Summary of recent advances
A family of small molecules called ascarosides act as pheromones to control multiple behaviors in the nematode Caenorhabditis elegans. At picomolar concentrations, a synergistic mixture of at least three ascarosides produced by hermaphrodites causes male-specific attraction. At higher concentrations, the same ascarosides, perhaps in a different mixture, induce the developmentally arrested stage known as dauer. The production of ascarosides is strongly dependent on environmental conditions, although relatively little is known about the major variables and mechanisms of their regulation. Thus, male mating and dauer formation are linked through a common set of small molecules whose expression is sensitive to a given microenvironment, suggesting a model by which ascarosides regulate the overall life cycle of C. elegans.
Introduction
The nematode Caenorhabditis elegans is one of the best-studied animals on earth. It has both self-fertilizing hermaphrodites and males, about 1000 cells, a simple nervous system with about 300 neurons, and is a nearly perfect genetic model organism with a 3.5 day development time from fertilized egg to adult [1]. Numerous fundamental biological and medical discoveries have been made using this model laboratory organism, including the development of the complete cell lineage [2], the structure of the nervous system [3], the discovery of the molecular basis of axon guidance [4], apoptosis [5,6], the identification of a ras protein involved in vulva development [7], the discovery of microRNAs [8,9], and RNAi [10]. C. elegans has also been a valuable model organism in fundamental areas such as chemotaxis and olfaction [11], aging [12], and in the in vivo application of the green fluorescent protein [13]. Until recently, relatively little was known about the small molecules that C. elegans uses for chemical signaling between individuals. However, a significant body of knowledge has been discovered in just the past four years, and the relatively new field of C. elegans chemical biology is enhancing the vast amount of genetic, anatomical, and cellular information that has made it such an outstanding model organism.
The word “pheromone” was proposed in 1959 by Karlson and Lüscher [14]. The word is derived from the Greek ϕερειν (to transfer, carry, bear) and oρμων (to excite, set in motion). Karlson and Lüscher recognized the need to distinguish pheromones from hormones, and they define pheromones as “substances which are secreted to the outside by an individual and received by a second individual of the same species, in which they release a specific reaction, for example, a definite behaviour or a developmental process” [14]. Wyatt has recently published a wonderful essay on the 50-year perspective of pheromone research [15]. Much of what we know about pheromones comes from insects, from the initial discovery nearly 50 years ago of the silk moth bombykol, a straight-chain16 carbon primary alcohol with 2 double bonds [16], to recent work identifying the cockroach blattellaquinone, a modified quinone [17]. Both bombykol and blattellaquinone are sex pheromones, but they are completely different chemically. Remarkably, many species of moth and female Asian elephants both use the same compound, (Z)-7-dodecen-1-yl acetate (a 12 carbon chain with one double bond and an acetate group), as a sex pheromone [18]. The extensive work in insect pheromones has lead to both great practical advances (for example, pheromone traps for moths are available at any home improvement store) and a deep understanding of the molecular genetics underlying insect pheromone behavioral responses [19].
Vanillic acid is a sex pheromone in the parasitic soybean cyst nematode [20], but this review focuses almost exclusively on the ascarosides, a family of small molecule pheromones that control two basic C. elegans traits: developmental arrest at the dauer stage and male mating behavior (Fig 1A). I will start by briefly summarizing dauer arrest and male mating behavior, and the bulk of the review will focus on the identification and biological characterization of C. elegans ascarosides (Table 1), starting with the discovery of daumone in 2005 [21]. I will briefly relate the C. elegans ascarosides to those originally found in the parasitic nematode Ascaris (Fig 1B), the animal from which the group of compounds got its name. I will close with a summary of what is known about C. elegans pheromones and propose a model to put these compounds into an ecological context (Fig 2).
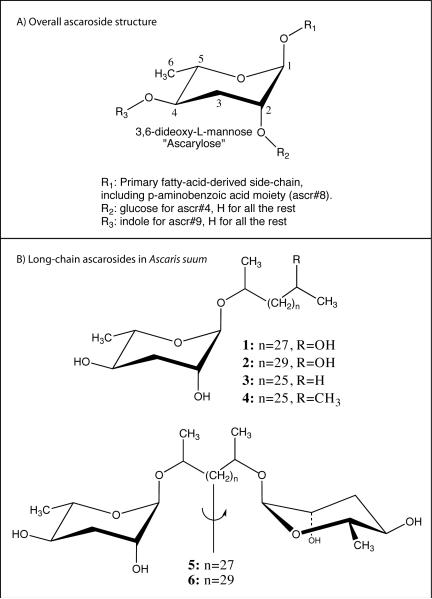
Figure 1. Ascaroside structures.
