Abstract
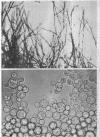
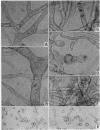
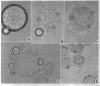
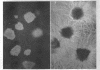
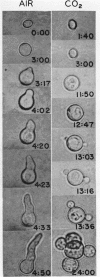
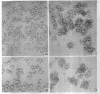
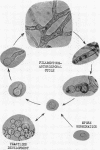
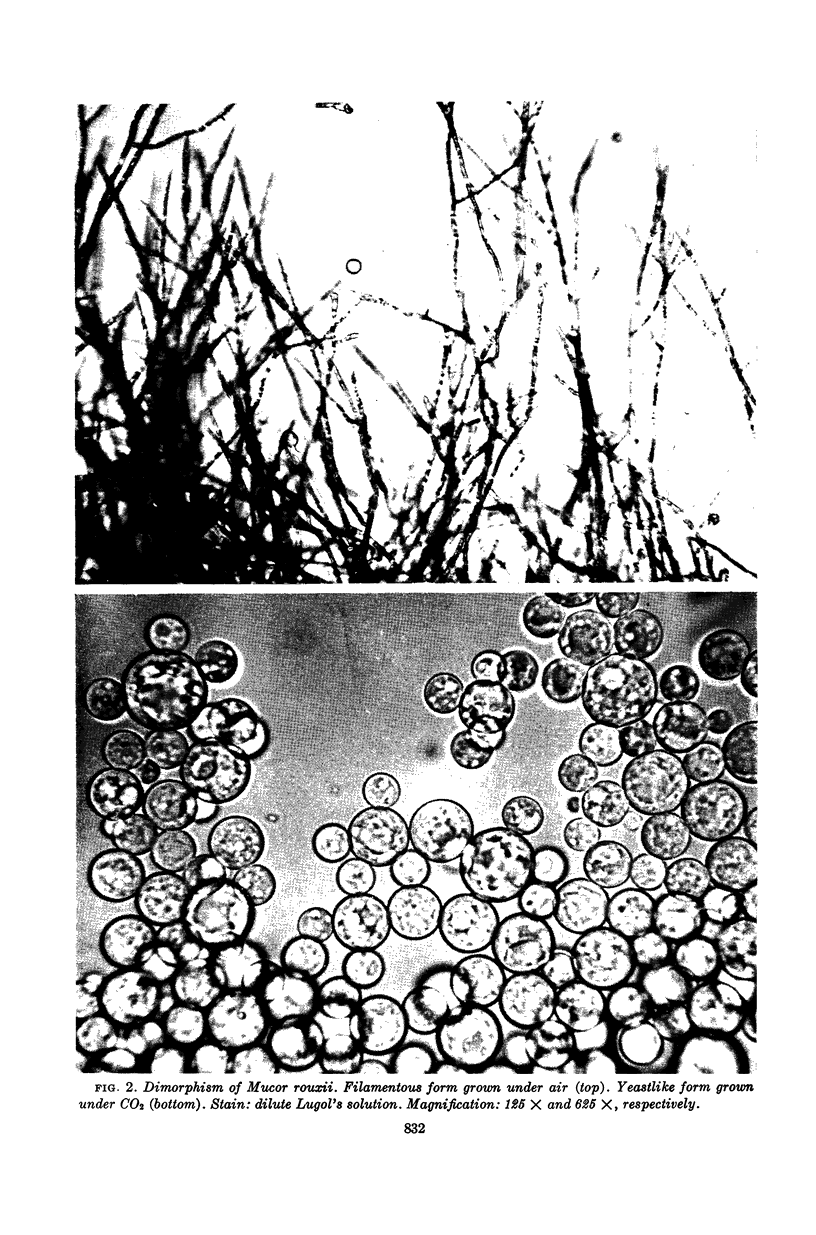
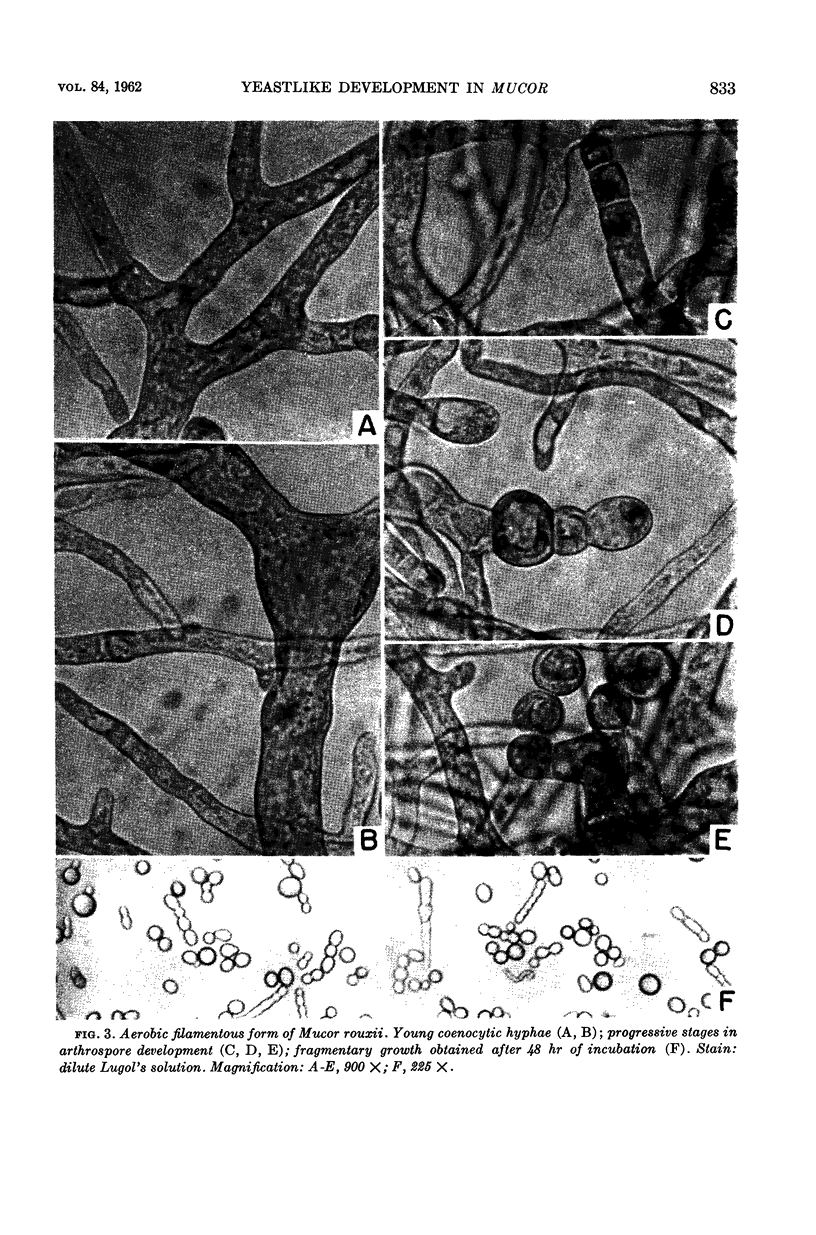
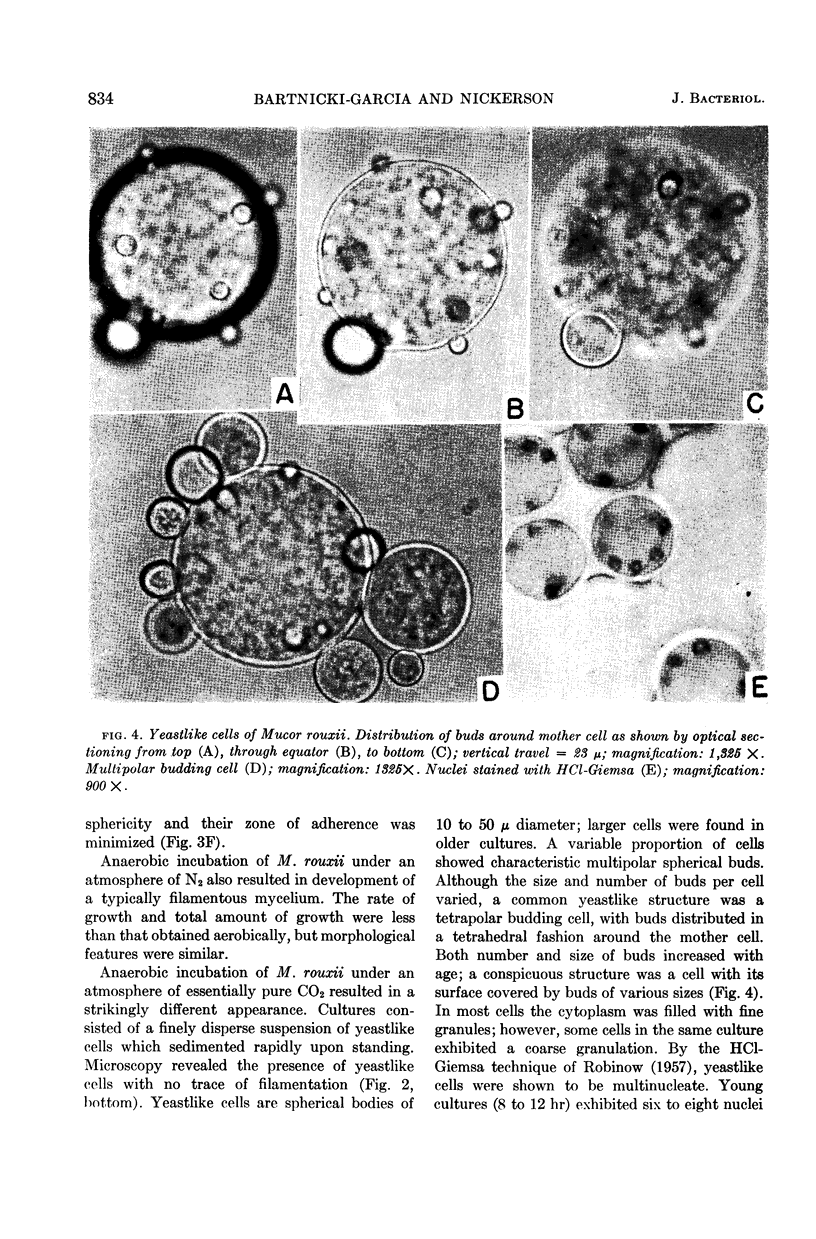
