Abstract
Background
The development of a gonorrhea vaccine is challenged by the lack of correlates of protection. The antigenically variable neisserial opacity (Opa) proteins are expressed during infection and have a semivariable (SV) and highly conserved (4L) loop that could be targeted in a vaccine. Here we compared antibodies to linear (Ablinear) and cyclic (Abcyclic) peptides that correspond to the SV and 4L loops and selected hypervariable (HV2) loops for surface-binding and protective activity in vitro and in vivo.
Methods/Findings
AbSV cyclic bound a greater number of different Opa variants than AbSV linear, including variants that differed by seven amino acids. Antibodies to the 4L peptide did not bind Opa-expressing bacteria. AbSV cyclic and AbHV2 cyclic, but not AbSV linear or AbHV2 linear agglutinated homologous Opa variants, and AbHV2BD cyclic but not AbHV2BD linear blocked the association of OpaB variants with human endocervical cells. Only AbHV2BD linear were bactericidal against the serum resistant parent strain. Consistent with host restrictions in the complement cascade, the bactericidal activity of AbHV2BD linear was increased 8-fold when rabbit complement was used. None of the antibodies was protective when administered vaginally to mice. Antibody duration in the vagina was short-lived, however, with <50% of the antibodies recovered 3 hrs post-administration.
Conclusions
We conclude that an SV loop-specific cyclic peptide can be used to induce antibodies that recognize a broad spectrum of antigenically distinct Opa variants and have agglutination abilities. HV2 loop-specific cyclic peptides elicited antibodies with agglutination and adherence blocking abilities. The use of human complement when testing the bactericidal activity of vaccine-induced antibodies against serum resistant gonococci is also important.
Introduction
Gonorrhea is the second most commonly reported disease in the United States with over 350,000 cases reported in 2006 [1] and over 62 million estimated annual cases worldwide [2]. The gonococcus colonizes many mucosal sites, including the cervix, urethra, rectum, and pharynx. Ascended reproductive tract infections are the major source of the morbidity and mortality associated with this pathogen. Ascended infection occurs in 10–20% of cervical infections, and can lead to pelvic inflammatory disease (PID) and the associated complications of involuntary infertility, ectopic pregnancy, and chronic pelvic pain [3]. Gonorrhea is also a co-factor for transmission of the human immunodeficiency virus [4]. The public health cost of gonococcal infections is significant; over 77 million dollars were spent in the U.S. in the year 2000 on the diagnosis and treatment of acute gonorrhea and post-infection sequelae in patients 15–24 years of age [5]. The rapid emergence of antibiotic-resistant strains [6] underscores the importance of identifying new preventive measures against gonorrhea as illustrated by the recent removal of fluoroquinolones from recommended treatments [7].
The development of a gonorrhea vaccine is challenged by the lack of known correlates of protection. Repeat infections are common even with the homologous strain [8] or serotype [9], [10], although evidence of partial immunity has been reported [11], [12]. N. gonorrhoeae does not express a capsule, which is the target of several effective meningococcal vaccines. Therefore, research towards a gonorrhea vaccine has focused on other surface antigens such as outer membrane proteins. The neisserial opacity (Opa) proteins are a family of outer membrane proteins that mediate adherence to and invasion of tissue culture cells [13]. Gonococci express 8–11 antigenically distinct proteins that are encoded by separate opa genes [14], [15]. Mature Opa proteins are predicted to have four surface-exposed loops, namely, one semi-variable (SV) loop, two hypervariable (HV1 and HV2) loops, and one conserved (4L) loop [16]. Sequence differences in the HV regions are responsible for the antigenic identity of each Opa protein as well as slight differences in molecular weight. Each opa gene undergoes phase variation via a frame shift mechanism, and therefore, a single gonococcus can express no Opa proteins, one Opa protein, or multiple Opa proteins simultaneously [17], [18].
The expression of Opa proteins by N. gonorrhoeae appears to be important during urogenital tract infections. The majority of urethral isolates from naturally [19] and experimentally infected men [20], [21] expressed one or more Opa proteins, and in women, mostly Opa-positive isolates were recovered from the cervix during certain stages of the menstrual cycle [19]. Evidence for Opa protein expression during infection is also supported by the detection of Opa protein-specific antibodies in serum and genital secretions from men and women with uncomplicated urogenital tract infections, PID, or disseminated gonococcal infection [22], [23]. The presence of antibodies to multiple Opa proteins is associated with a reduced risk of PID in commercial sex workers [24], and therefore, Opa proteins may be protective vaccine antigens.
While the HV loops are highly variable among Opa proteins, the SV and 4L loops are relatively and highly conserved, respectively and could be targeted in a vaccine. Immunization with whole Opa proteins may prevent generation of high levels of antibodies against the conserved loops due to the immunodominance of the HV loops [25]. Additionally, Opa-mediated interactions with CEACAM1 on B and T lymphocytes during immunization may decrease the effector functions of these immune cells and thus prevent a robust vaccine-induced immune response [26], [27], although this hypothesis was not supported experimentally [28]. To avoid these potential pitfalls and to test whether the SV and 4L loops might carry broadly reactive, protective epitopes, here we utilize peptide-based immunization strategies to generate antibodies against the SV and 4L loops. Both linear and cyclic peptides were used to generate antibodies based on evidence that cyclic peptides induced bactericidal, conformation-dependant antibodies against meningococcal outer membrane proteins [29]. Opa loop-specific antibodies were tested for specificity, surface-binding, in vitro activity against N. gonorrhoeae and the capacity to protect female mice from experimental genital tract infection when delivered topically.
Results
Antibodies against the SV Loop and 4L Loop Are Broadly Reactive
The 4L loop sequence of strain FA1090 is highly conserved with only a single amino acid difference among the 8 Opa proteins expressed by this strain. The SV loop is relatively well conserved with the OpaA and OpaK proteins sharing the same SV loop sequence and only 2–12 amino acid variations among the other 6 proteins (Figure 1). To test the hypothesis that antibodies against the 4L and SV loops would be broadly reactive, we examined the specificity of affinity-purified rabbit polyclonal antibodies against linear peptides that correspond to the OpaA/K SV (AbSV linear) and 4L (Ab4L linear) loop sequences by western blot. Ab4L linear strongly recognized all of the Opa proteins of strain FA1090 except OpaE (Figure 2C) as reported previously [30]. AbSV linear bound strongly to denatured OpaA and OpaK, and also to OpaF and OpaI, which have an SV loop that is predicted to differ from the OpaA/K SV sequence by 2 and 7 amino acids, respectively. AbSV linear weakly recognized OpaD, which is predicted to differ from the OpaA/K SV loop by 4 amino acids. When room temperature-treated samples were analyzed, AbSV linear recognized only OpaA, OpaK and OpaF. OpaD and OpaI proteins were not recognized. AbSV linear did not recognize OpaB, which differs by 6 amino acids from the target peptide or OpaC or OpaE under either condition, which differ from the OpaA/K SV sequence by 11–12 amino acids (Figures 2A and D).
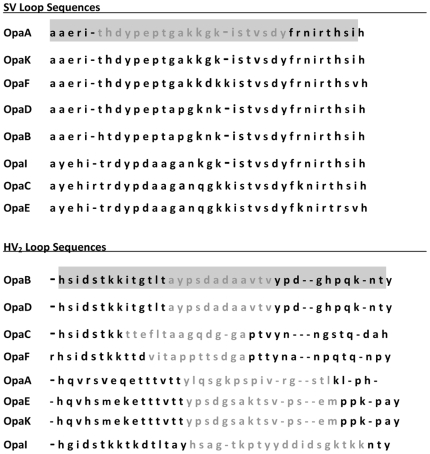
Figure 1. Conservation of SV and HV2 loop sequences.