A) Overall structure of ascarosides. The ascarylose sugar is numbered and locations of known modifications are indicated. The known C. elegans ascaroside pheromones are shown in Table 1. B) Six long-chain ascarosides from the eggs of the parasitic nematode Ascaris suum [46]. Structures 4and 5are symmetric—indicated by the vertical line and arrow— and contain two ascarylose sugars [46]. Similar long-chain structures have been reported in both N2 (wild type) and daf-22 C. elegans [35].
Table 1. C. elegans ascarosides.
| Name | Alternate Name(s) | Chemical Structure | Dauer Activity | Mating Activity | Reference |
|---|---|---|---|---|---|
| ascr#1 | Daumone C7 | 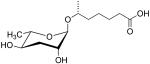 |
Very Low | None | [21] |
| ascr#2 | C6 | 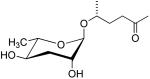 |
High | Moderate, Synergy with ascr#3 and ascr#4 | [31] |
| ascr#3 | C9 | 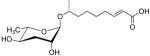 |
High | High, Synergy with ascr#2 and ascr#4 | [31] |
| ascr#4 | 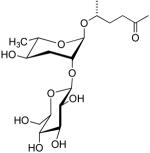 |
Low | None alone, Synergy with ascr#2, ascr#3 | [30] | |
| ascr#5 | C3 | 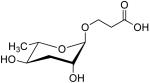 |
High, some synergy with ascr#2 and ascr#3 | None [35] | [36] |
| ascr#6.1 | 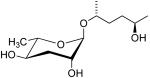 |
None | Moderate | [35] | |
| ascr#6.2 | 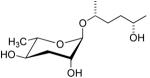 |
Not tested | Not tested | [35] | |
| ascr#7 | 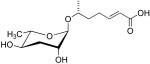 |
None | None | [35] | |
| ascr#8 | Moderate | High, synergy with ascr#2 and ascr#3 | [35] | ||
| ascr#9 | indolecarboxyl-ascaroside C5 | 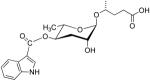 |
Moderate, Bell-shaped response | Not tested | [37] |
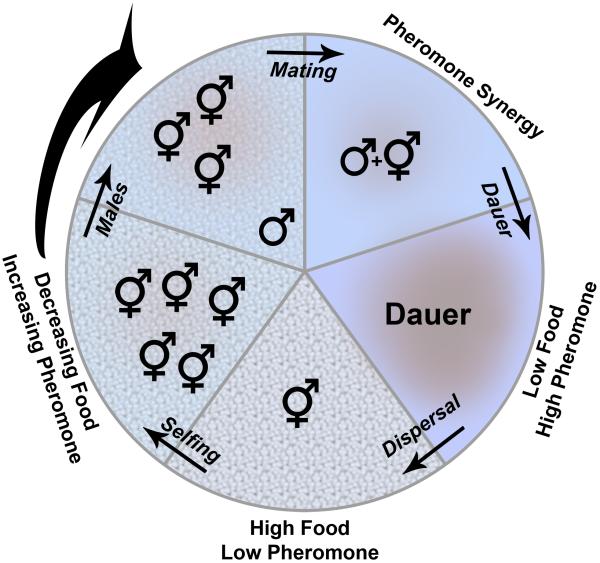
Figure 2. General model for C. elegans ascaroside pheromone function.
Starting with the bottom wedge in the circle that represents a microenvironment with high food and low pheromone, food levels decrease and pheromone increases going clockwise around the circle. Food is represented by the pattern on the surface and the colors represent pheromone. Discussion and overall rationale for each stage is given in the text.
Dauer
Dauer has been recently reviewed [22] and will be only briefly summarized here. In the presence of ample food and low worm density, C. elegans progresses through four larval stages (L1 to L4) to become a reproductive adult in about 3.5 days. If the ratio of food to worm density becomes too low, L2 animals enter the dauer stage [23]. Animals in dauer cease feeding and development, become radially shrunken, move only when prodded, and are capable of a specialized behavior called nictation, a waving motion of the anterior body in the air that is thought to aid in dispersal through increased interactions with insects [24]. Dauer animals can survive for months, and when conditions become favorable for growth (high food, low pheromone), dauer larvae resume normal development as L4 larvae [24,25].
Golden and Riddle showed that supernatants of liquid cultures of C. elegans could induce dauer formation [23]. In this initial publication, Golden and Riddle indicated that the “pheromone is present in very small amounts, and that it may be a hydroxylated, short-chain fatty acid or a mixture of closely related compounds” [23]. As I will show below, they got it right.
