54.3 |
| Age of start of infant formula, mean ± SD | 8.6 ± 3.9 |
| Age of start of solid food, mean ± SD | 5.9 ± 0.6 |
| Maternal age, mean ± SD | 25.9 ± 6.4 |
| Maternal gestations, mean ± SD | 2.2 ± 1.3 |
| Maternal education, % (n) | |
| Only Grammar school | 98 (9.5) |
| High school | 739 (71.5) |
| Technical school or University | 187 (18.1) |
| Paternal age, mean ± SD | 29.1 ± 7.3 |
| Paternal education, % (n) | |
| Only Grammar school | 55 (5.3) |
| High school | 771 (74.6) |
| Technical school or University | 189 (18.3) |
936 stool samples were collected during diarrhea (87.9% of all episodes) and 424 control samples were collected from children without diarrhea (82.2% of 516 planned controls). The most common pathogens in diarrheal samples (table 3) were the diarrheagenic E. coli (31.0%), Campylobacter (18.6%) and rotavirus (17.2%). Among the diarrheagenic E. coli, EAEC (15.1%) and EPEC (7.6%) were the most common. All the pathogens were more frequently isolated from older infants (> 6 months of age) than younger infants (p<0.001). Atypical EPEC were much more common than typical EPEC; bfp- strains represented 87.3% (62/71) of all eae+ strains in diarrhea samples and 88.1% (37/42) of all eae+ control samples. Among ETEC strains, ETEC-LT was most common (13/30, 43.3%), followed by ETEC-ST (11/30, 36.7%) and ETEC-LT-ST (6/30, 20%). All STEC strains were eae+ and stx1+; there were no sxt2+ strains. No EIEC, Vibrio cholerae or intestinal parasites were detected. The incidence rate for all diarrheagenic E. coli was 0.25 episodes/child/year; the incidence rate by age group for each pathogen is listed in table 4.
Table 3.
Pathogens isolated in diarrhea and control samples (without diarrhea) in Peruvian infants.
| Pathogen | Children < 6 months | Children ≥ 6 months | All children | |||
|---|---|---|---|---|---|---|
| Diarrhea (n=406) n (%) |
Control (n=153) n (%) |
Diarrhea (n=530) n (%) |
Control (n=271) n (%) |
Diarrhea (n=936) n (%) |
Control (n=424) n (%) |
|
| EAEC | 47 (11.6) | 22 (14.4) | 94 (17.7) | 54 (19.9) | 141 (15.1) | 76 (17.9) |
| EPEC | 12 (3.0) | 10(6.5) | 59 (11.1) | 32 (11.8) | 71 (7.6) | 42 (9.9) |
| DAEC | 17 (4.2) | 5 (3.2) | 26 (4.9) | 4 (1.5) a | 43 (4.6) | 9 (2.1) |
| ETEC | 9 (2.2) | 2 (1.3) | 21 (4.0) | 3 (1.1) b | 30 (3.2) | 5 (1.2) |
| STEC | 1 (0.3) | 0 (0) | 4 (0.8) | 5 (1.8) | 5 (0.5) | 5 (1.2) |
| All diarrheagenic E. coli | 86 (21.2) | 39 (25.5) | 204 (38.5) | 96 (35.4) | 290 (31.0) | 135 (31.8) |
| Campylobacter | 48 (11.8) | 9 (5.9) c | 126 (23.7) | 54 (19.9) | 174 (18.6) | 63 (14.9) |
| Salmonella | 0 (0) | 1 (0.7) | 0 (0) | 1 (0.4) | 0 (0) | 2 (0.5) |
| Shigella | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.1) | 0 (0) |
| Rotavirus d | 24 (5.9)e | - | 137 (28.1)f | - | 161 (17.2) | - |
p=0.023,
p=0.026 and
p=0.043, for the comparison between diarrhea and control samples for each pathogen.
Studies for rotavirus were not done in control samples. Stools were evaluated for rotavirus in:
382 samples of children < 6m with diarrhea;
469 samples in children ≥ 6m because there was not sufficient stool sample in all cases.
Table 4.
