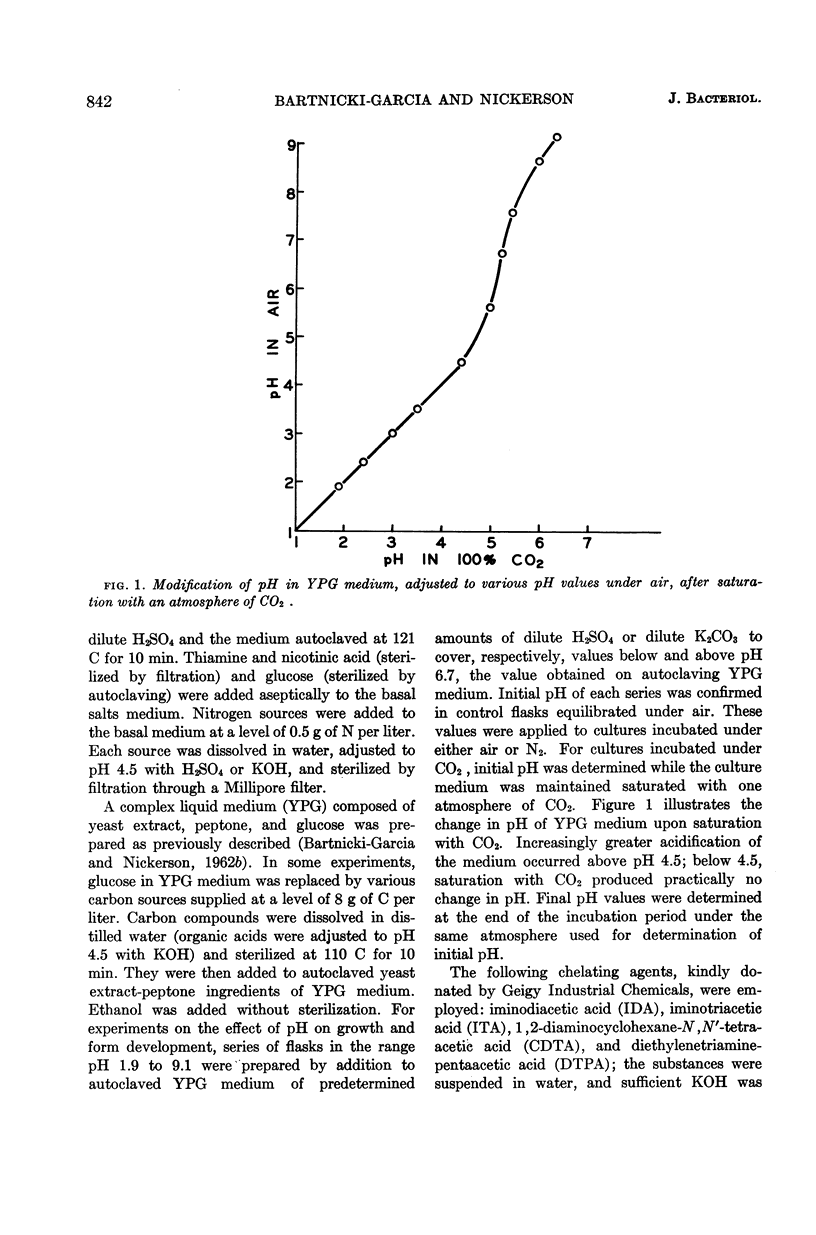
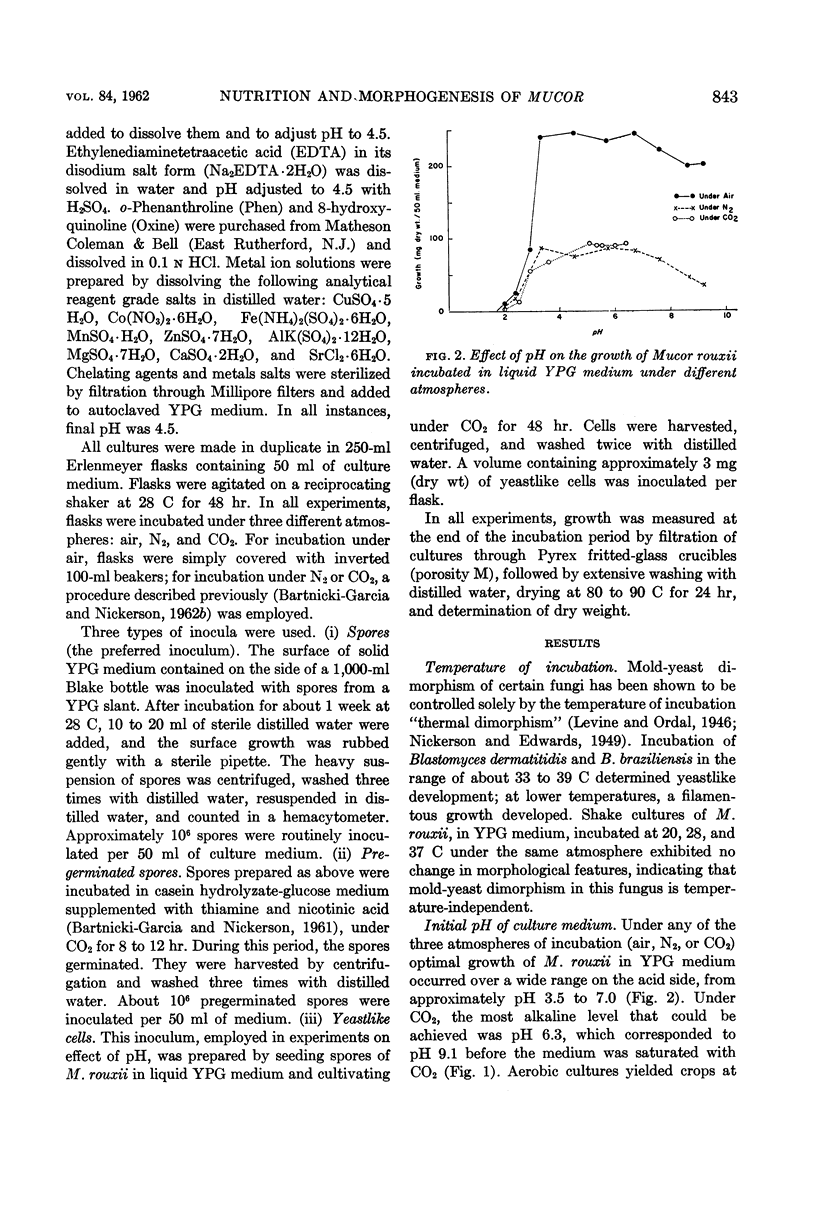
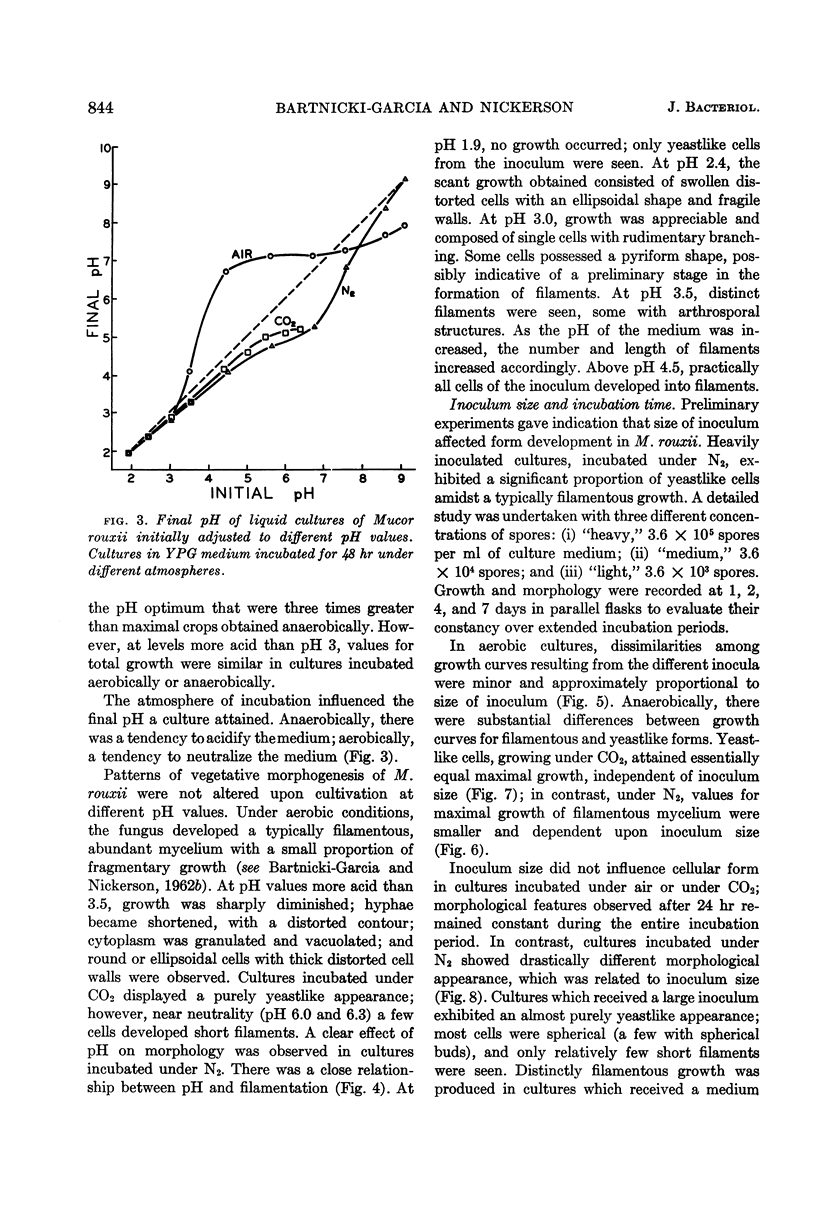
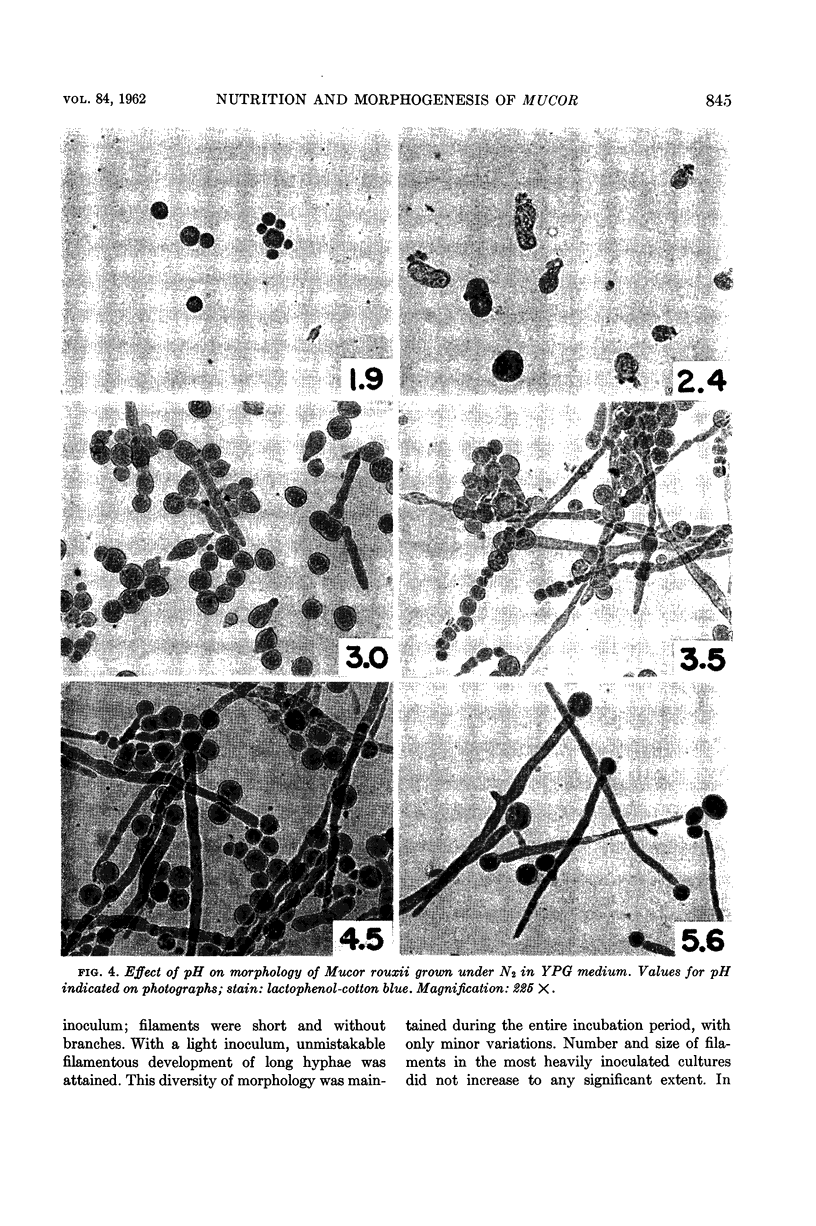
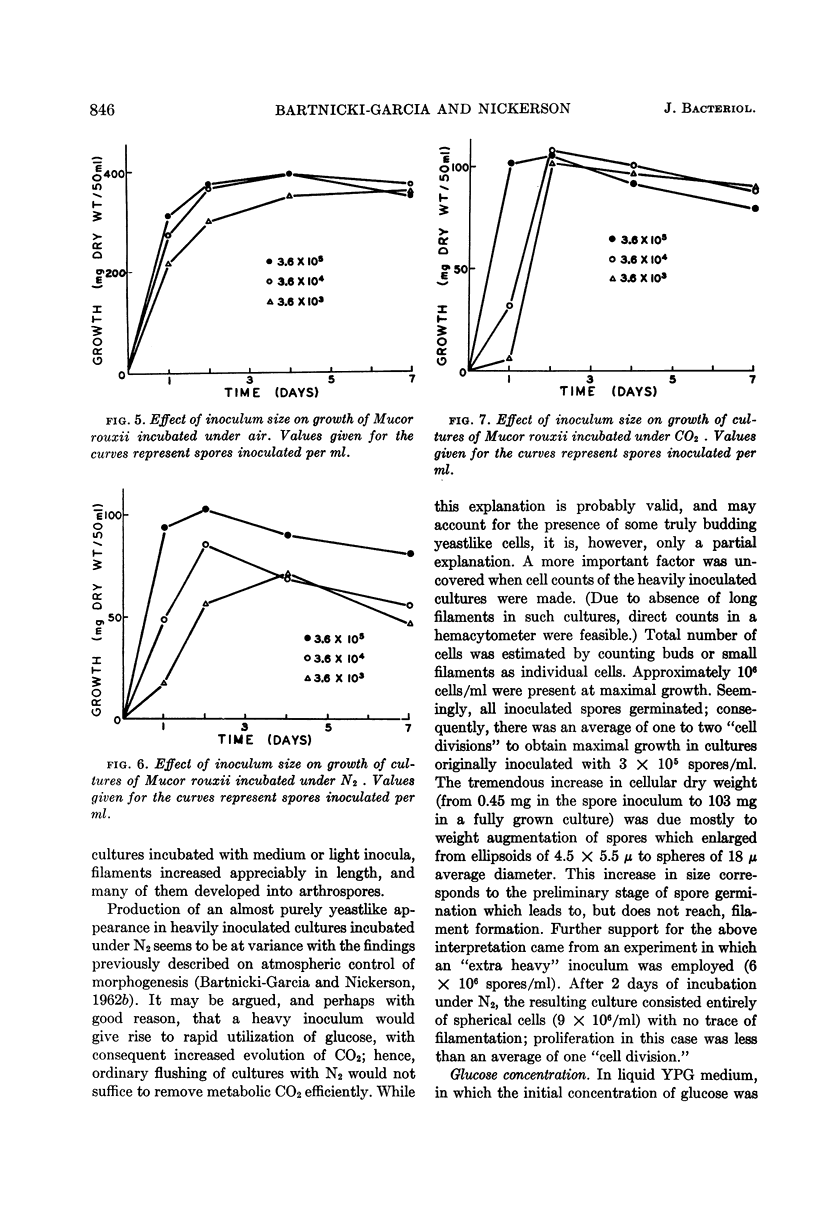
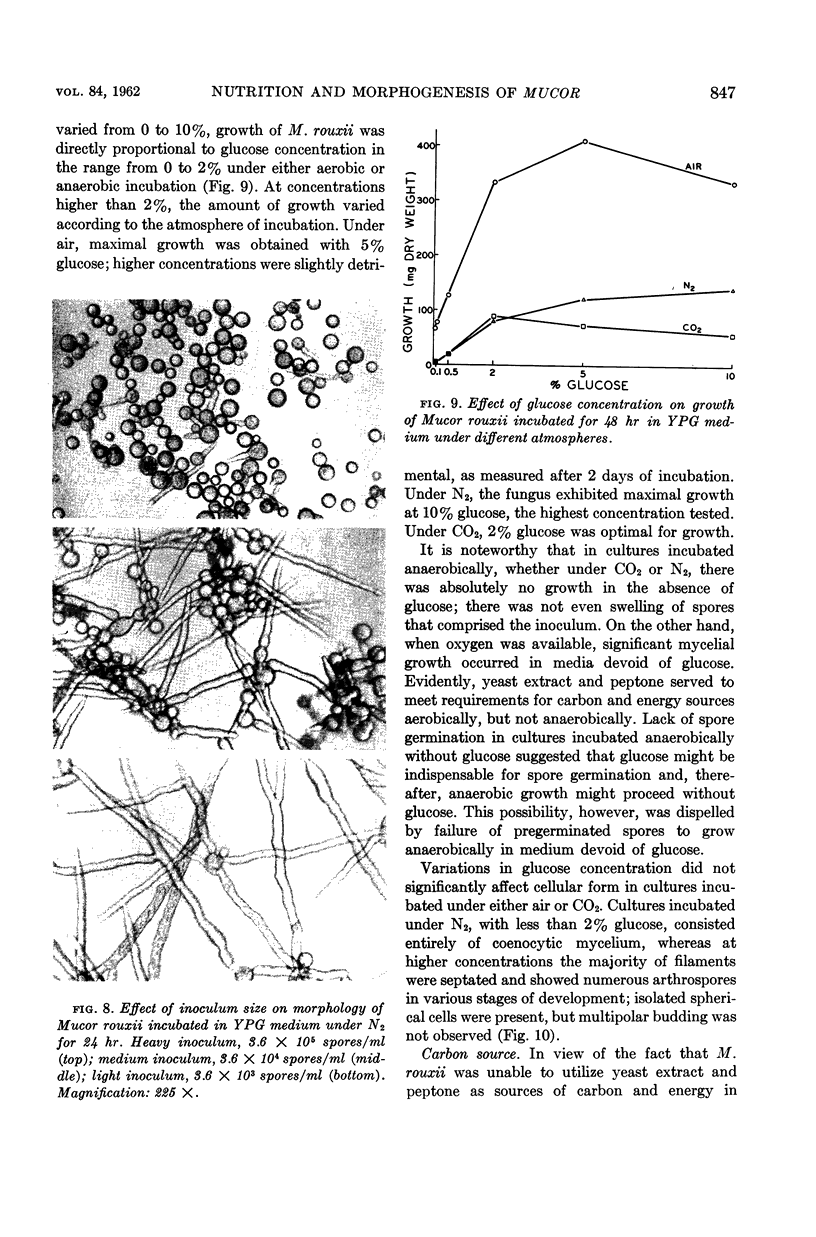
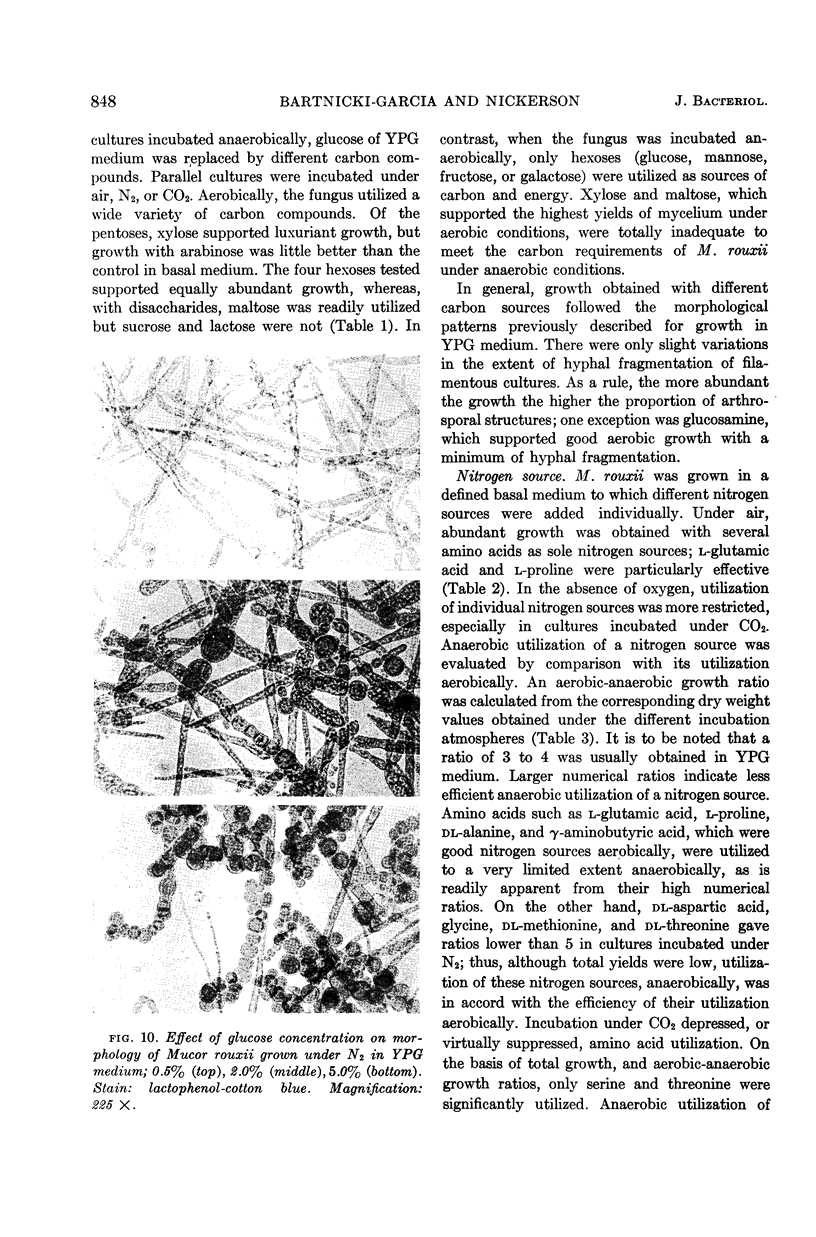
Abstract
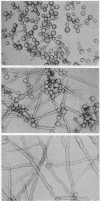
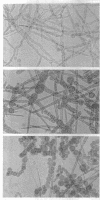
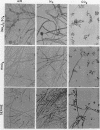
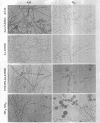