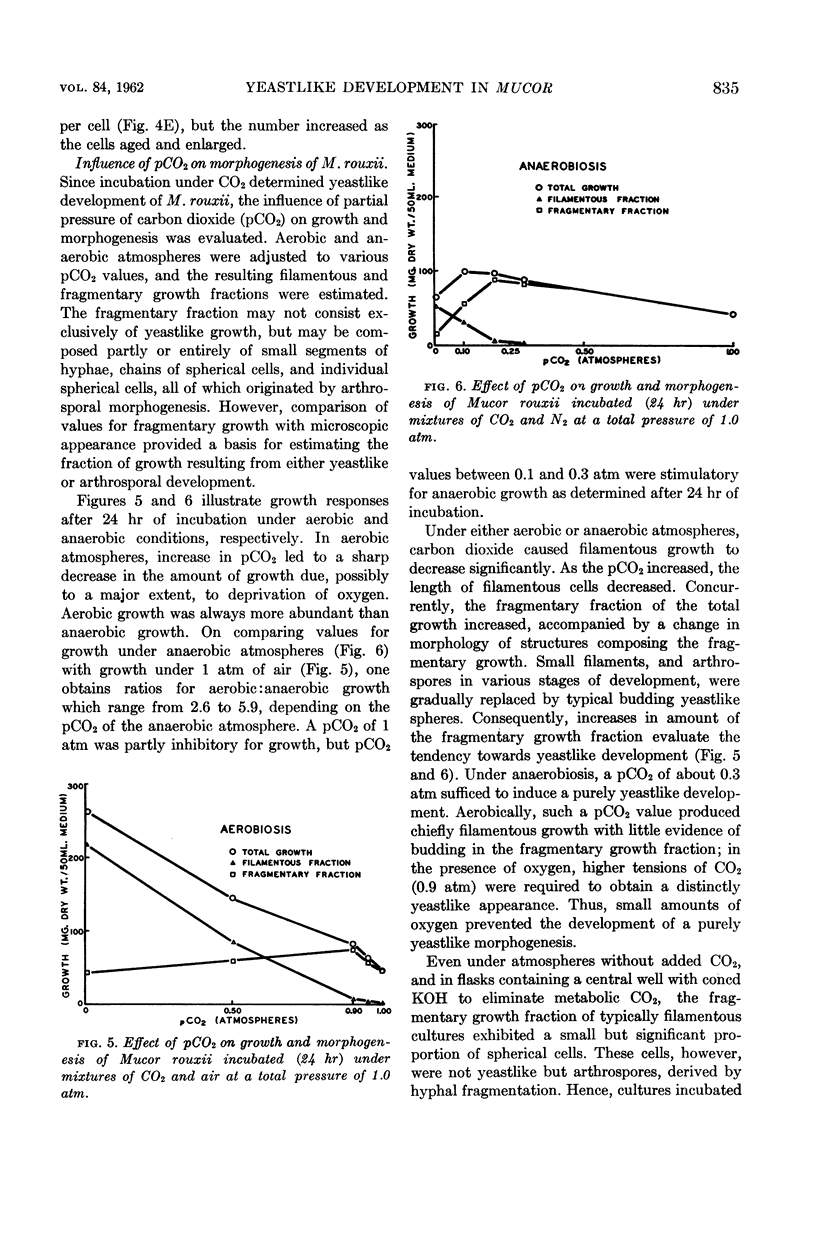
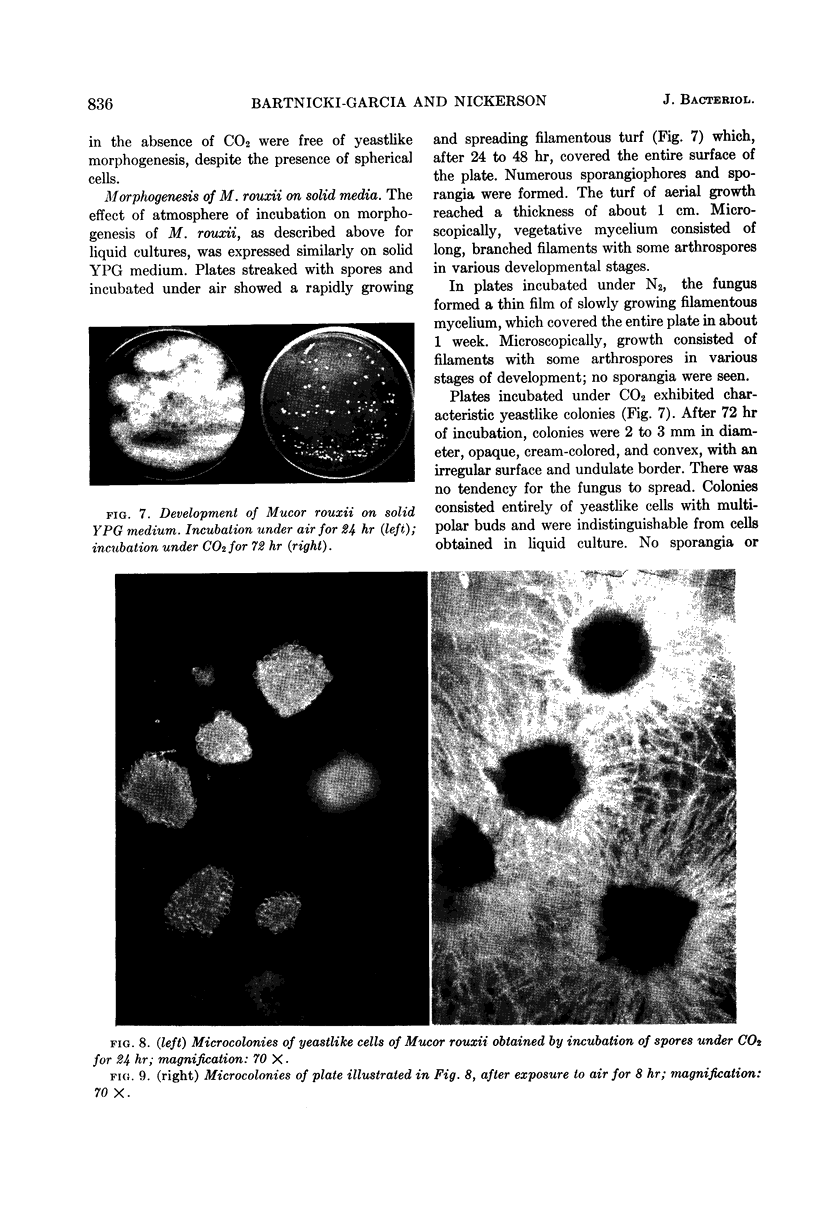
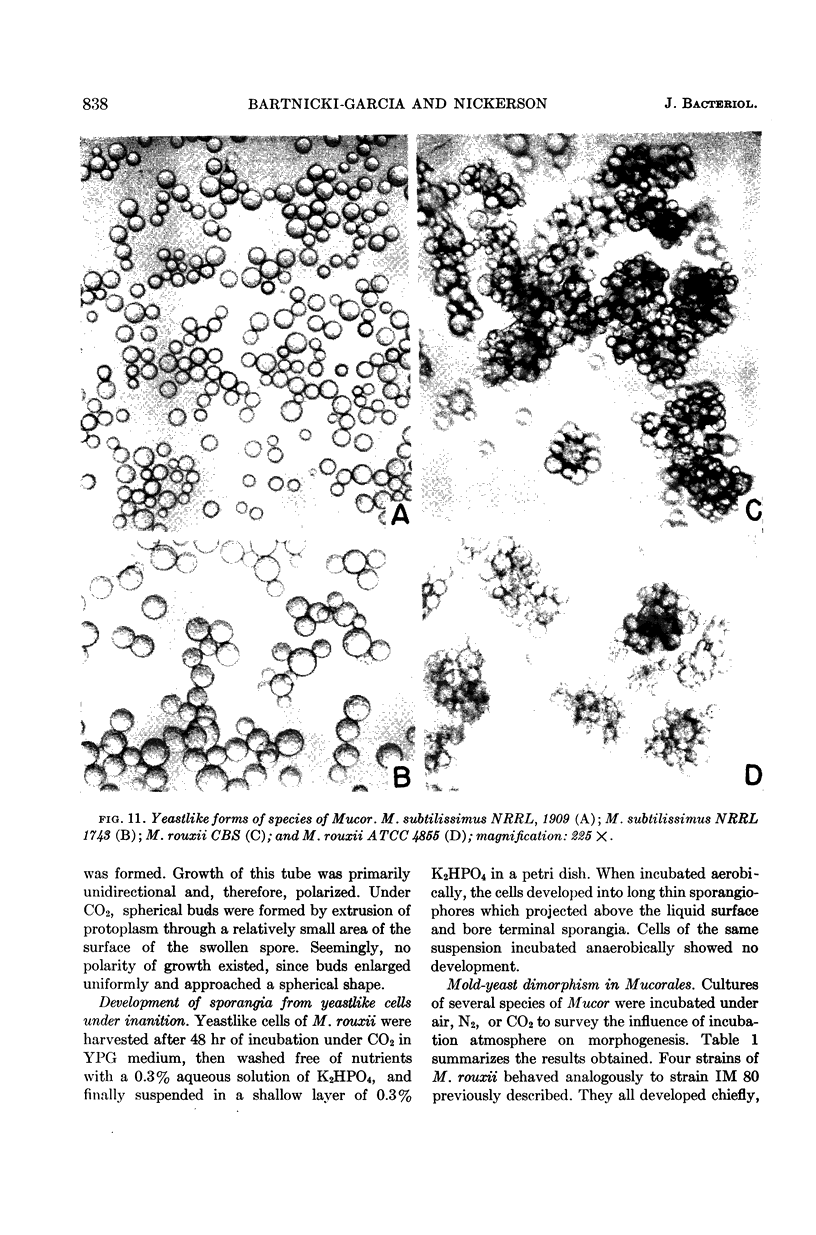
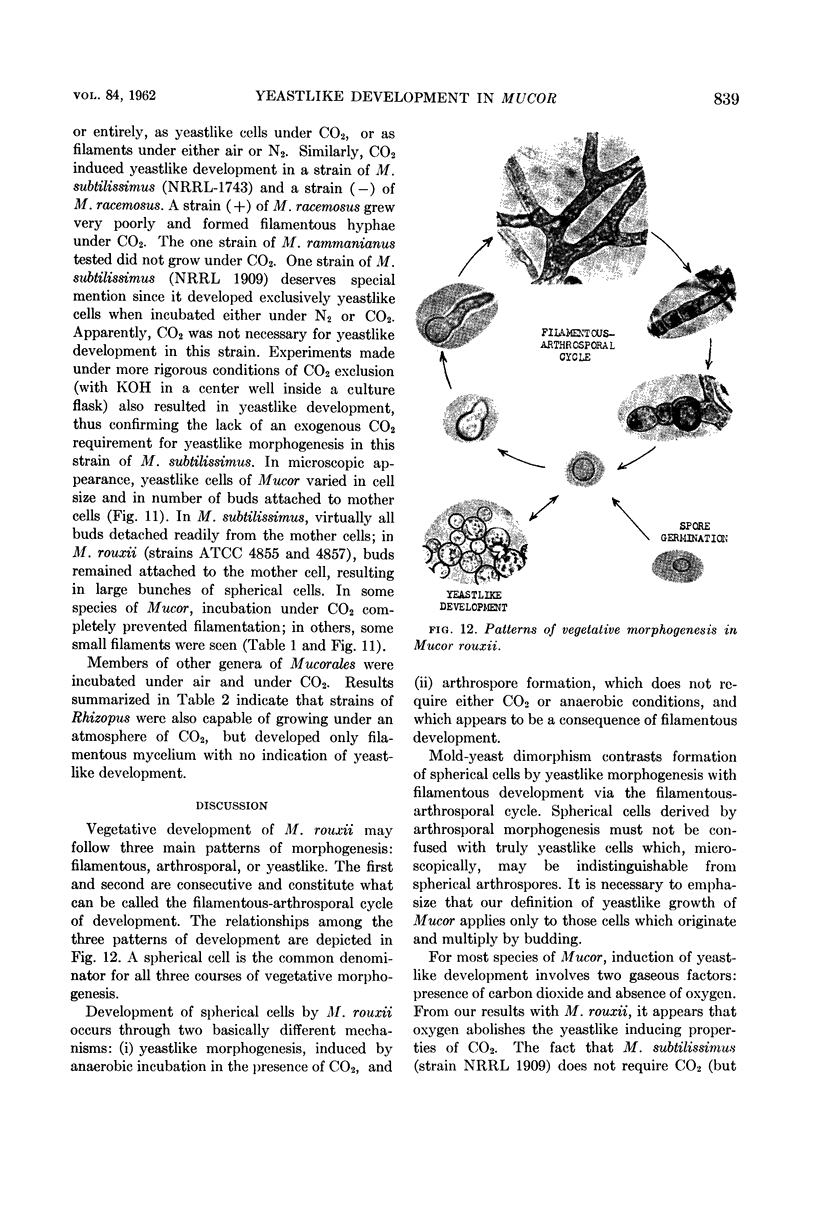
Bartnicki-Garcia, S. (Rutgers, The State University, New Brunswick, N. J.) and Walter J. Nickerson. Induction of yeastlike development in Mucor by carbon dioxide. J. Bacteriol. 84:829–840. 1962—Vegetative development of Mucor rouxii may follow either one