Incidence rate by age group for each isolated pathogen in diarrhea episodes.
| Pathogen b | Children < 6 m a | Children ≥ 6 m a | All children a |
|---|---|---|---|
| EAEC | 0.13 | 0.13 | 0.13 |
| EPEC | 0.03 | 0.08 | 0.07 |
| DAEC | 0.05 | 0.04 | 0.04 |
| ETEC | 0.02 | 0.03 | 0.03 |
| All diarrheagenic E. coli | 0.23 | 0.26 | 0.25 |
| Campylobacter | 0.13 | 0.18 | 0.16 |
| Rotavirus | 0.07 | 0.19 | 0.15 |
Incidence rate (episodes/child/year);
Rates for STEC, Salmonella and Shigella, not included due to very low number of samples.
The comparison of isolation rates (diarrhea vs. control) showed that all pathogens were isolated at similar rates with the exception of three groups: ETEC, DAEC, and Campylobacter. ETEC and DAEC were more frequently isolated from diarrheal samples than controls in older infants (Table 3). In contrast, Campylobacter was significantly associated with diarrhea in younger infants, although frequency of infection increased with age. Children older than 6 months of age infected with ETEC had a 4.56-fold increased risk for diarrhea (95% CI, 1.20 to 17.28, p=0.026). In the same age group, children infected with DAEC had a 3.81-fold increase risk for diarrhea (95%CI, 1.20 to 12.12, p=0.023).
Co-infections were common, accounting for 13.3% of diarrheal and 5% of control samples (p<0.001). The most common co-infections in diarrhea samples were rotavirus/EAEC (24 cases), rotavirus/campylobacter (15 cases) and campylobacter/EAEC (14 cases). Among control samples the most common co-infections were campylobacter/EAEC (11 cases) and campylobacter/EPEC (6 cases). Among diarrheagenic E. coli positive samples, co-infections with other pathogens were significantly more common in diarrhea than in control samples (40.1% vs. 15.6%, p<0.001) (Figure 1).
Figure 1.
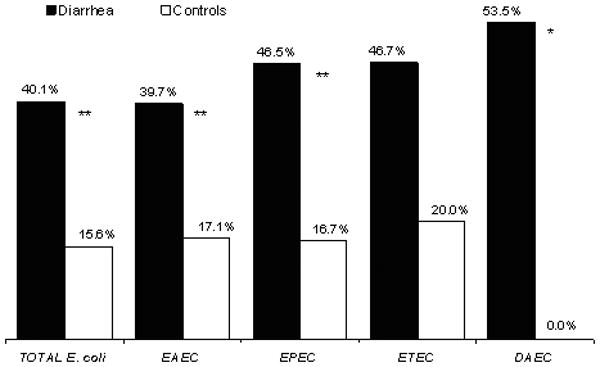
Mixed infections in which a diarrheagenic E. coli were isolated in diarrhea and control samples. Black bars, percentage of co-infections among diarrhea samples for each diarrheagenic E. coli group: all E. coli (n=272), EAEC (n=141), EPEC (n=71), ETEC (n=30), DAEC (n=43). White bars, percentage of co-infections among control samples: all E. coli (n=135), EAEC (n=76), EPEC (n=42), ETEC (n=5), DAEC (n=9). * p< 0.01 and ** p< 0.001, for the comparison of co-infections among diarrhea and control samples for each pathogen. STEC data not shown because of small numbers.
For episodes due to a single pathogen (excluding co-infections) DAEC tended to cause longer illness (Table 5). One fifth of DAEC episodes had blood in the stools, and half had fever. The episodes with the maximum number of loose stools per day were with EPEC, ETEC, campylobacter and rotavirus. Half of the children with ETEC received ORS, a rate similar to rotavirus cases; however, only the later required IV fluids. Episodes due to rotavirus had the highest severity scores, followed by episodes due to mixed infections and EPEC. More than half of the rotavirus episodes had moderate or severe scores (Table 5). A third of diarrhea episodes received an antibiotic empirically.
Table 5.
Clinical characteristics and severity of diarrheal episodes due to a single or multiple pathogens.