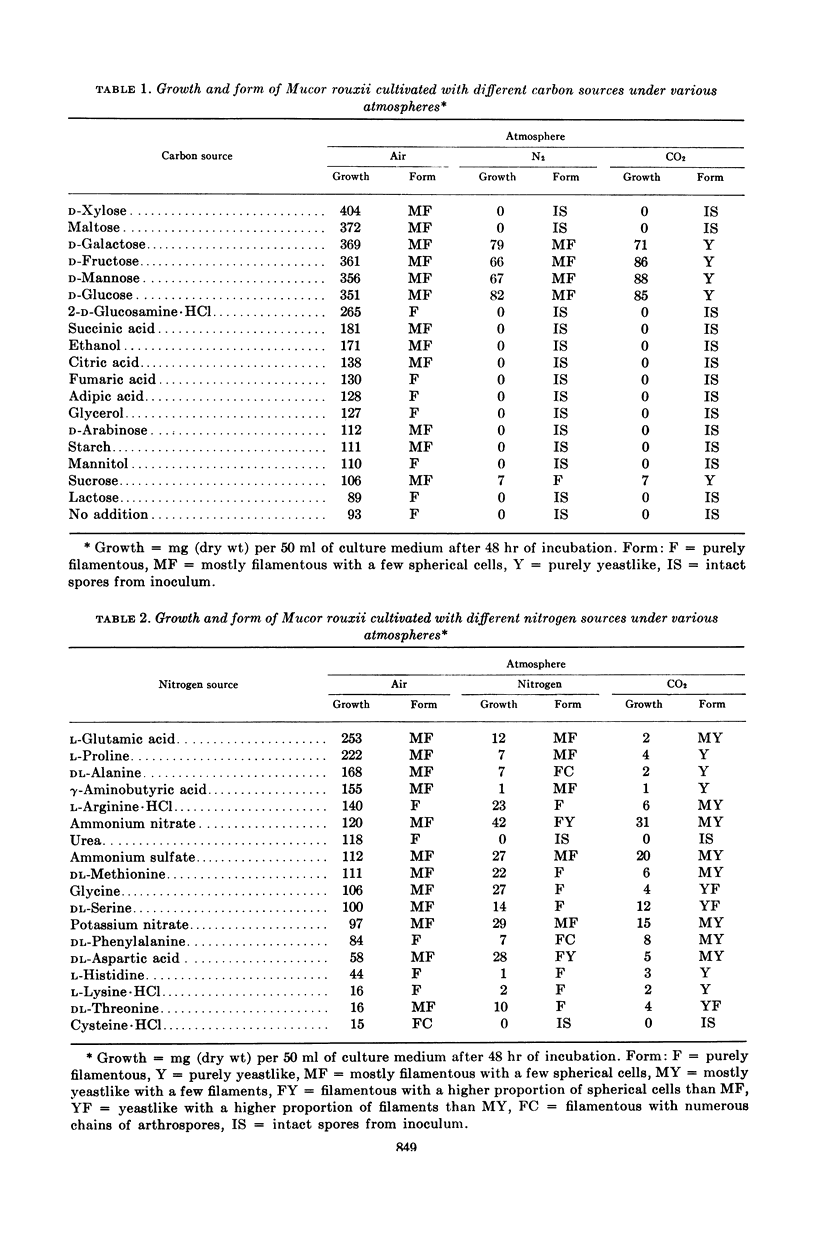
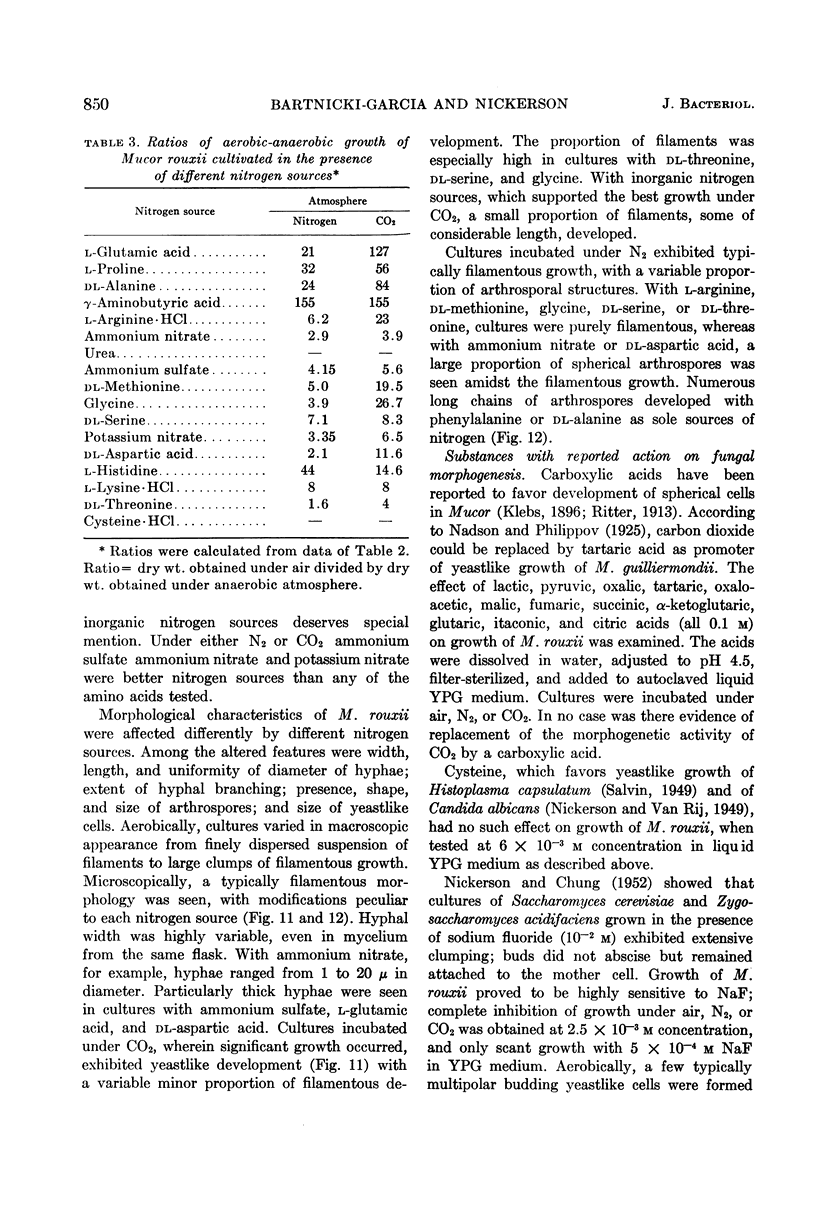
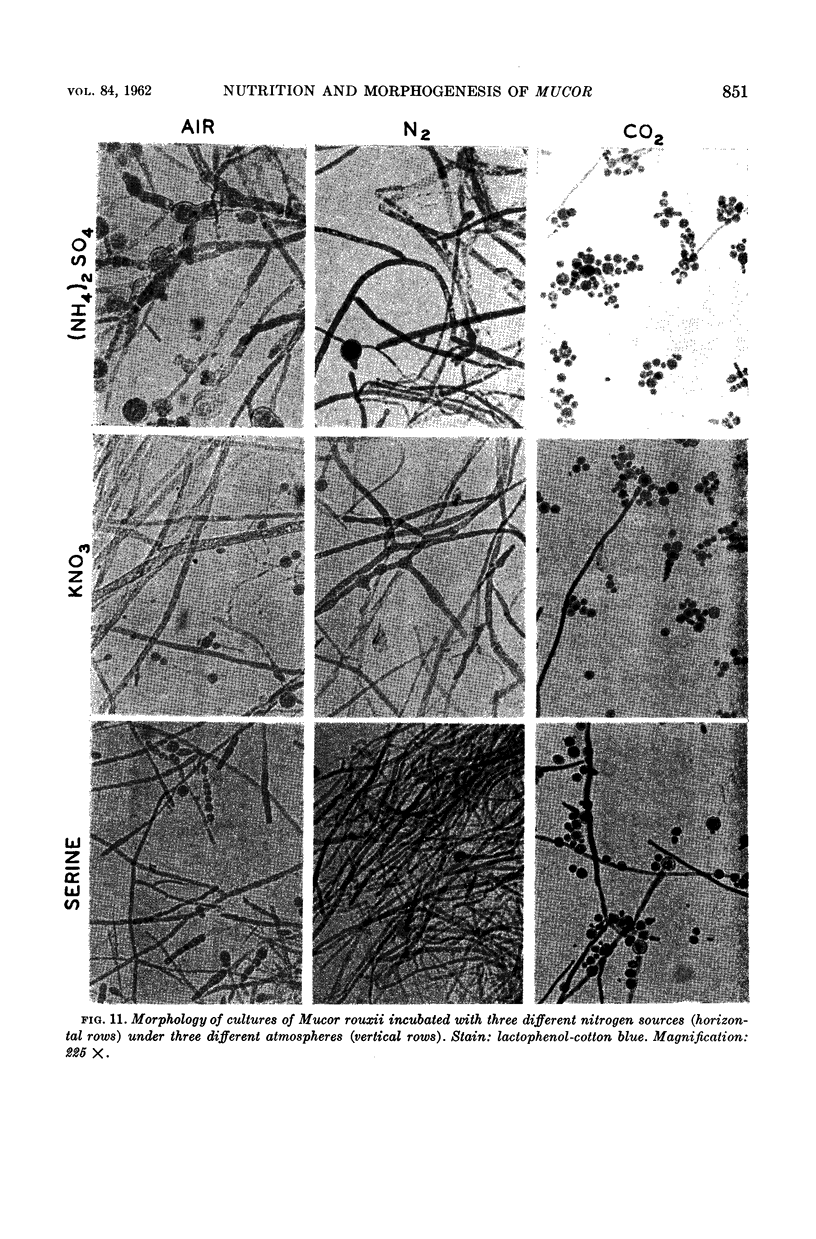
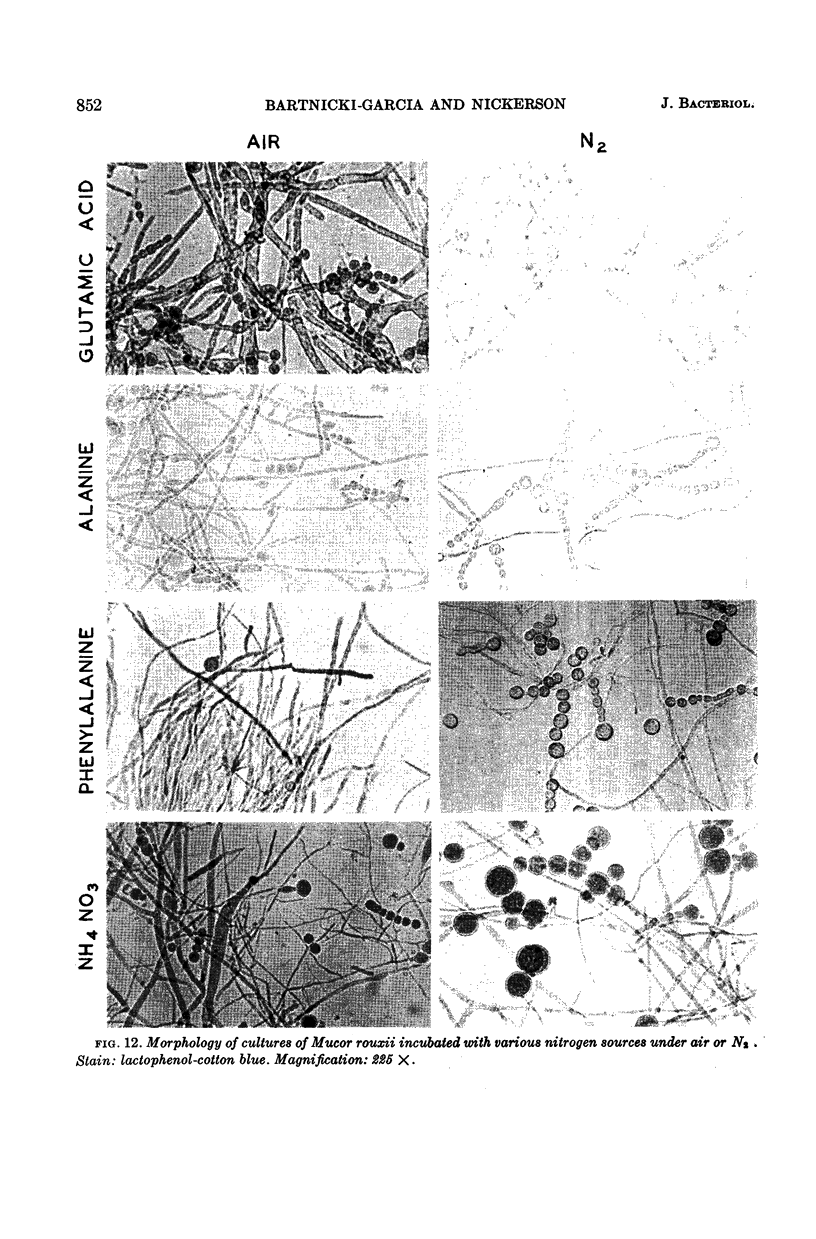
Bartnicki-Garcia, S. (Rutgers, The State University, New Brunswick, N.J.) and Walter J. Nickerson. Nutrition, growth, and morphogenesis of Mucor rouxii. J. Bacteriol. 84:841–858. 1962.—Mucor rouxii was grown under three different atmospheres of incubation: air, N2, and CO2 in parallel cultures. The atmosphere of incubation markedly