C. elegans Mating Behavior
Simon and Sternberg published the first evidence for a chemical cue produced by C. elegans hermaphrodites that attracts males [26]. They demonstrated that males were attracted to a region of an agar plate that had been conditioned by an unc-52 hermaphrodite, which is essentially motionless at the young adult stage but otherwise normal and able to reproduce. They also showed that the cue was diffusible by analyzing reversal frequencies of males as they approached the conditioned spot. A vulvaless mutant hermaphrodite could also attract males, showing that the cue was not secreted by or dependent on a vulva, and the sensory mutations osm-5 and osm-6 eliminated the male response. This effect was not reciprocal, as a conditioned spot from unc-52 males would not elicit a response by N2 hermaphrodites. Finally, Simon and Sternberg showed that several C. elegans isolates produced the mating cue, demonstrating that it is robust and evolutionarily stable [26].
White and coworkers have also shown that C. elegans hermaphrodites produce a male-specific mating cue and have identified male-specific cells that are necessary for the response [27]. In contrast to Simon who used immobile hermaphrodites as the source of cue [26], White et al. used extracts from hermaphrodite liquid cultures and showed that C. elegans males were attracted to spots on a plate from the hermaphrodite cultures [27], consistent with the results of Simon and Sternberg [26]. Using a combination of genetics and laser ablation studies, White et al. also showed that the male response was mediated by a TRPV (transient receptor potential vanilloid) channel that is produced by the gene products of osm-9, ocr-1, and ocr-2. They also demonstrated that the male-specific cephalic companion (CEM) neurons, along with the AWA and AWC olfactory neurons, were necessary. Interestingly, they were able to overexpress fem-3, which allowed a fraction of CEM neurons to avoid apoptosis in hermaphrodites, and they showed that these “masculinized” hermaphrodites behaved like males in response to hermaphrodite-conditioned media [27].
Chasnov and colleagues have published two papers [28,29] that are in apparent disagreement with the Simon and Sternberg [26] and White et al. [27] studies described above. In these studies, Chasnov et al. compared the male-specific attraction and mating behavior between different Caenorhabditis species that are either hermaphrodite/male (C. elegans and C. briggsae ) or female/male (C. remanei and Caenorhabditis sp. strain CB5161). In both studies, Chasnov et al. showed that C. elegans males had a greater attraction to females of other species compared to C. elegans hermaphrodites, and they concluded that C. elegans hermaphrodites have lost their ability to produce a mating pheromone [28,29]. There are several experimental differences between Chasnov et al. studies [28,29] and the Simon and Sternberg [26] or White et al. [27] studies. It is likely that females in species such as C. remanei and Caenorhabditis sp. strain CB5161 produce chemical signals for male attraction that are not present in C. elegans, which could potentially explain the observed stronger attraction of C. elegans males to females of these other species. As I will describe below, the C. elegans mating pheromone has now been identified [30], and I will describe a few properties of the pheromone that may also help to account forthese discrepancies.
C. elegans Dauer Pheromone Identification
Over twenty years passed between the demonstration that dauer is regulated by a pheromone [23] and the isolation and identification of the first dauer pheromone from C. elegans. The first chemical identification of a C. elegans pheromone was the discovery of “daumone” by Jeong and coworkers in 2005 [21]. These investigators conducted an activity-guided purification of the dauer pheromone by growing large-scale liquid cultures in a 300 L fermenter and extracting the aqueous culture with ethyl acetate. They conducted chromatographic separations, and the fractions were tested for their activity with a dauer formation bioassay. When they obtained a significantly pure fraction, they used standard NMR and mass spectrometry techniques to identify ascr#1 (Table 1: a.k.a. daumone and C7: see side bar for a discussion on nomenclature) [21]. For a short time it was assumed by many C. elegans investigators that the dauer pheromone story was nearly complete.
Jeong and coworkers provided the first clue that there was more to the dauer story. The investigators reported that during the activity-guided fractionation, they were able to purify daumoneby >15,000-fold based on dauer formation activity. However, the same activity-guided fractionation purified daumone by 128,300-fold based on mass [21]. Thus, 88% of the activity was lost during the purification, and the investigators speculated that perhaps “purified or synthetic daumone might be more active when it is associated with other unidentified molecules (for example, membrane lipids)” [21].
Butcher et al. found that at least some of Jeong and coworkers “other unidentified molecules” were additional, more potent, dauer pheromones [31]. Using a similar approach taken by Jeong et al., Butcher et al. used a dauer recovery assay to purify ascr#2 (a.k.a. C6). In contrast to the dauer formation assay, which is used to test the ability of a compound to cause worms to enter dauer, the dauer recovery assay uses worms that are already in dauer and tests for compounds that keep the animals in dauer; consequently, these two assays may be sensitive to different compounds. As seen in Fig 1, ascr#1 and ascr#2 are similar and have a common feature of the ascarylose sugar. Butcher and colleagues then looked for additional ascarosides in a targeted assay using primarily NMR spectroscopy, and they were able to identify ascr#3, yet another ascaroside. They then tested the activities of all the compounds in a dauer formation assay and discovered that ascr#2 and ascr#3 were approximately 100 times more potent in dauer formation than ascr#1, which was also identified in their targeted identification [31]. Thus, Butcher et al. both verified Jeong and coworker’s identification of daumone but also showed that it is, at best, a minor component in dauer formation.