| EAEC |
EPEC |
DAEC |
ETEC |
Campya |
Rotavirus |
Mixed infectionsb |
|
|---|---|---|---|---|---|---|---|
| n=85 | n=38 | n=20 | n=16 | n=103 | n=85 | n=109 | |
| Duration in days, median (range) | 5 (1-31) | 5.5 (1-24) | 7 (1-25) | 5 (1-21) | 5 (1- 21) | 4 (1-14) | 4 (1-17) |
| Persistent diarrhea, % | 14.1 | 7.9 | 15.0 | 18.8 | 2.9 | 1.2 | 3.7 |
| Blood in stools, % | 9.5 | 5.3 | 20.0 | 12.5 | 35.9 | 2.4 | 16.5 |
| Fever, % | 36.9 | 34.2 | 50.0 | 37.5 | 40.8 | 55.3 | 49.5 |
| Maximum number of stools/day during episode, mean (range) | 5.5 (3-10) | 6.0 (3-12) | 5.5 (3-10) | 6.0 (3-9) | 6.2 (2-15) | 6.3 (3-15) | 6.0 (1-20) |
| Maximum number of emesis/day during episode, mean (range) | 1.0 (0-7) | 1.2 (0-7) | 0.7 (0-5) | 0.6 (0-5) | 1.0 (0-8) | 2.9 (0-12) | 1.7 (0-10) |
| Received ORS, % | 29.8 | 42.1 | 25.0 | 56.3 | 35.9 | 54.1 | 45.9 |
| Required IV fluids, % | 2.4 | 0 | 0 | 0 | 1.9 | 7.1 | 3.7 |
| Received antibiotics empirically, % | 24.7 | 34.2 | 30.0 | 31.3 | 60.2 | 35.3 | 42.2 |
| Severity score c, mean ± SD | 6.6 ± 3.3 | 7.7 ± 3.1 | 6.8 ± 3.1 | 7.2 ± 2.0 | 7.0 ± 2.9 | 9.2 ± 3.9 | 8.0 ± 3.9 d |
| % with moderate and severe score | 31.9 | 32.2 | 40.9 | 25.0 | 28.4 | 53.0 | 38.2 |
Campylobacter;
Mixed infections (multiple pathogen) in which a diarrheagenic E. coli was isolated in addition to rotavirus (45%), campylobacter(44%) and both (11%);
Modified Vesikari score; moderate and severe score ≥ 9 points;
p<0.05 for the comparison of the severity score between mixed infections and rotavirus, and mixed infections and EAEC. STEC data not shown because of small numbers.
Discussion
In this study the incidence of diarrhea that required medical attention was approximately 1 episode/child/year, with diarrheagenic E. coli accounting for a fourth of these episodes. This incidence is lower than current worldwide estimates (≈4 episodes/child/year) [8]; however, this was a passive surveillance study, in which only diarrhea episodes that required outpatient or inpatient care were studied. Thus, pathogens causing more severe illnesses are undoubtedly over represented in the diarrheal cases as suggested by the duration of illness data. The most commonly isolated pathogens in diarrheal and control samples were the diarrheagenic E. coli. These bacteria were more frequently isolated from older infants, reflecting increased exposure to pathogens after six months of age as potentially contaminated foods are introduced into the diet [10]. In this population solid foods were started on average at 6 months of age and infant formula at 8 months; however, half of the children continued some breastfeeding at 12 months of age. Therefore, in a setting with a high frequency of exposure to pathogens and high frequency of breastfeeding, the major age-related flora differences may result from complex interactions.
The interpretation of pathogen frequency in diarrhea versus control samples is complicated by these interactions. Colonization rather than illness results from the interaction of several factors: Pathogens are sometimes heterogeneous, sharing specific virulence genes used for categorizing them in a group, but having accessory genes that affect virulence. Host susceptibility to infection is determined by the child's age, presence of protective maternal factors such as trans-placental antibodies and breastfeeding (sIgA, oligosaccharides, lactoferrin, lysozyme) [17], nutritional and immunological status, prior exposure and acquired immunity, and genetic susceptibility. Environmental factors such as poor hygiene and high fecal contamination, result in early and frequent exposure with development of acquired immunity. We hypothesize that the high frequency of pathogens in samples from asymptomatic young children reflects these factors.
Several epidemiological studies have compared the isolation rate of diarrheagenic E. coli among diarrhea and control samples, with different results [7, 9-12, 18, 19]. The factors mentioned above, should be taken into consideration in interpretation and comparison of studies. For example, a recent case-control study in children (<5 years of age) in Vietnam found diarrheagenic E. coli to be significantly more associated with diarrheal samples than controls [20]. Several methodological issues could explain the difference between these results and the current study. However the most important difference is likely to be the inclusion of older children in the Vietnamese study. Asymptomatic colonization of breastfed infants may be the major difference between these studies.