affected nutritional requirements, growth, and morphogenesis. Absence of oxygen greatly reduced growth and increased the nutritional demands of the fungus. Presence of a high tension of CO2 resulted in a change from filamentous to yeastlike morphogenesis. Aerobically, a large variety of carbon sources was utilized; anaerobically, only hexoses served to meet requirements for carbon and energy. Aerobically, various amino acids supported abundant growth; anaerobically, they were poorly utilized. Ammonium and nitrate ions were better sources of nitrogen for anaerobic growth. In general, incubation under either air or N2 resulted in development of coenocytic filamentous mycelium, whereas incubation under CO2 resulted in development of budding yeastlike cells. Variations in temperature and time of incubation, inoculum size, type and concentration of carbon source, type of nitrogen source, and presence of various substances with known action on fungal morphogenesis altered growth in many cases, but did not significantly affect the patterns of vegetative morphogenesis conditioned by each atmosphere of incubation. However, vegetative morphogenesis was strongly affected by addition of certain chelating agents. Yeastlike development of M. rouxii was prevented by ethylene-diaminetetraacetic acid (EDTA) in concentrations which were also partially inhibitory for growth; under these conditions, development was filamentous. Chemically related chelating agents were similarly active. The growth-inhibitory and morphogenetic effects of EDTA were reversed by transition-group metal ions. Yeastlike development of M. subtilissimus, which does not require CO2 for its induction, was also inhibited by EDTA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Nickerson W. J. THIAMINE AND NICOTINIC ACID: ANAEROBIC GROWTH FACTORS FOR MUCOR ROUXII. J Bacteriol. 1961 Jul;82(1):142–148. doi: 10.1128/jb.82.1.142-148.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE TERRA N., TATUM E. L. Colonial growth of Neurospora. Sorbose and enzymes alter the composition of the cell wall and induce morphological changes. Science. 1961 Oct 13;134(3485):1066–1068. doi: 10.1126/science.134.3485.1066. [DOI] [PubMed] [Google Scholar]
- HARRIS G., THOMPSON C. C. The uptake of nutrients by yeasts. III. The maltose permease of a brewing yeast. Biochim Biophys Acta. 1961 Sep 2;52:176–183. doi: 10.1016/0006-3002(61)90915-5. [DOI] [PubMed] [Google Scholar]
- Levine S., Ordal Z. J. Factors Influencing the Morphology of Blastomyces dermatitidis. J Bacteriol. 1946 Dec;52(6):687–694. doi: 10.1128/jb.52.6.687-694.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum E. L., Barratt R. W., Cutter V. M., Jr Chemical Induction of Colonial Paramorphs in Neurospora and Syncephalastrum. Science. 1949 May 20;109(2838):509–511. doi: 10.1126/science.109.2838.509. [DOI] [PubMed] [Google Scholar]