of two patterns of morphogenesis (mold-yeast dimorphism), depending on the atmosphere of incubation. Under air or N2, a filamentous (moldlike) growth developed, commonly followed by fragmentation of hyphae into spherical cells (arthrospores). Introduction of CO2 into an anaerobic atmosphere induced development of spherical, budding yeastlike cells. Anaerobically, a pCO2 of 0.3 atm or higher produced a purely yeastlike development. Presence of oxygen annulled the effect of CO2 On germination, spores gave rise directly to either type of vegetative development, depending on the atmosphere of incubation. Induction of yeastlike development by CO2 occurred in five strains of M. rouxii, and in most species of Mucor tested. M. subtilissimus, however, did not require CO2; it developed in the yeastlike form under anaerobic conditions. Strains of Rhizopus grew under CO2, but developed only filamentous mycelium. Members of other genera of Mucorales were unable to grow under an atmosphere of CO2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- DROUHET E., MARIAT F. Etude des facteurs déterminant le développement de la phase levure de Sporotrichum schencki. Ann Inst Pasteur (Paris) 1952 Oct;83(4):506–514. [PubMed] [Google Scholar]
- LONES G. W., PEACOCK C. L. Role of carbon dioxide in the dimorphism of Coccidioides immitis. J Bacteriol. 1960 Feb;79:308–309. doi: 10.1128/jb.79.2.308-309.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINOW C. F. The structure and behavior of the nuclei in spores and growing hyphae of Mucorales. I. Mucor hiemalis and Mucor fragilis. Can J Microbiol. 1957 Aug;3(5):771–789. doi: 10.1139/m57-087. [DOI] [PubMed] [Google Scholar]