In developing countries several epidemiological studies have been conducted in children to determine the age-related difference of diarrheagenic E. coli [7, 9-12, 18, 19]. The most commonly isolated E. coli in children less than one year of age are EPEC, EAEC and ETEC although in some studies DAEC are common in this age group [9, 12, 18]. The relative frequencies of pathogens vary among studies. Few epidemiological studies have compared the isolation rate of diarrheagenic E. coli in children less than 6 months of age and older infants [9-12]. In general, there is a tendency for higher isolation of pathogens, especially ETEC, in older infants. Previous studies in Peruvian children looked for some classes of diarrheagenic E. coli [21-23]. This, plus the size of this study and its focus on more severe illness make it particularly relevant.
In this study EAEC were the most prevalent pathogens in both diarrhea and control samples. This contrasts with several studies showing a difference in prevalence of EAEC compared to controls [24-27]. However, EAEC are heterogeneous [28]. The genes used in our multiplex PCR, are for an initial screening of diarrheagenic E. coli strains. Other virulence markers may be worth studying to further define more virulent strains.
The second most commonly isolated E. coli was EPEC. Atypical EPEC were more frequent than typical EPEC in diarrhea cases, which is in concordance with current data suggesting that atypical EPEC are more prevalent than typical EPEC in both developed and developing countries [29-32]. However, the frequency of typical and atypical EPEC and their association with acute diarrhea, persistent diarrhea, or asymptomatic carriage is not fully defined. Several recent pediatric studies have found atypical EPEC to be significantly associated with diarrhea [24, 25, 33, 34]. The full virulence profiles of most atypical EPEC strains are unknown [35].
The role of DAEC in diarrheal disease has been controversial since some studies reported similar frequency of isolation from diarrhea and control samples. However, other case-control studies have found a correlation between DAEC infection and diarrhea especially in children >12 months of age [12, 36]. Although DAEC accounted for only 5% of our cases, it was significantly associated with diarrhea in infants 6-12 months of age. This age-association differs from two previous studies in Brazilian children, in which DAEC was associated with diarrhea in children 13-24 months of age [12, 36]. In those studies the isolation rate of DAEC in younger children was high.
The prevalence of ETEC was low; however children older than 6 months of age infected with ETEC had a 4.5-fold increased risk for diarrhea. Most epidemiological studies with data on isolation rates by age have reported ETEC prevalence rates around 10-20% in children <12 months of age [7, 9, 23, 26, 37]. However, several studies [12, 19, 26] reported rates similar to the current study. Fifty percent of ETEC episodes had dehydration and required ORS, similar to the rates with rotavirus infection. ORS use is consistent with the dehydrating nature of ETEC infection, the high morbidity rates in the community setting, and elevated mortality rates in the inpatient setting [5]. LT was present in almost two thirds of ETEC infections, either alone or in combination with ST; this is important from a vaccine perspective [38].
STEC were isolated infrequently from children with diarrhea. However, this study shows that these pathogens are present in the peri-urban communities of Lima, and therefore children could potentially develop more severe disease and complications associated with STEC infection. All STEC strains isolated in this study were stx1 and eae positive. Strains that only have genes for stx1 are known to be less virulent than those that are stx2 positive [39]. The lack of EIEC and low isolation of Shigella could be explained by the age of the patients, as well as the high frequency of empiric antibiotic use in this population prior to stool sample collection.
Mixed infections are an evolving problem in diarrhea epidemiology. With better diagnostics tools there are more mixed infections being found. This makes it complicated to determine which pathogen is responsible for the disease, or if there is an additive effect of each pathogen present in a co-infection. Some studies had found a significantly greater number of children with diarrhea than control children with co-infections, suggesting associations of these potential enteropathogens in the etiology of diarrhea [11, 19]. In this study, mixed infections were common, especially in diarrheal samples that included a diarrheagenic E. coli. These episodes tended to have more dehydration in comparison with episodes due to a single diarrheagenic E. coli.
There are methodological limitations in this study. The lack of intestinal parasites could be explained by suboptimal stool sample volume as well as the diagnostic methods used. It is possible that some patients may have been ill with non rotaviral viral enterocolitis. Since this was a passive surveillance study, we do not have information on milder illness not requiring medical attention. Thus, this study does not provide an estimate of total diarrhea incidence in this population. Even with these limitations, the data demonstrate that a variety of diarrheagenic E. coli strains are common in Peru and that they are associated with sporadic diarrhea in children requiring medical attention.