Baiga et al. have made fluorescent derivatives of ascr#1 and ascr#3 and have used these compounds as probes to track the uptake and localization of dauer compounds in C. elegans [32]. They showed that the fluorescent probes had activity in dauer formation; that the probes first concentrate on the cuticle of the pharynx and, for the ascr#3 derivative, move to amphid neurons; that the fluorescence could be seen in the cuticle and amphid neurons of L4 animals after dauer recovery; and that the probes can penetrate C. elegans eggs [32]. Gallo and Riddle [33] recently compared the physiological properties of synthetic ascr#1 [21] to natural C. elegans pheromone extracts [34]. Consistent with Butcher et al., Gallo and Riddle found that ascr#1 is not a major component of the dauer-forming pheromone and that at concentrations reported to induce dauer formation [21], ascr#1 is toxic and will lower the life span of animals that are exposed to it [33].
Mating Pheromone Identification: Overlap with Dauer
While others were looking for dauer pheromone, Srinivasan, Kaplan and coworkers were conducting activity-guided fractionation of the mating pheromone in C. elegans [30]. Whereas Jeong et al. and Butcher et al. both used standard liquid bacterial/worm co-cultures to make the starting material, the Srinivasan and Kaplan team started with synchronized liquid co-cultures but then transferred worms at defined developmental stages to water to collect exclusively worm-derived compounds that exclude bacterial contaminants. This strategy accomplished three important goals. First, it greatly simplified the male-specific chemotaxis bioassay, because worms are attracted to many bacterial small molecules. Second, it simplified the chemical composition of the starting material for identification. Third, it allowed for the collection of material produced specifically at each developmental stage, as opposed to synchronized cultures that accumulate all compounds over time. The male activity only showed up at L4, young adult, and adult stages, increasing confidence that it was a biologically relevant signal [30].
The activity-guided fractionation of the mating pheromone was complicated by the complete loss of activity in individual fractions following an ion-exchange chromatography step [30]. Srinivasan, Kaplan et al. found that no individual chromatographic fraction was sufficient to generate a male-specific response but that recombining fractions recovered activity. This indicated that the male-attracting pheromone consisted of more than one compound. The major component of one of these fractions was identified as a new ascaroside based on the known ascr#2 but modified with glucose. This compound was named ascr#4 (Table 1). The next step was to analyze the known dauer pheromones ascr#1, ascr#2, and ascr#3 for male-specific attraction, and they then discovered that at low concentrations ascr#2 and ascr#3 [31], but not ascr#1 [21], also functioned as mating pheromones [30]. They showed that ascr#2, ascr#3, and ascr#4 act synergistically and are active at picomolar concentrations in specifically attracting males. The mating response was bell-shaped, and at higher concentrations necessary for dauer formation, males were no longer attracted. At higher concentrations of pheromone, hermaphrodites were strongly repelled. Srinivasan, Kaplan et al. also found that the amphid single-ciliated sensory neuron type K (ASK) and the male-specific CEM neuron, are required for male attraction by ascr#3 [30].
As noted above, Chasnov and colleagues questioned the existence of a C. elegans hermaphrodite mating cue [28,29]. At least two characteristics of the pheromone might help to account for this discrepancy. First, the male mating response is bell-shaped, with a maximal response near 1 picomole of material. There is a relatively narrow range of activity in male response, and it would be easy to have either too much or too little material. Second, the male response is strongly synergistic, involving not only ascr#2, ascr#3, and ascr#4 described above but at least one additional ascaroside ascr#8 described below [35]. The ratios of the compounds are important, and it is also clear (see below) that both development and environmental conditions significantly influence the expression of ascarosides.
More Ascarosides
In an attempt to discover why Jeong et al. identified ascr#1 [21] and Butcher et al. identified ascr#2 and ascr#3 [31] as dauer pheromones, Butcher and coworkers examined the temperature dependence of ascaroside expression and did activity-guided fractionations using dauer formation assays of cultures grown at 20 C (like Jeong et al.) and 25°C (like Butcher et al.). Like their earlier study [31], they found that ascr#2 and ascr#3 were most abundant at 25°C. However, they also found that at 20°C, ascr#5 (a.k.a. C3) and ascr#1 were expressed, but ascr#1 was not active in their dauer recovery assays [36]. They went on to characterize the dauer formation activity and found modest synergy between ascr#5 and either ascr#2 or ascr#3, especially at 20°C. More recently, Butcher and colleagues have identified a novel indole-containing dauer pheromone that they named indolecarboxyl-ascaroside C5 and which I have named ascr#9 (Table 1) [37]. Ascr#9 is interesting, both structurally and functionally. It is a relatively minor component of the dauer pheromone mixture, but it has dauer-forming activity at low nanomolar concentrations and has a bell-shaped curve, with activity dropping significantly at higher concentrations [37]. This is similar to the mating activity profile observed by Srinivasan, Kaplan and coworkers with ascr#2 and ascr#3 [30] and is the only current example of this sort of dauer-formation profile [37].