The predicted amino acid sequences of the SV and HV2 loops of the 8 Opa proteins of strain FA1090 are shown. The sequences of the 36-mer cyclic peptides used to generate SV- and HV2BD-specific antisera are highlighted in grey. The sequences of the linear peptides used to generate affinity-purified rabbit antibodies against the SV and HV2 loops are shown in light grey font.
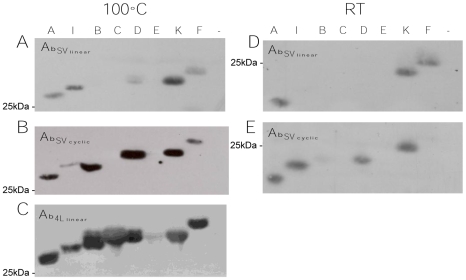
Figure 2. Specificity of antibodies against the conserved Opa loops.
Cell lysates of an Opa-negative variant and each of the 8 Opa–positive variants of strain FA1090 were incubated at RT or 100°C prior to fractionation and incubated with (A and D) AbSV linear (1∶75,000), (B and E) AbSV cyclic (1∶6,000), and (C) Ab4L linear (1∶6,000). The broader reactivity of AbSV cyclic compared to AbSV linear is clearly shown whether native or denatured Opa proteins are analyzed. Ab4L linear is even more broadly reactive in that it recognizes all denatured Opa proteins well except OpaE. None of the antibodies recognize a protein in the Opa-negative lane (−). The location of a 25 kDa molecular weight marker is indicated, and the denatured Opa proteins migrated at a slightly higher molecular weight than those incubated at RT, which is consistent with well characterized heat modifiable nature of these proteins. The Ab4L linear immunoblot was kindly provided by Dr. Amy Simms.
We also immunized mice with cyclic peptides that correspond to a longer surface-exposed region of the OpaA/K SV loop that does not include any of the predicted transmembrane regions (Figure 1) to increase the likelihood of generating conformation-dependent antibodies [29]. The resultant antiserum, AbSV cyclic, was more broadly reactive than AbSV linear and recognized all but 2 Opa proteins of strain FA1090 when denatured samples were analyzed (Figure 2B). AbSV cyclic recognized Opa proteins with up to 7 amino acid differences compared to the cyclic SV peptide, but not OpaC or OpaE, which are the most divergent from OpaA/K in the SV region (Figure 1). AbSV cyclic recognized OpaA, OpaK, OpaD and OpaI proteins when RT-treated samples were used.
SV-Specific but Not 4L-Specific Antibodies Bind the Bacterial Surface
The semi-quantitative surface-binding immunoblot (SBI) assay, which utilizes whole gonococci, was used to compare the concentration of antibodies needed for detectable binding to the different Opa variants of strain FA1090. Affinity-purified rabbit antibodies against linear HV2 loop peptides (AbHV2 linear) bound homologous Opa variants in a dose-dependent manner (Figure 3A) and not heterologous or Opa-negative variants. AbSV linear bound only OpaA, OpaK and OpaF variants (Figure 3B), which is consistent with western blot results against unheated lysates in which native conformations are preserved. Approximately 10-fold higher concentrations of AbSV linear were required to detect binding to whole bacteria compared to AbHV2 linear (compare Figures 3A and 3B). No specific binding was seen with Ab4L linear at any concentration tested (Figure 3C).
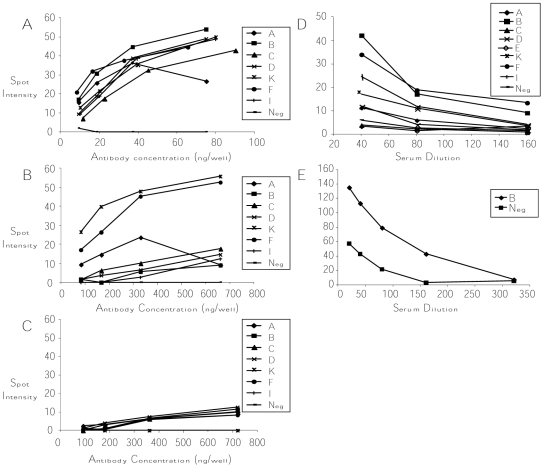
Figure 3. Detection of antibody surface-binding by the SBI assay.
Opa-specific antibodies or antisera were serially diluted and incubated with defined Opa variants spotted onto nitrocellulose filters and binding was detected as described in the Materials and Methods. Shown are the normalized spot intensities plotted against the concentration of affinity-purified rabbit antibodies (AbHV2 linear, AbSV linear, and Ab4L linear) or dilution of antisera (AbSV cyclic and AbHV2BD cyclic): (A) AbHV2 linear, (B) AbSV linear, (C) Ab4L linear (D) AbSV cyclic, (E) AbHV2BD cyclic. All results shown are representative of at least two independent experiments. None of the antibodies bound Opa-negative gonococci. The results for AbHV2BD linear when tested against Opa-negative variants are shown in panel A as an example.
The reactivity of the mouse antiserum against the cyclic SV loop peptide in the SBI assay mirrored the broader reactivity observed with this antiserum on western blots. AbSV cyclic bound intact OpaA, OpaB, OpaD, OpaF, OpaK and OpaI variants above background in the SBI assay at a dilution of 1∶40, while OpaC, OpaE, and Opa-negative variants were not recognized by AbSV cyclic (Figure 3D). We also tested mouse antisera against a cyclic peptide that corresponds to the HV2 loop of OpaB/D. As predicted, AbHV2BD cyclic bound to OpaB variants better than Opa-negative variants (Figure 3E). Consistent with the greater surface exposure of the HV2 loop, a 5-fold higher dilution of AbHV2BD cyclic produced a spot of similar intensity when tested against OpaB variants as that of the AbSV cyclic antiserum (compare Figures 3D and 3E).
IFA staining was performed as a second measure of surface-binding. Consistent with the results obtained by SBI, AbSV linear (2.2 µg/mL) bound OpaA variants as well as OpaK and OpaF-expressing gonococci, but none of the other Opa variants. AbSV cyclic bound the same set of Opa variants as recognized in the SBI assay, but not OpaC or Opa-negative variants (Table 1). As predicted from the SBI results, mouse antiserum against the cyclic HV2BD peptide bound OpaB variants at a higher dilution (1∶100) than antiserum against the cyclic SV loop peptide (1∶30). Ab4L linear did not bind any Opa-positive gonococci at concentrations of 1.2 µg/mL or 2.4 µg/mL (data not shown), a result that confirms the negative SBI results with these antibodies (Table 1).
Table 1. Surface-binding of SV loop-specific antibodies as assessed by SBI and IFA.
| AbSV linear a | AbSV cyclic b | |||
| IFA | SBI | IFA | SBI | |
| OpaA | + | + | + | + |
| OpaB | − | − | + | + |
| OpaC | − | − | +/− | − |
| OpaD | − | − | + | + |
| OpaE | ntc | ntc | ntc | − |
| OpaF | + | + | + | + |
| OpaK | + | + | + | + |
| OpaI | − | − | + | + |
affinity-purified polyclonal rabbit antibodies.
mouse antisera.
not tested.