Acknowledgments
Dr. T. Ochoa is supported by 1K01TW007405; Dr. T. Cleary is supported by R01-HD051716. This work has been partially funded by Dr. C. Lanata's Institutional Research Funds and by the United States Military Infectious Disease Research Program (MIDRP), work unit number 60000.000.0.B0017.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government, nor the National Institutes of Health and other funding institutions.
Financial Support: This work has been partially funded by 1K01TW007405 grant (Dr. Ochoa); R01-HD051716 grant (Dr. Cleary); by Dr. Lanata's Institutional Research Funds and by the United States Military Infectious Disease Research Program (MIDRP), work unit number 60000.000.0.B0017.
Footnotes
Summary: Diarrheagenic E. coli were the most commonly isolated pathogens in a passive surveillance diarrhea cohort study of children < 1y in Lima. These pathogens were more frequently isolated in older infants, reflecting the increased pathogen exposure after 6m of age.
There is no conflict of interest for all authors.
References
- 1.Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ. 2008;86:710–7. doi: 10.2471/BLT.07.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125–36. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Nataro JP, Mai V, Johnson J, et al. Diarrheagenic Escherichia coli infection in Baltimore, Maryland, and New Haven, Connecticut. Clin Infect Dis. 2006;43:402–7. doi: 10.1086/505867. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MB, Nataro JP, Bernstein DI, Hawkins J, Roberts N, Staat MA. Prevalence of diarrheagenic Escherichia coli in acute childhood enteritis: a prospective controlled study. J Pediatr. 2005;146:54–61. doi: 10.1016/j.jpeds.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 5.Lanata CF, Mendoza W, Black RE. Improving diarrhoea estimates. WHO. [21 April 2009];2002 http://www.who.int/child_adolescent_health/documents/pdfs/improving_diarrhoea_estimates.pdf.
- 6.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458–64. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 9.Porat N, Levy A, Fraser D, Deckelbaum RJ, Dagan R. Prevalence of intestinal infections caused by diarrheagenic Escherichia coli in Bedouin infants and young children in Southern Israel. Pediatr Infect Dis J. 1998;17:482–8. doi: 10.1097/00006454-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Quiroga M, Oviedo P, Chinen I, et al. Asymptomatic infections by diarrheagenic Escherichia coli in children from Misiones, Argentina, during the first twenty months of their lives. Rev Inst Med Trop Sao Paulo. 2000;42:9–15. doi: 10.1590/s0036-46652000000100002. [DOI] [PubMed] [Google Scholar]
- 11.Ratchtrachenchai OA, Subpasu S, Hayashi H, Ba-Thein W. Prevalence of childhood diarrhoea-associated Escherichia coli in Thailand. J Med Microbiol. 2004;53:237–43. doi: 10.1099/jmm.0.05413-0. [DOI] [PubMed] [Google Scholar]
- 12.Spano LC, Sadovsky AD, Segui PN, et al. Age-specific prevalence of diffusely adherent Escherichia coli in Brazilian children with acute diarrhoea. J Med Microbiol. 2008;57:359–63. doi: 10.1099/jmm.0.47660-0. [DOI] [PubMed] [Google Scholar]
- 13.Morris SS, Cousens SN, Lanata CF, Kirkwood BR. Diarrhoea - defining the episode. Int J Epidemiol. 1994;23:617–23. doi: 10.1093/ije/23.3.617. [DOI] [PubMed] [Google Scholar]
- 14.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 15.Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol. 2008;46:1752–7. doi: 10.1128/JCM.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacher DW, Steinsland H, Blank TE, Donnenberg MS, Whittam TS. Molecular evolution of typical enteropathogenic Escherichia coli: clonal analysis by multilocus sequence typing and virulence gene allelic profiling. J Bacteriol. 2007;189:342–50. doi: 10.1128/JB.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–8. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Levine MM, Ferreccio C, Prado V, et al. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am J Epidemiol. 1993;138:849–69. doi: 10.1093/oxfordjournals.aje.a116788. [DOI] [PubMed] [Google Scholar]
- 19.Orlandi PP, Magalhães GF, Matos NB, et al. Etiology of diarrheal infections in children of Porto Velho (Rondonia, Western Amazon region, Brazil) Braz J Med Biol Res. 2006;39:507–17. doi: 10.1590/s0100-879x2006000400011. [DOI] [PubMed] [Google Scholar]