Pungaliya and coworkers took a different approach to finding additional ascarosides in C. elegans [35]. They applied a new method developed by Schroeder et al. called differential analysisby 2D NMR spectroscopy (DANS), which compares material from two different biological sources [38], for example a mutant animal with wild type. In the C. elegans study, Pungaliya et al. compared liquid cultures generated from wild type N2 and daf-22 animals [35], which had previously been shown to lack the short-chain ascarosides [30,39]. By comparing NMR data of N2 and daf-22 animals, they were able to identify ascr#5, ascr#6.1, ascr#6.2 (a diastereomer of 6.1), ascr#7, and ascr#8 (Fig 1). They synthesized the new ascarosides and tested them for activity using both dauer formation and male attraction assays and found that ascr#8 potently attracts males and is synergistic with ascr#2 and combinations of ascr#2 and ascr#3 (but not ascr#3 alone) [35]. Significantly, the addition of ascr#8 was able to fully account for male mating activity [35], which was only partially reconstructed with ascr#2, ascr#3, and ascr#4 [30]. Ascr#8 is a unique ascaroside in that it features an amide linkage and a p-aminobenzoic acid (PABA) subunit.
The C. elegans mating pheromone is a fascinating example of the synergistic action of small molecules, as a blend of three different ascarosides produces full activity at concentrations at which each individual component is completely inactive. At higher concentrations ascarosides also synergistically induce dauer formation, perhaps to a lesser extent than mating. Whereas synergistic action of sex pheromone components is well known from other animal species, especially insects (e.g. [40,41]), C. elegans presents an ideal opportunity to study synergy on the molecular level and to discover how multiple signals converge into a specific behavior.
Ascaroside Biosynthesis
Butcher and coworkers used daf-22 animals, which lack the dauer pheromone [42], to characterize some of the steps in ascaroside biosynthesis [39]. They showed that the daf-22 encodes a homolog of human sterol carrier protein, SCPx, an enzyme that catalyzes the final step in peroxisomal fatty acid β-oxidation reactions [39]. Based on this information, they also examined a dhs-28 mutant C. elegans. The dhs-28 gene encodes a protein homolog of human D-bifunctional protein, which biosynthetically acts just upstream of SCPx. They showed that these proteins are expressed in the intestine and suggest that this is the site of ascaroside biosynthesis in C. elegans [39]. This pathway degrades short-, medium-, and long-chain fatty acids into acetyl-CoA and bile acid intermediates. Butcher et al. grew liquid cultures of daf-22 and dhs-28 animals for both 10 and 20 days, conducted dauer formation assays, and identified ascarosides in the long-term dhs-28 cultures. Interestingly, both mutants were relatively inactive in dauer formation at 10 days but had significant dauer-forming activity at 20 days. They identified several longer-chain ascarosides up to 15 carbons in length in the 20-day dhs-28 culture, suggesting that these were intermediates ascaroside pheromone biosynthesis. Many steps in ascaroside biosynthesis remain to be elucidated, both in the initial formation of long-chain ascarosides and in details regarding specific functional groups, especially the aromatic amide in ascr#8 [35] or the indole in ascr#9 [37].
Common themes between C. elegans and Ascaris
Many years before the contemporary work in C. elegans pheromone biology, ascarosides were identified in the eggs of Ascaris species, which are parasitic nematodes commonly known as large intestinal roundworms. A variety of long-chain ascarosides were initially discovered in the horse parasitic nematode Parascaris equorum [43] and subsequently characterized and identified in Ascaris lumbricoides (the human parasite) and Ascaris suum (the pig parasite) (Fig 1B) [44-46]. Ascaris eggs are amazingly resilient, can survive for several years in damp soil, and resist degradation by many chemicals [47]. This remarkable stability contributes to the infection by Ascaris lumbricoides of approximately 1.5 billion people in the world in 2002 (http://www.cdc.gov/ncidod/dpd/parasites/ascaris/factsht_ascaris.htm). Ascaris eggs have three layers: a protein coat, a chitin middle layer, and an inner layer of mixture of long-chain ascarosides, which have been shown to be responsible for the unusual impermeability [48]. Although both A. lumbricoides and C. elegans are in the phylum Nematoda, the orders Ascaridida and Rhabditida are significantly diverged; however, nematodes appear to be the only eukaryotes from which the sugar ascarylose has been isolated [46]. The chemical properties of the very long-chain ascarosides identified from Ascaris (Fig 1B) and Parascaris differ from those of the C. elegans pheromone components (Table 1), but long-chained ascarosides very similar to those identified from Ascaris and Parascaris have now also been found in C. elegans [35,39].