In summary, the IFA and SBI results were identical and confirmed the predicted surface-exposure of the SV and HV2 loops. The need for increased concentration of SV loop-specific antibodies to detect surface binding suggests the SV loop is less accessible than the HV2 loop. We also conclude that the fourth loop may not be accessible despite its predicted surface-exposure. Alternatively, the linear 4L peptide may not induce antibodies that recognize conformational epitopes present in the native protein. Finally, we also demonstrated that the cyclic SV peptide induced more broadly reactive antibodies compared to a linear peptide.
Bactericidal and Agglutination Activities
We next investigated whether cyclic or linear peptide-derived antibodies against the SV or HV2-loops would be bactericidal. Disappointingly, AbSV linear were not bactericidal against any of the variants tested at concentrations ranging from 4.5–120 µg/mL and the use of cyclic peptides to induce bactericidal antibodies against the SV or HV2BD loops was also not successful when a dilution as low as 1∶9 was tested. Analysis of mouse antisera to cyclic loop peptides showed IgG2a>IgG1>IgG2b>IgG3. In contrast to mouse antisera against cyclic peptides, rabbit antibodies against the linear HV2BD peptide, AbHV2BD linear, were bactericidal against OpaB variants with a bactericidal50 concentration of 40.4 µg/mL. Antibodies against the HV2 loop of Opa I, AbHV2I linear, also demonstrated bactericidal activity against a homologous variant with a bactericidal50 concentration of 26 µg/mL. No other antibodies against HV2 linear peptides were bactericidal against the corresponding homologous Opa variant.
Strain FA1090 is inherently resistant to the bactericidal activity of NHS due to the binding of the complement regulatory protein human C4b-binding protein (hC4BP) to its porin [31]. This interaction is host-restricted [39]. Therefore, to better mimic events that might occur when testing the activity of these antibodies in the mouse infection model, we utilized strain FA1090F62 por5–8, which produces a recombinant hybrid porin that does not bind hC4BP and is thus serum sensitive. As observed with wild type FA1090 bacteria, AbHV2BD linear but not the AbHV2BD cyclic or AbSV cyclic mouse antisera was bactericidal against OpaB variants of strain FA1090F62 por5–8 in the presence of NHS. Control antibodies AbHV2A linear, which like AbHV2BD linear are affinity-purified rabbit polyclonal antibodies, were not bactericidal for OpaB variants of this serum sensitive strain (Figure 4A). To further investigate this host restriction, we also compared NHS with BBS as a nonhuman complement source. For these experiments we utilized a constitutive OpaB-expressing derivative of wild type strain FA1090 and a strain in which all the opa genes have been inactivated. Consistent with the host restriction for hC4BP, AbHV2BD linear exhibited 8-fold higher activity against the OpaB-expressing strain when BBS was used (bactericidal50 titers 1∶36 (28 µg/mL) with NHS versus 1∶288 (3.5 µg/mL) with BBS) (Figure 4B). AbHV2BD linear showed no bactericidal activity against the Opa-deficient strain with either complement source (data not shown).
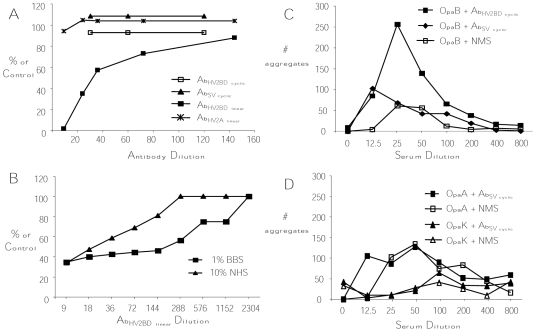
Figure 4. Bactericidal and agglutination activities.
The bactericidal activity of SV and HV2 loop-specific antibodies was measured against (A) OpaB variants of the serum sensitive strain FA1090F62por 5–8 in the presence of 1% NHS and (B) the constitutively OpaB-expressing strain of FA1090 (SR) incubated with AbHV2BD linear in the presence of NHS or BBS. Results are expressed as the percent of bacteria recovered compared to wells with no antibody present. Only AbHV2BD linear was bactericidal against the OpaB-expressing serum sensitive strain. The use of a non-human complement source (BBS) resulted in ∼8-fold greater bactericidal activity for AbHV2BD linear against serum resistant (wild type) OpaB-expressing variants. AbHV2BD linear was not bactericidal when HI-NHS and HI-BBS were used (data not shown). Agglutination activity was measured as the average number of bacterial aggregates following incubation of (C) OpaB variants with increasing dilutions of AbHVBD cyclic, AbSV cyclic, or NMS and (D) OpaA or OpaK variants with AbSV cyclic or NMS. Antibodies raised to the cyclic SV peptides showed agglutinating activity except when tested against OpaK variants.
The capacity to agglutinate bacteria may facilitate shedding of bacteria in vaginal secretions and thus may be another important effector function of antibodies. AbHV2BD cyclic agglutinated OpaB variants at a titer of 1∶200 with ∼40 aggregates per 40X field compared to only ∼4 aggregates per field with the same dilution of NMS. (Figure 4C). In contrast, AbHV2BD linear did not agglutinate OpaB variants at dilutions as low as 1∶2 (500 µg/mL) (data not shown). AbSV cyclic agglutinated OpaA and OpaB variants but not OpaK variants at a titer of 1∶12.5 compared to NMS (Figures 4C and D) although AbSV cyclic bound OpaK variants. AbSV linear did not agglutinate any Opa variants tested (data not shown).
HV2-Specific but Not SV-Specific Antibodies Reduce Adherence to Cultured Cervical Cells
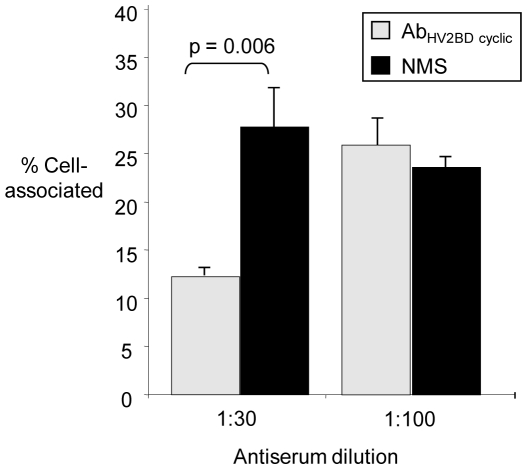
Antibody-mediated inhibition of Opa-mediated adherence and invasion may also be protective. Most Opa proteins mediate invasion of human cells via binding to human carcinoemybryonic cellular adhesion molecules (CEACAMs) [13]. To test whether Opa loop-specific antibodies can block gonococcal interactions with human CEACAM-expressing endocervical cells, ME180 cells were inoculated with the constitutive OpaB-expressing strain following incubation with AbHV2BD linear, AbHV2BD cyclic, or AbSV cyclic. Treatment with AbHV2BD cyclic (Figure 5) but not AbSV cyclic (data not shown) resulted in a dose-dependent decrease in the number of cell-associated bacteria as compared to NMS. In contrast there was no decrease in the number of cell-associated bacteria when bacteria were treated with 0.25 µg/mL or 2.5 µg/mL of AbHV2BD linear versus AbHV2C linear, which does not bind OpaB variants (data not shown). We conclude AbHV2BD cyclic, but not AbHV2BD linear block OpaB-mediated interactions with human endocervical cells.
Figure 5. Antibody-mediated inhibition of gonococcal association with tissue culture cells.
Pre-incubation with AbHV2BD cyclic but not AbSV cyclic decreased the total number of ME180 cell-associated OpaB-expressing gonococci when an antiserum dilution of 1∶30 was used. Shown is the average percent of cell-associated bacteria that were preincubated with AbHV2BD cyclic (test) or NMS (control) based on combined data from three independent assays. A two-tailed, unpaired Student's t-test was used to assess statistical differences between test and control wells.