- 20.Hien BTT, Scheutz F, Dac Cam P, et al. Diarrheagenic E. coli and Shigella strains isolated from children in a hospital case-control study in Hanoi, Vietnam. J Clin Microbiol. 2008;46:996–1004. doi: 10.1128/JCM.01219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanata CF, Black RE, Maurtua D, et al. Etiologic agents in acute vs persistent diarrhea in children under three years of age in peri-urban Lima, Peru. Acta Paediatr Suppl. 1992;381:32–8. doi: 10.1111/j.1651-2227.1992.tb12369.x. [DOI] [PubMed] [Google Scholar]
- 22.Lopez Marin D, Sagaro Gonzales E, Valdes Depena M, Fragoso Arbelo T, Albizu-Campos JC. Enteropathogenic agents isolated in persistent diarrhoea. Rev Gastroenterol Peru. 1996;16:214–21. [PubMed] [Google Scholar]
- 23.Cama RI, Parashar UD, Taylor DN, et al. Enteropathogens and other factors associated with severe disease in children with acute watery diarrhea in Lima, Peru. J Infect Dis. 1999;179:1139–44. doi: 10.1086/314701. [DOI] [PubMed] [Google Scholar]
- 24.Moreno AC, Filho AF, Gomes TD, et al. Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diagn Microbiol Infect Dis. 2008 May 26; doi: 10.1016/j.diagmicrobio.2008.03.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Araujo JM, Tabarelli GF, Aranda KR, et al. Typical enteroaggregative and atypical enteropathogenic types of Escherichia coli are the most prevalent diarrhea-associated pathotypes among Brazilian children. J Clin Microbiol. 2007;45:3396–9. doi: 10.1128/JCM.00084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TV, Le Van P, Le Huy C, Gia KN, Weintraub A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J Clin Microbiol. 2005;43:755–60. doi: 10.1128/JCM.43.2.755-760.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarantuya J, Nishi J, Wakimoto N, et al. Typical enteroaggregative Escherichia coli is the most prevalent pathotype among E. coli strains causing diarrhea in Mongolian children. J Clin Microbiol. 2004;42:133–9. doi: 10.1128/JCM.42.1.133-139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12–8. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa TJ, Barletta F, Contreras C, Mercado E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg. 2008;102:852–6. doi: 10.1016/j.trstmh.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508–13. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afset JE, Bergh K, Bevanger L. High prevalence of atypical enteropathogenic Escherichia coli (EPEC) in Norwegian children with diarrhoea. J Med Microbiol. 2003;52:1015–9. doi: 10.1099/jmm.0.05287-0. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen RN, Taylor LS, Tauschek M, Robins-Browne RM. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg Infect Dis. 2006;12:597–603. doi: 10.3201/eid1204.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estrada-Garcia T, Lopez-Saucedo C, Thompson-Bonilla R, et al. Association of diarrheagenic Escherichia coli Pathotypes with infection and diarrhea among Mexican children and association of atypical Enteropathogenic E. coli with acute diarrhea. J Clin Microbiol. 2009;47:93–8. doi: 10.1128/JCM.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bueris V, Sircili MP, Taddei CR, et al. Detection of diarrheagenic Escherichia coli from children with and without diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz. 2007;102:839–44. doi: 10.1590/s0074-02762007005000116. [DOI] [PubMed] [Google Scholar]
- 35.Afset JE, Bruant G, Brousseau R, et al. Identification of Virulence Genes Linked with Diarrhea Due to Atypical Enteropathogenic Escherichia coli by DNA Microarray Analysis and PCR. J Clin Microbiol. 2006;44:3703–11. doi: 10.1128/JCM.00429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scaletsky IC, Fabbricotti SH, Carvalho RL, et al. Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in Northeast Brazil: a case-control study. J Clin Microbiol. 2002;40:645–8. doi: 10.1128/JCM.40.2.645-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albert MJ, Faruque SM, Faruque AS, et al. Controlled study of Escherichia coli diarrheal infections in Bangladeshi children. J Clin Microbiol. 1995;33:973–7. doi: 10.1128/jcm.33.4.973-977.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svennerholm AM, Tobias J. Vaccines against enterotoxigenic Escherichia coli. Expert Rev Vaccines. 2008;7:795–804. doi: 10.1586/14760584.7.6.795. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa TJ, Cleary TG. Epidemiology and spectrum of disease of Escherichia coli O157. Curr Opin Infect Dis. 2003;16:259–63. doi: 10.1097/00001432-200306000-00013. [DOI] [PubMed] [Google Scholar]