Are there any similarities in the functions of ascarosides in Ascaris spp. and C. elegans ? Whereas C. elegans hatches from its egg as an L1 larvae, A. suum undergoes two molts in the egg. The Ascaris L3 stage emerges from the hatched egg when the egg is ingested into a new host, and the Ascaris L3 appears homologous to C. elegans dauer larvae [49]. It is likely no coincidence that ascarosides line Ascaris eggs and are in direct contact with the larval animals, so compounds may play a similar dauer-forming (or at least dauer-maintaining) role in Ascaris as they do in C. elegans. Investigations of the functions of ascarosides in Ascaris and Parascaris thus may provide new insights into the control of parasitic nematode infections.
More Pheromone Functions
De Bono and colleagues have discovered that mutations in the neuropeptide Y-like receptor (NPR-1) are responsible for different naturally occurring strains of C. elegans to exhibit either solitary or social feeding behavior [50]. Wild-type N2 animals are solitary feeders, but several natural isolates form aggregates at the edges of bacterial lawns. A null mutation of npr-1 can convert solitary into social feeders. Recent work by Macosko and coworkers has shown that the RMG interneuron is the primary site of action of the npr-1 gene [51]. They found that RMG forms gap junctions with several other neurons, most notably the ASK sensory neuron, which Srinivasan, Kaplan et al. had shown to be responsible for male-specific attraction to ascr#3 [30]. Srinivasan, Kaplan etal. also showed that N2 hermaphrodites were repelled by ascarosides, and Macosko verified these results. Interestingly, Macosko also found that npr-1 loss-of-function hermaphrodites were attracted to ascarosides. They went on to show that calcium levels in ASK neurons decrease in response to a blend of ascr#2, ascr#3, and ascr#5 and that ascaroside-generated signals in ASK can propagate to other cells [51]. High RMG activity promotes social aggregating behavior and also enhances the ASK response to ascarosides, and high NPR-1 activity diminishes the response [51]. Thus, ascarosides appear to modulate aggregation behavior in a strain-specific manner, adding to the dauer and mating functions previously established.
Dauer Conditions Increase Males
Morran and coworkers recently published an interesting study analyzing the frequency of males in three different C. elegans strains exposed to dauer conditions [52]. They observed that male frequency increases following exposure to dauer conditions for two naturally occurring strains (CB4856 and JU440) but not for the common laboratory strain N2. The increase in male frequency in CB4856 extended to several generations following exposure to dauer conditions. The authors identified two mechanisms that accounted for the increased males: differential male survival during dauer and an increase in male mating (outcrossing) rates following exposure to dauer [52]. Several other potential mechanisms including sexual conversion, differential migration, and increases in X chromosome nondisjunction rates were explored but ruled out as contributing to increased males [52]. It must be pointed out that this study did not explicitly identify the dauer pheromone or any other chemical cue as the mechanism responsible for increased males, but as discussed below, an increase in male frequency in response to dauer conditions reinforces the dual roles of ascarosides in dauer and mating.
General Model of Ascaroside Function
It took 25 years to identify the first dauer pheromone [21], and in the past three years a tremendous amount has been discovered: there are currently ten known ascarosides produced by C. elegans [21,30,31,35-37], the dauer pheromone is a mixture of ascarosides [31,36], the mating pheromone is based on the same chemical compounds as the dauer pheromone, but at much lower concentrations and perhaps with different ascaroside components [30], environmental conditions influence ascaroside expression [35,36], solitary hermaphrodites are repelled by ascarosides [30,51], and social hermaphrodites are attracted to ascarosides [51]. We know that CEM [27,30] and ASK [30,51] cells mediate the response to ascarosides, that ascarosides are biosynthesized through the peroxisomal β-oxidation pathway [39], and that male frequency increases with dauer conditions [52].