Passive Protection Studies
The CEACAM residues that are important for Opa-mediated adherence are not conserved in the murine CEACAM1 [30], [40], and consistent with this host restriction, we have not observed Opa-mediated adherence to two different murine epithelial cell lines (J. G. Cole et al, manuscript submitted). However, female mice can be experimentally infected with N. gonorrhoeae despite the absence of human CEACAMs [30], [32], and Opa-specific antibodies could prevent or reduce colonization of mice through bactericidal activity, opsonophagocytic uptake, or agglutination with subsequent shedding. Additionally, because selection for Opa expression occurs during experimental infection of BALB/c mice for reasons that are not known [30], Opa-specific antibodies may block other Opa-mediated functions [38]. We therefore tested the capacity of Opa loop-specific antibodies to reduce gonococcal colonization of BALB/c mice when administered vaginally as done in protection studies for other sexually transmitted pathogens [37], [41]–[43].
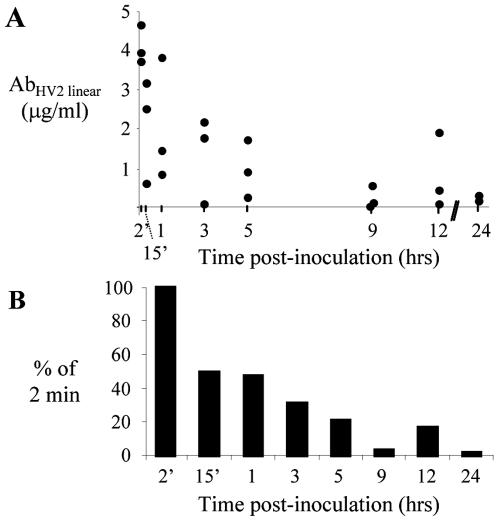
First, to determine how long antibodies remain in the vagina after topical administration, groups of mice were inoculated vaginally with 20 µl of PBS containing 10 µg of AbHV2 linear, and the amount of rabbit IgG in vaginal washes was measured over time. A 50% decrease in the amount of rabbit IgG recovered was observed between 2 and 15 min post-inoculation. Antibody levels were maintained for at least another 45 min, after which a gradual decline was observed over the next 4 hours. Low levels of antibody were detected in all but one mouse at 9, 12, and 24 hrs post-inoculation (Figure 6). No rabbit IgG was detected in vaginal washes from untreated control mice and there was no correlation between persistence of antibody and the stage of estrous at the time of inoculation. Based on these data we concluded that antibody must function within the first 24 hrs to be effective, and thus chose to analyze colonization loads only at early time points (days 1, 2, and 3 post-inoculation). We also chose a challenge dose of 103 CFU for subsequent protection experiments based on pilot experiments that showed this dose resulted in colonization by strain FA1090 for at least three days in 85% percent of mice.
Figure 6. Duration of topically applied antibodies in the vagina.
A fifty percent reduction in the concentration of topically applied rabbit antibodies was detected in vaginal washes collected 1 hr post-inoculation compared to a 2 min time point. Antibody levels decreased further over time. The limit of detection was 7.8 ng/ml. (A) Concentration of rabbit IgG (µg/ml) in mouse vaginal washes was determined in duplicate by capture ELISA. (B) The percent of antibody remaining relative to the average 2 minute value is shown for each time point. Results are combined from two experiments and each animal was used for a single time point (n = 2–3 mice per time point).
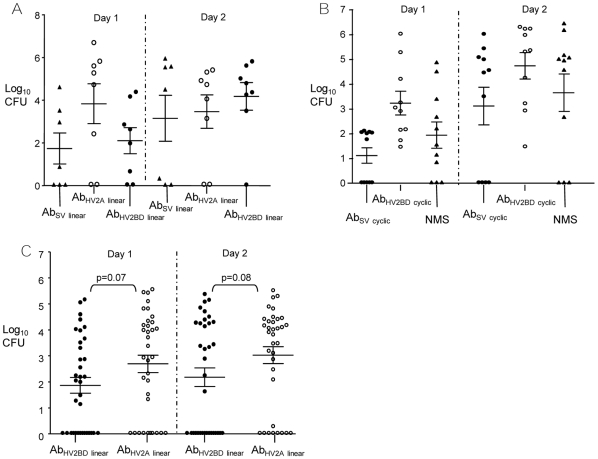
We first tested AbSV linear to address our main objective of developing broadly-reactive protective antibodies against N. gonorrhoeae. Mice were inoculated with 103 OpaA variants that were preincubated with AbSV linear, AbHV2A linear, or AbHV2BD linear. OpaA variants were used since AbSV linear bound OpaA variants (Figure 3B and Table 1). There was no significant difference in the number of gonococci recovered on days 1 and 2 post-inoculation when results from the AbSV linear and AbHV2A linear groups were compared to the AbHV2BD linear control group (Figure 7A). We also tested AbSV cyclic, which may recognize conformational epitopes among Opa proteins compared to AbSV linear. AbSV cyclic bound OpaB variants well in the SBI and IFA assay, and we therefore assessed the capacity of AbSV cyclic and AbHV2BD cyclic to passively protect mice from OpaB variants. Incubation of OpaB variants with AbHV2BD cyclic or AbSV cyclic did not result in a significant difference in colonization load (Figure 7B) or percent of test mice colonized on day 3 compared to the NMS control group (data not shown). In summary, despite binding to the bacterial surface, antibodies against the SV loop do not reduce N. gonorrhoeae colonization, even when a cyclic peptide is used to better mimic the loop conformation.
Figure 7. Passive protection experiments.
Mice were inoculated with OpaA or OpaB variants that were preincubated with antibodies against linear or cyclic Opa SV or HV2-loop sequences. (A) OpaA variants with AbSV linear, AbHV2A linear, or AbHV2BD linear. There was no difference in the number of gonococci recovered from mice in the AbSV linear and AbHV2A linear test groups compared to the AbHV2B linear control group on days 1 or 2 post-inoculation (p≤0.14). (B) OpaB variants with AbHV2BD cyclic, AbSV cyclic or NMS. There was no difference in the number of gonococci recovered from mice treated with AbSV cyclic compared to the control (NMS) group (p≤0.20) on days 1 or 2 post-inoculation. (C) OpaB variants with AbHV2BD linear and AbHV2A linear. Shown are combined data from three independent experiments that showed no difference in the number of bacteria recovered from each group on days 1 and 2 post-inoculation at the level of p = 0.07 and p = 0.08, respectively (n = 7–15 mice/group in each experiment; total number = 35 mice/group). Symbols indicate a single animal and horizontal bars indicate the group mean with the SEM shown. Statistical differences were analyzed by a two-tailed Student's t test.
None of the HV2-specific antibodies that we tested as potential positive controls in studies with SV loop-specific antibodies showed protection. Results from initial pilot studies suggested inoculation of mice with 103 CFU of OpaB variants incubated with 5 µg AbHV2BD linear would be protective in passive protection studies. Unlike AbHV2BD cyclic, AbHV2BD linear were bactericidal, and therefore, we performed a larger scale experiment to test the capacity of AbHV2BD linear to protect mice challenged with OpaB variants. Significantly fewer bacteria were recovered from mice in the AbHV2BD linear test group compared to mice for which AbHV2A linear was used as a negative control (p≤0.03) on days 1 and 2 post-inoculation. However, this difference was not observed in two subsequent experiments and statistical analysis of combined data from all three experiments showed no significant decrease in recovery of gonococci from mice inoculated with AbHV2BD linear-treated OpaB variants on days 1 and 2 post-inoculation compared to AbHV2A linear-treated bacteria (p = 0.07 and p = 0.08, respectively) (Figure 7C). There was also no difference in the number of mice colonized in each group with 60% of the AbHV2BD linear and 71% of the AbHV2A linear-treated mice infected on day 1 (p = 0.45). We considered the possibility that subpopulations of gonococci that express a different Opa protein than that of target variant were responsible for the colonization of test groups. However, the distribution of Opa phenotypes of vaginal isolates on day 1 did not differ significantly from that of the inoculum in any experiment. We conclude that none of the HV2-specific antibodies tested are protective against N. gonorrhoeae colonization, including antibodies with bactericidal activity, and the lack of effectiveness of these antibodies in vivo is not due to escape variants establishing infection.
Discussion
N. gonorrhoeae is a highly successful Gram-negative bacterium that is noted for the antigenic variability of its surface and the frequency by which it causes repeat infections. Opa proteins are expressed during infection and have two conserved loops, the SV and 4L loops, which could be targeted in a vaccine. Here we analyzed antibodies against peptides that correspond to the SV and 4L loops for the capacity to bind to gonococci that express different Opa proteins and for correlates of antibody-mediated protection. Antibodies against linear or cyclic peptides that correspond to the SV loop recognized intact gonococci as assessed by two different methods, and promisingly, antibodies generated against cyclic SV peptides bound the surface of 6 of the 8 Opa variants of strain FA1090, including Opa variants with 7 amino acid differences from the target peptide. In contrast, 4L-specific antibodies did not bind the bacterial surface. The cyclic SV loop peptide also induced antibodies with agglutination ability, while the SV linear peptide did not. The broader reactivity and agglutinating ability of AbSV cyclic may be explained by the fact that the cyclic SV peptide was longer (36 amino acids) than the linear peptides (20 amino acids) and included more conserved regions of the loop. Cyclic peptides may therefore carry more conserved epitopes, T-cell epitopes, and possibly conformational epitopes that are shared by Opa proteins with a less related primary sequence. Interestingly, bactericidal activity was only exhibited by antibodies against linear peptides. This result was in contrast to the demonstration that cyclic peptides were more successful for inducing bactericidal antibodies against the class 1 protein of N. meningitidis [29].
With regard to the difference in bactericidal activity between AbHV2BD linear and AbHV2BD cyclic, one should consider the fact that the antibodies were produced in different animal species and that AbHV2BD linear were high titer affinity-purified antibodies. Antibodies generated by the linear or cyclic SV loop peptides were not bactericidal, however, even when rabbit serum was used as a complement source to by-pass the host-restricted serum resistance of this strain. It is possible that a different adjuvant might improve the potential of the SV loop as a vaccine target by promoting the induction of bactericidal antibodies.
We also showed that the species used as the complement source in bactericidal assays is important when examining the bactericidal activity of vaccine-induced antibodies against SR strains of N. gonorrhoeae. Some P1B strains, like strain FA1090 are resistant to NHS due to the binding of human C4BP to the P1B molecule [31], which reduces activation of the classical pathway and subsequent bacteriolysis [44]. Here we demonstrated that AbHV2BD linear killed OpaB variants of a SS derivative of strain FA1090 better than SR wild type OpaB variants when NHS was used as the complement source. AbHV2BD linear was also more bactericidal against OpaB variants of strain FA1090 when rabbit serum was used. These results illustrate the importance of considering the complement source in bactericidal assays designed to predict vaccine efficacy in humans versus laboratory animals. Ideally, antibodies that are strongly bactericidal against SR strains in the presence of NHS are desired.
A vaccine-induced immune response against Opa proteins could also block Opa-mediated adherence and invasion, which has been the focus of vaccine studies on meningococcal Opa proteins. de Jonge et al. [25], [28] reported that antibodies raised against purified recombinant Opa proteins, outer membrane protein vesicles containing Opa proteins, or liposomes containing recombinant Opa proteins from N. meningitidis elicited an antibody response, which while not always bactericidal, blocked Opa-CEACAM interactions on tissue culture cells [25]. Here we showed AbHV2BD cyclic but not SV-specific antibodies decreased the total number of bacteria associated with CEACAM-expressing endocervical cells. The inability to block adherence with SV-specific antibodies is in accordance with studies on N. meningitidis Opa proteins that show the SV loop is not involved in CEACAM-binding [46]. Other Opa protein functions are likely to exist, however, that could perhaps be blocked by an SV loop-specific immune response. For example, Opa-expressing gonococci are preferentially recovered from experimentally infected male volunteers [20], [21] and during lower genital tract infection of female mice [30]. The basis for this selection is not known, and the inability to detect CEACAMs on primary male urethral cells [47] and the dissimilarities between human and murine CEACAM1 [40], [48] suggest Opa proteins may play other important roles during infection [49].
Finally, SV loop-specific and selected HV2 loop-specific antibodies showed no protection in mice when mixed with the homologous variant prior to vaginal inoculation. We conclude the SV or HV2 Opa protein loops may not be effective targets for antibody-mediated protection. It is possible, however, that technical limitations may have prevented us from detecting a protective effect. The concentration of antibodies may not have been high enough and similar to that reported by Sherwood et al. [50], antibodies were gradually lost within 24 hrs post-inoculation. Clearance via opsonophagocytosis might not occur during this time frame since phagocytes are not detected in infected murine tissue until 2 to 5 days post-inoculation [38]. Systemic delivery of antibodies to the vagina has been successful for others. For example, monoclonal IgA against Chlamydia trachomatis was detected in mouse vaginal secretions for up to 48 hrs when delivered intraperitoneally [37], and Parr et al. [41] reported intraperitoneal administration of IgG resulted in the detection of specific IgG 48 hours later in vaginal secretions at levels equal to 3% of that found in the vaginal secretions of immunized mice. We have detected rabbit IgG in vaginal washes for as long as 60 hrs after intraperitoneal or intravenous injection of high titer rabbit polyclonal antiserum against whole gonococci (B.T. Mocca and A.E. Jerse, unpublished data). However, we did not detect gonococcal-specific antibodies in vaginal washes following intravenous injection of the affinity-purified HV2-specific rabbit antibodies used in this study, and we therefore chose to deliver the antibodies topically.
In summary, we have demonstrated that broadly-reactive antibodies can be generated against a relatively conserved Opa protein loop that bind to the bacterial surface and have agglutination ability. These antibodies could potentially recognize many Opa variants produced by different gonococcal strains and therefore, the use of different adjuvants or other strategies to induce high titered SV loop-specific antibodies with bactericidal activity that can be delivered systemically is warranted. The in vitro and in vivo experiments described here should be useful in the development of other vaccine antigens against gonorrhea.