However, much remains unknown: we have no ascaroside receptors, we know relatively little about how environmental factors influence ascaroside expression, we know nothing about the mechanisms of ascaroside release (constitutive secretion, regulated secretion, defecation, and other possible mechanisms), and we know relatively little about the mechanisms of synergy of this large family of signaling molecules. It is no small task to unravel the overlapping functions of ten or more ascarosides that act synergistically and in a concentration-dependent manner with modulation from several additional cellular and environmental inputs. Zwaal and coworkers have identified two G-protein alpha subunits, GPA-2 and GPA-3, that are involved in the signal transduction by dauer pheromone [53], but neither dauer nor mating receptors havebeen reported. The physiological function of the original daumone [21] (a.k.a ascr#1 and C7) remains a mystery. Many details of ascaroside biosynthesis and its regulation in response to environmental cues remain unknown. We also know very little about the role of ascarosides in other nematode species, despite the fact that they were discovered in Ascaris. Significant gaps remain in our knowledge, but it is possible to piece together a general model that unifies the role of ascarosides in the life history of C. elegans.
Figure 2 illustrates several key points in an ecological cycle of C. elegans. It is necessarily simplified, and each panel could last from one to several generations depending on environmental factors, especially food supply. The bottom wedge of the circle represents abundant food and little or no pheromone. Pheromone levels increase and food decreases clockwise from the bottom, which specifies a single hermaphrodite in this region as a limiting case of dispersal into a new microenvironment. Under conditions of abundant food and low worm population, hermaphrodites would self-fertilize and thus produce primarily hermaphrodites as offspring, represented by the second wedge clockwise from the bottom. Morran and coworkers found that male frequency increased after exposure to dauer conditions [52]. In the model in Fig 2, I assume that ascaroside pheromones are responsible for the increased male frequency and that the effect will increase as overall local pheromone levels increase, even at levels too low to induce dauer. This assumption clearly needs to be tested experimentally. Males are attracted to a synergistic mixture of ascarosides [30,35]. A second untested assumption of this model is that out of the known ascarosides (Table 1), some will be constitutively expressed to monitor population density and others will be regulated according to environmental or developmental factors. For example, ascr#4 is produced at high levels when worms are incubated in water or media withno food [30], but in standard liquid bacterial co-cultures, ascr#4 levels are extremely low [35]. Moreover, when Srinivasan, Kaplan et al. tested material generated from worms at distinct developmental stages, only exudates from L4, young adult, and adult animals caused male-specific attraction [30]. Thus, the existing data support the idea that males will be potently attracted to hermaphrodites when the right combination of ascarosides is produced. The strong synergy observed in male attraction allows for population-sensing (e.g. dauer-forming) ascaroside(s) to accumulate in the microenvironment without leading to male attraction. Then, small amounts of different ascaroside(s) could be released by a hermaphrodite that is developmentally ready to mate, and this additional cue would provide directionality, allowing the male to find the fertile hermaphrodite in a relatively uniform level of dauer pheromone.
Since it appears that some ascarosides, most notably ascr#4, are regulated by food, this provides a mechanism for C. elegans hermaphrodites to outcross with males as the environmental conditions worsen. This would increase the number of offspring from about 300 for a sperm-limited self-fertilizing hermaphrodite to about 1000 for a hermaphrodite who mates with males [54]. Importantly, it would also provide a mechanism for genetic variability as conditions deteriorate. As the levels of pheromone increase and the food decreases, the offspring that arise from outcrossing will become dauer. As shown by Morran et al., the dauer state will lead to increased males [52]. The dauer state will allow these offspring to survive for several months and disperse to a fresh microenvironment with abundant food, thus completing the cycle (Fig. 2). I have illustrated the limiting case of a single hermaphrodite dispersing to new food, but depending on the density of dispersed animals, outcrossing with males could still be significant in the initial fresh microenvironment [52].
Clearly, as we discover new ascarosides, receptors, regulators, and functions, the general model proposed in Figure 2 will be refined (or replaced all together!). However, the accumulated knowledge over the past three years is nicely consistent and points to a critical role of pheromones in nematode ecology.
Acknowledgements
I thank all the members of my research group, especially Ramadan Ajredini and Aaron Dossey for a stimulating environment. My worm colleagues in the Sternberg and Schroeder laboratories have been wonderful, and I especially thank Jagan Srinivasan, Andrea Choe, Paul Sternberg, and Frank Schroeder for very fulfilling research collaborations. Fatma Kaplan, Hans Alborn, and Peter Teal at the USDA laboratory provide great collaborations and helpful discussions. Paul Sternberg, Frank Schroeder, Fatma Kaplan, Charlie Baer, Ian Castro-Gamboa, and Patrick Phillips all provided helpful feedback on this review. ASE was supported by the NIH (1R01GM085285-01) and the Human Frontiers Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Sidebar: Nomenclature) The original daumone was named according to its function in promoting dauer formation. This is reasonable for a single compound with a single function. A second nomenclature in use is to name the compounds by the length of the carbon side-chains on the ascarylose sugar. Under this nomenclature, daumone is C7. This system avoids limitations when naming according to its function, but it has its own limitations when dealing with modifications such in ascr#4, the glycosylated form of ascr#2 or ascr#9, an indole derivative (Table 1). Another limitation would be if a new compound were identified with, for example, a six carbon carboxylic acid chain rather than a methyl ketone. For these reasons, I favor the system in which the ascarosides are named sequentially in order of their discovery, without reference to function or molecular architecture. In this system, daumone is ascr#1, C6 is ascr#2, C9 is ascr#3, and the glycosylated form of c6 is ascr#4. This system was developed to be easily searchable and to be distinct from nomenclature for phenotypes, genes or proteins [30]. Note that there are no spaces in the names in order to improve automated text searching. Frank Schroeder has established a C. elegans Small Metabolic Molecule Identifiers Database (http://www.smmid.org/) that has up-to-date information on pheromones and other metabolic signaling molecules in nematodes.