Materials and Methods
Strains and Culture Conditions
Neisseria gonorrhoeae strain FA1090 [porB1b, streptomycin resistant, serum resistant (SR)] was originally isolated from a female patient with disseminated gonococcal infection. Strain FA1090 expresses 8 antigenically distinct Opa proteins: OpaA, OpaB, OpaC, OpaD, OpaE, OpaF, OpaI and OpaK. Frozen stocks of each Opa variant were prepared as described [30]. Stock Opa variants expressed LOS species with the same banding pattern on silver stained tricine gels (data not shown) and stocks composed of either mostly piliated or nonpiliated variants were maintained. Strain FA1090F62por5–8 is a serum sensitive (SS) derivative of strain FA1090 in which porin loops 5–8 were replaced with loops 5–8 of the SS strain F62 as described by Ram et al. (kindly provided by Sanjay Ram, University of Massachusetts) [31]. Strain FA1090F62 por5–8 is sensitive to NHS, and does not bind human C4b-binding protein (hC4BP). OpaA, OpaF, and Opa-negative variants were isolated from OpaB-expressing FA1090F62por5–8 bacteria after 2–3 serial passages of individual colonies that were screened by colony suspension immunoblots with HV2-specific antibodies as described [30], [32]. Where indicated, recombinant strains of FA1090 that express no Opa proteins or that constitutively express only OpaB were used (kindly provided by Janne Cannon, University of North Carolina). N. gonorrhoeae was cultured on GC agar (Difco) with Kellogg's supplement [33] and 0.2 µM Fe(NO3)3 at 37°C under 7% CO2. GC-VCNTS agar (GC agar with vancomycin, colistin, nystatin, trimethoprim, and streptomycin sulfate) was as described [32].
Generation of Antibodies
Two general types of antibodies were evaluated in this study, specifically affinity-purified rabbit polyclonal antibodies against linear peptides (Ablinear) and mouse antisera raised against cyclic peptides (Abcyclic). Ab4L linear, AbHV2A linear, AbHV2BD linear, AbHV2C linear, AbHV2F linear, AbHV2I linear, and AbHV2K linear antibodies were previously described [30]; here we obtained SV-loop specific rabbit antibodies (AbSV linear), which were generated against a linear 20 amino acid peptide (DYPEPTGAKKGKISTVSDYF) that corresponds to the SV loop of OpaA and OpaK (OpaA/K) of strain FA1090 (Figure 1). Peptide synthesis, rabbit immunizations, and affinity purification were performed by Bethyl Laboratories (Montgomery, Texas). Rabbit antibodies were dialyzed (50 kDa exclusions pore size) (Spectrum Laboratories Inc, Racho Dominguez, CA) to remove the 0.1% sodium azide that was added during preparation. We also generated mouse antisera (AbSV cyclic and AbBD cyclic) to two cyclic peptides that correspond to the SV loop sequence of OpaA/K (AAERITHDYPEPTGAKKGKISTVSDYFRNIRTHSIH; 36-mer) or the HV2 loop sequence that is common to OpaB and OpaD (OpaB/D) (IDSTKKITGTLTAYPSDADAAVTVYPDGHPQKNTYQ; 36-mer). Cyclic peptides were synthesized by Celtek Peptides (Nashville, TN) through the addition of a disulfide bond between added terminal cysteine residues, and six week-old female BALB/c mice were immunized subcutaneously three times at three week intervals with 50 µg of peptide suspended in TiterMax Gold (Sigma Chemical Co, St. Louis, MO). Blood was collected two weeks after the final boost, and individual samples were analyzed by enzyme linked immunosorbent assay (ELISA) for peptide-specific antibody titers essentially as described [34]. Briefly, 96-well plates were coated with 5 µg of the cyclic peptide in 50 mM NaHCO3 (pH 9.6), and incubated with three-fold dilutions of mouse sera followed by goat anti-mouse IgG (γ chain-specific) conjugated to horseradish peroxidase (HRP) and HRP substrate (Sigma). Absorbance was read at 405 nm on an EL800 Universal Microplate Reader (Bio-Tek Instruments, Winooski, VT) and analyzed with KC Junior software (Bio-Tek Instruments). Background was set at 3 standard deviations above the average A405 readings of 3 wells to which no primary antibody was added. Sera with titers >1∶7,290 were pooled and frozen at −20°C. The relative levels of IgG isotypes within Opa loop-specific mouse antisera were measured with a mouse antibody isotyping kit (Bio-Rad Laboratories, Hercules, CA) as per the manufacturer's instructions.
Immunoblots
For western blots, bacteria were suspended in lithium acetate buffer and incubated in Laemmli buffer (Sigma) containing sodium dodecyl sulfate (SDS) and β-mercaptoethanol for 10 min at 100°C to denature the samples or at room temperature (RT) to preserved native conformations. Samples were fractionated on 11.5% SDS polyacrylamide gels and transfered to polyvinylidene fluoride (PVDF) membranes. After blocking in 0.5% Tween 20, membranes were incubated with AbSV linear (1∶75,000), Ab4L linear (1∶6,000), or AbSV cyclic (1∶6,000), followed by goat anti-rabbit IgG HRP (1∶50,000) (Bethyl Laboratories) or anti-mouse IgG HRP (1∶50,000) (Sigma). Primary and secondary antibodies were diluted in block, and blots were washed three times in PBS with 0.05% Tween 20 after each incubation. Detection was with ECL detection reagent (Amersham Biosciences) as per the manufacturer's instructions. Attempts to utilize an ELISA with whole bacteria as the antigen to measure surface-binding were not successful due to the bacteria not adhering well enough to the wells. Therefore, a semi-quantative surface-binding immunoblot (SBI) similar to that described by Afonina et al. [35] was used to measure the binding of antibodies to intact gonococci. Bacteria of the Opa phenotype to be tested were suspended in PBS to an A600 of 0.20 and diluted 1∶20 in PBS. One hundred microliters (∼5×105 CFU) of the final suspensions were applied to a nitrocellulose membrane via a 96-well vacuum manifold apparatus (Schleicher & Schuell, Keene, NH). The membrane was dried at RT, incubated for 30 min at 37°C, and then blocked for 1 hr in PBS with 3% BSA (Sigma). The filter was returned to the manifold and individual wells were incubated for 1 hr with 100 µL of two-fold serial dilutions of affinity-purified rabbit antibodies (AbSV linear, Ab4L, AbHV2 linear; range 8.2–720 ng) or mouse antisera (Absv cyclic, AbHV2BD cyclic; range 1∶20–1∶320). Positive control wells were incubated with serial dilutions of rabbit polyclonal antiserum against heat-killed FA1090 bacteria (1∶4,000–1∶64,000) or the porin-specific monoclonal antibody B2E8 (1∶250–1∶4,000) (A.E. Jerse and Mary Petzke, unpublished data). All antibodies were diluted in PBS with 3% BSA, and secondary detection, washes, and exposure of the membranes to substrate were as for western blots. Spot intensities were quantified by densitometry (Image J Version 1.37v) and the mean intensity of three wells incubated without primary antibody was subtracted from that of wells with Opa-specific antibodies (test wells) or anti-whole bacteria or B2E8 antibodies (control wells). The spot intensities of the control wells were plotted against the antibody concentration (Ablinear, rabbit) or antiserum dilution (Abcyclic, mouse), and values that fell within the linear regions of the curves were used to normalize for slight differences in the number of bacteria in each spot. Normalized data were obtained by dividing the mean intensity of the test wells by that of the appropriate control well (mouse or rabbit antibody).
Indirect Fluorescent Antibody (IFA) Staining
Single colonies of FA1090 Opa variants were suspended in water, applied to IFA slides (Electron Microscopy Sciences, Hatfield, PA), and fixed in 100% methanol at −20°C after drying at RT. Slides were blocked for 1 hr in PBS with 0.1% immunoglobulin-free BSA (Sigma) (blocking buffer). Slides were incubated with primary antibodies for 1 hr at the following final concentrations or dilutions, which were determined empirically: AbHV2 linear (0.87–1.2 µg/mL), AbSV linear (2.2 µg/mL), Ab4L linear (2.4 µg/ml), and AbHV2 cyclic (1∶100) and AbSV cyclic (1∶30). Secondary antibodies were goat anti-rabbit or goat anti-mouse IgG conjugated to AlexaFluor 488 (Invitrogen, Carlsbad, CA) (1∶500) and incubations were for 30 min. All antibodies were diluted in blocking buffer and wells were washed five times with PBS after each incubation. Antibodies specific for the HV2 loop of each Opa variant were used as positive controls (0.87–1.2 µg/mL) in all IFA assays; polyclonal rabbit antisera against heat-killed FA1090 (1∶1,000) was used as a positive control for Opa-negative variants; negative controls were antibodies against heterologous HV2 loops and wells that were not incubated with primary antibodies. Slides were examined with an Olympus BX60 system microscope with a BX-FLA reflected light fluorescence attachment and Olympus U-M41001 filter. All images were obtained with a SPOT charge-coupled-device digital camera (Diagnostic Instruments Inc., Sterling Heights, MI).
Bactericidal Assay
Bactericidal assays against wild type FA1090 Opa variants were performed in microtiter plates using 10% normal human serum (NHS) (Quidel Corporation, San Diego, CA) or 1% baby bunny serum (BBS) (AbD Serotec, Raleigh, NC) as the complement source. For assays that used strain FA1090F62por5–8, 1% NHS was used. These serum concentrations are >2-fold lower than that concentration that showed no killing of the target strains in the absence of added antibody as determined by Garvin et al [36]. For testing Opa loop-specific antibodies, Ablinear or Abcyclic were serially diluted in minimal essential medium (MEM) (final volume 150 µl), and 50 µl of diluted NHS or BBS were added to each well to achieve the final serum concentrations stated above based on a final 250 µl volume. Bacteria to be tested were harvested from solid GC agar after 20–22 hrs growth, suspended in MEM, and 50 µl containing 1.5–2.5×103 CFU were added to each well. Plates were incubated at 37°C in 7% CO2 for 1 hr, after which 50 µl of GCB were added and mixed. Two 50 µl aliquots were cultured on GC agar and the average number of CFU recovered was determined. The bactericidal50 titer was that concentration of antibody that resulted in a 50% reduction in the number of CFU recovered from wells that contained serum but no added test antibodies. Polyclonal rabbit antiserum against whole gonococci, which showed high level bactericidal activity against all Opa variants tested, was used as a positive control and antibodies that do not bind the target strain were used as negative controls. Heat-inactivated (HI) NHS and BBS were prepared by incubation at 56°C for 30 min, and were tested in parallel for each assay; none of the test or control antibodies had activity when HI serum was used. At least two independent experiments were performed for each test antibody, and the results were similar.
Agglutination Assay
Agglutination titers were determined by the method of Pal et al [37]. Bacteria from primary subcultures of frozen stocks of Opa variants were suspended in PBS and passed through a 1.2 µm pore to remove aggregates. Filter suspensions were adjusted to an A600 of 0.4. Test antibodies or antisera were serially diluted 2-fold and 10 µL of each dilution were incubated with 10 µL of bacteria (∼5×106 CFU) at 37°C in a microtiter plate for 45 min. Bacteria were incubated with the same dilutions of normal mouse sera (NMS) or affinity-purified polyclonal rabbit antibodies against heterologous HV2 loops in parallel. After incubation, 5 µL were spotted on a glass slide and stained with HEMA3 (Exaxol Corp). The average number of aggregates per at least three 40X fields was determined under light microscopy. Agglutination titers were defined as the highest dilution of test antibodies that caused greater than three times the number of aggregates seen in the same dilution of NMS or control antibodies. Comparisons between piliated and non-piliated variants of the same Opa type were also performed and identical results were obtained (data not shown).
Passive Protection Experiments
Female BALB/c mice 6–8 weeks of age (National Cancer Institute, Frederick, MD) were treated with 1.5 mg water-soluble 17β-estradiol (Sigma) and antibiotics to promote susceptibility to N. gonorrhoeae as described [38]. In pilot experiments, 5–8 mice per group were inoculated vaginally with 103, 104, or 105 CFU of predominantly OpaB-variants that were pre-incubated in PBS with 250 or 500 µg/ml of AbHV2BD linear or in PBS alone for 20 min at 37°C. In subsequent experiments with loop-specific affinity purified rabbit antibodies, bacteria (∼5×104 CFU/ml) were preincubated in PBS with 250 µg/ml of AbHV2A linear, AbHV2BD linear, or AbSV linear and 20 µl of the suspension (∼103 CFU and 5 µg of antibodies) were inoculated vaginally into mice (n = 7–15 mice per group). In studies with mouse antisera against cyclic peptides, mice were inoculated with a 20 µl suspension containing 6×103 CFU that were preincubated with AbHV2BD cyclic, AbSV cyclic, or NMS (final dilution of antiserum or NMS, 1∶30) (n = 10–11 mice per group). For all experiments, vaginal mucus was quantitatively cultured for N. gonorrhoeae daily for 3 days as described [32].
Measurement of Antibody Duration
In separate experiments, the amount of topically applied antibody recovered from the vagina was measured over time by inoculating 23 untreated, 6 week-old female BALB/c mice vaginally with 20 µL of PBS containing 10 µg of affinity-purified rabbit polyclonal Opa-specific antibodies. The vaginas of 2–3 mice per time point were washed 3 times with 40 µL PBS and the three samples from each mouse were pooled (∼120 µL) and centrifuged at 13,000 rpm for 3 min. Supernatants were frozen at −20°C. Control samples were collected from 3 untreated mice. The concentration of rabbit IgG in murine vaginal washes was measured with the Rabbit IgG Quantitative Kit ELISA (Bethyl Laboratories). Animal experiments were conducted in the laboratory animal facility at USUHS, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, under a protocol approved by the USUHS Institutional Animal Care and Use Committee.
Adherence Assay
ME180 cervical epithelial cells (ATCC, Manassas, VA) were grown to near confluency in 24-well tissue culture plates in McCoy's 5A medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Quality Biological Inc., Gaithersburg, MD) and 2.2 g/L sodium bicarbonate. Non-piliated OpaB-expressing bacteria were subcultured from the freezer and passed once to GC agar before being suspended in McCoy's 5A medium supplemented with 2.2 g/L sodium bicarbonate and 5 mg/L Fe(NO3)3 to an A600 of 0.07. Bacterial suspensions were diluted 1∶10 and pre-incubated for 5 min with mouse antisera against HV2 or SV cyclic peptides (test) or NMS (negative control) (final dilutions 1∶30 and 1∶100), or with 0.25 µg/mL or 2.5 µg/mL of AbHV2BD linear (test) or AbHV2C linear (negative control). Bacterial suspensions (500 µl) were applied to cells (multiplicity of infection, 10∶1) in triplicate wells. After 2 hrs at 37°C in 7% CO2, monolayers were washed four times with PBS to remove nonadherent bacteria. Cells were lysed with 0.5% saponin (Sigma) and the number of cell-associated bacteria was determined by serial dilution and culture of the saponin-treated supsensions. Results are expressed as the number of cell-associated bacteria divided by the number of bacteria in the inoculum (% cell-associated). The average percent of cell-associated bacteria recovered from in test and control wells was calculated from three independent experiments that were each performed in triplicate. Standard error bars are shown.
Statistical Analysis
A Fishers Exact test was used to compare the number of mice colonized in each experimental group in passive protection experiments. Differences in the number of gonococci recovered from mice and the recovery of cell-associated gonococci in tissue culture experiments were analyzed by the Student's t-test.
Acknowledgments
We thank Afrin Begum and Jana Jones for expert technical assistance with bactericidal assays and the SBI assay, respectively, and Dr. Cara Olsen for help with statistical analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by NIH/NIAID STI-TM-CRC grant U19 AI31496. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McNabb SJ, Jajosky RA, Hall-Baker PA, Adams DA, Sharp P, et al. Summary of notifiable diseases–United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;55:1–92. [PubMed] [Google Scholar]
- 2.Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74(Suppl 1):S12–16. [PubMed] [Google Scholar]
- 3.Hook EW, Handsfield HH. Gonococcal Infections in the adult. In: Holmes KK, Mardh P-A, Sparling PF, Lemon SM, Stamm WE, et al., editors. Sexually Transmitted Diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 451–466. [Google Scholar]
- 4.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 5.Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health. 2004;36:11–19. doi: 10.1363/psrh.36.11.04. [DOI] [PubMed] [Google Scholar]
- 6.Tapsall JW. Antibiotic resistance in Neisseria gonorrhoeae. Clin Infect Dis. 2005;41(Suppl 4):S263–268. doi: 10.1086/430787. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332–336. [PubMed] [Google Scholar]
- 8.Faruki H, Kohmescher RN, McKinney WP, Sparling PF. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally mediated resistance). N Engl J Med. 1985;313:607–611. doi: 10.1056/NEJM198509053131004. [DOI] [PubMed] [Google Scholar]
- 9.Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, et al. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. 1999;149:353–358. doi: 10.1093/oxfordjournals.aje.a009820. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs MM, Alcorn TM, Davis RH, Fischer W, Thomas JC, et al. Molecular typing of Neisseria gonorrhoeae causing repeated infections: evolution of porin during passage within a community. J Infect Dis. 1999;179:371–381. doi: 10.1086/314608. [DOI] [PubMed] [Google Scholar]
- 11.Plummer FA, Simonsen JN, Chubb H, Slaney L, Kimata J, et al. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989;83:1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan TM, Eschenbach DA, Knapp JS, Holmes KK. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980;138:978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- 13.Dehio C, Gray-Owen SD, Meyer TF. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey JA, Litaker W, Madhure A, Snodgrass TL, Cannon JG. Physical map of the chromosome of Neisseria gonorrhoeae FA1090 with locations of genetic markers, including opa and pil genes. J Bacteriol. 1991;173:5476–5486. doi: 10.1128/jb.173.17.5476-5486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat KS, Gibbs CP, Barrera O, Morrison SG, Jahnig F, et al. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 16.Malorny B, Morelli G, Kusecek B, Kolberg J, Achtman M. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. J Bacteriol. 1998;180:1323–1330. doi: 10.1128/jb.180.5.1323-1330.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy GL, Connell TD, Barritt DS, Koomey M, Cannon JG. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989;56:539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- 18.Stern A, Brown M, Nickel P, Meyer TF. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 19.James JF, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19:332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson J, Barrera O, Sola J, Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988;168:2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammel CJ, Sweet RL, Rice PA, Knapp JS Schoolnik GK, et al. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J Infect Dis. 1985;152:990–1001. doi: 10.1093/infdis/152.5.990. [DOI] [PubMed] [Google Scholar]
- 23.Zak K, Diaz JL, Jackson D, Heckels JE. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984;149:166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- 24.Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, et al. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest. 1994;93:1748–1755. doi: 10.1172/JCI117159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jonge MI, Vidarsson G, van Dijken HH, Hoogerhout P, van Alphen L, et al. Functional activity of antibodies against the recombinant OpaJ protein from Neisseria meningitidis. Infect Immun. 2003;71:2331–2340. doi: 10.1128/IAI.71.5.2331-2340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 27.Pantelic M, Kim YJ, Bolland S, Chen I, Shively J, et al. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun. 2005;73:4171–4179. doi: 10.1128/IAI.73.7.4171-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jonge MI, Hamstra HJ, Jiskoot W, Roholl P, Williams NA, et al. Intranasal immunisation of mice with liposomes containing recombinant meningococcal OpaB and OpaJ proteins. Vaccine. 2004;22:4021–4028. doi: 10.1016/j.vaccine.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Christodoulides M, McGuinness BT, Heckels JE. Immunization with synthetic peptides containing epitopes of the class 1 outer-membrane protein of Neisseria meningitidis: production of bactericidal antibodies on immunization with a cyclic peptide. J Gen Microbiol. 1993;139:1729–1738. doi: 10.1099/00221287-139-8-1729. [DOI] [PubMed] [Google Scholar]
- 30.Simms AN, Jerse AE. In vivo selection for Neisseria gonorrhoeae opacity protein expression in the absence of human carcinoembryonic antigen cell adhesion molecules. Infect Immun. 2006;74:2965–2974. doi: 10.1128/IAI.74.5.2965-2974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–295. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria Gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Ree JM, Schwillens P, van den Bosch JF. Monoclonal antibodies for serotyping the P fimbriae of uropathogenic Escherichia coli. J Clin Microbiol. 1986;24:121–125. doi: 10.1128/jcm.24.1.121-125.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afonina G, Leduc I, Nepluev I, Jeter C, Routh P, et al. Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA protects against infection in the swine model of chancroid. Infect Immun. 2006;74:2224–2232. doi: 10.1128/IAI.74.4.2224-2232.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garvin LE, Bash MC, Keys C, Warner DM, Ram S, et al. Phenotypic and genotypic analyses of Neisseria gonorrhoeae isolates that express frequently recovered PorB PIA variable region types suggest that certain P1a porin sequences confer a selective advantage for urogenital tract infection. Infect Immun. 2008;76:3700–3709. doi: 10.1128/IAI.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 38.Song W, Condron S, Mocca BT, Veit SJ, Hill D, et al. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngampasutadol J, Ram S, Blom AM, Jarva H, Jerse AE, et al. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci U S A. 2005;102:17142–17147. doi: 10.1073/pnas.0506471102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villullas S, Hill DJ, Sessions RB, Rea J, Virji M. Mutational analysis of human CEACAM1: the potential of receptor polymorphism in increasing host susceptibility to bacterial infection. Cell Microbiol. 2007;9:329–346. doi: 10.1111/j.1462-5822.2006.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parr EL, Parr MB. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 43.Whaley KJ, Zeitlin L, Barratt RA, Hoen TE, Cone RA. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 44.Ram S, Mackinnon FG, Gulati S, McQuillen DP, Vogel U, et al. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol Immunol. 1999;36:915–928. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 45.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, et al. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J Immunol. 2008;180:3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 46.Bos MP, Kao D, Hogan DM, Grant CC, Belland RJ. Carcinoembryonic antigen family receptor recognition by gonococcal Opa proteins requires distinct combinations of hypervariable Opa protein domains. Infect Immun. 2002;70:1715–1723. doi: 10.1128/IAI.70.4.1715-1723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol. 2001;42:659–672. doi: 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 48.Han E, Phan D, Lo P, Poy MN, Behringer R, et al. Differences in tissue-specific and embryonic expression of mouse Ceacam1 and Ceacam2 genes. Biochem J. 2001;355:417–423. doi: 10.1042/0264-6021:3550417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bos MP, Hogan D, Belland RJ. Selection of Opa+ Neisseria gonorrhoeae by limited availability of normal human serum. Infect Immun. 1997;65:645–650. doi: 10.1128/iai.65.2.645-650.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherwood JK, Zeitlin L, Chen X, Whaley KJ, Cone RA, et al. Residence half-life of IgG administered topically to the mouse vagina. Biol Reprod. 1996;54:264–269. doi: 10.1095/biolreprod54.1.264. [DOI] [PubMed] [Google Scholar]