References
- 1.Brenner S. Genetics of Caenorhabditis-Elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sulston JE, Horvitz HR. Post-Embryonic Cell Lineages of Nematode, Caenorhabditis-Elegans. Developmental Biology. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 3.White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous-System of the Nematode Caenorhabditis-Elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 4.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 5.Ellis RE, Yuan JY, Horvitz HR. Mechanisms and Functions of Cell-Death. Annual Review of Cell Biology. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 6.Yuan JY, Horvitz HR. The Caenorhabditis-Elegans Cell-Death Gene Ced-4 Encodes a Novel Protein and Is Expressed During the Period of Extensive Programmed Cell-Death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- 7.Han M, Sternberg PW. Let-60, a Gene That Specifies Cell Fates During C-Elegans Vulvar Induction, Encodes a Ras Protein. Cell. 1990;63:921–931. doi: 10.1016/0092-8674(90)90495-z. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. The evolution of our thinking about microRNAs. Nat Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 9.Ruvkun G. The perfect storm of tiny RNAs. Nat Med. 2008;14:1041–1045. doi: 10.1038/nm1008-1041. [DOI] [PubMed] [Google Scholar]
- 10.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 12.Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 14.Karlson P, Luscher M. Pheromones’: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt TD. Fifty years of pheromones. Nature. 2009;457:262–263. doi: 10.1038/457262a. [DOI] [PubMed] [Google Scholar]
- 16.Butenandt A, Beckmann R, Hecker E. [On the sexattractant of silk-moths. I. The biological test and the isolation of the pure sex-attractant bombykol.] Hoppe Seylers Z Physiol Chem. 1961;324:71–83. doi: 10.1515/bchm2.1961.324.1.71. [DOI] [PubMed] [Google Scholar]
- 17.Nojima S, Schal C, Webster FX, Santangelo RG, Roelofs WL. Identification of the sex pheromone of the German cockroach, Blattella germanica. Science. 2005;307:1104–1106. doi: 10.1126/science.1107163. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen LE, Lee TD, Zhang A, Roelofs WL, Daves GD., Jr. Purification, identification, concentration and bioactivity of (Z)-7-dodecen-1-y1 acetate: sex pheromone of the female Asian elephant, Elephas maximus. Chem Senses. 1997;22:417–437. doi: 10.1093/chemse/22.4.417. [DOI] [PubMed] [Google Scholar]
- 19.Vosshall LB. Scent of a fly. Neuron. 2008;59:685–689. doi: 10.1016/j.neuron.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe H, Huettel RN, Demilo AB, Hayes DK, Rebois RV. Isolation and Identification of a Compound from Soybean Cyst Nematode, Heterodera-Glycines, with Sex-Pheromone Activity. Journal of Chemical Ecology. 1989;15:2031–2043. doi: 10.1007/BF01207435. [DOI] [PubMed] [Google Scholar]
- 21.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 22.Hu PJ. Dauer. WormBook. 2007:1–19. doi: 10.1895/wormbook.1.144.1. http://www.wormbook.org/ [DOI] [PMC free article] [PubMed]
- 23.Golden JW, Riddle DL. A Pheromone Influences Larval Development in the Nematode Caenorhabditis-Elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 24.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 25.Klass M, Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- 26.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The sensory circuitry for sexual attraction in C. elegans males. Current Biology. 2007;17:1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Chasnov JR, Chow KL. Why are there males in the hermaphroditic species Caenorhabditis elegans? Genetics. 2002;160:983–994. doi: 10.1093/genetics/160.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 32.Baiga TJ, Guo H, Xing Y, O’Doherty GA, Dillin A, Austin MB, Noel JP, La Clair JJ. Metabolite induction of Caenorhabditis elegans dauer larvae arises via transport in the pharynx. ACS Chem Biol. 2008;3:294–304. doi: 10.1021/cb700269e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallo M, Riddle DL. Effects of a Caenorhabditis elegans Dauer Pheromone Ascaroside on Physiology and Signal Transduction Pathways. J Chem Ecol. 2009;35:272–279. doi: 10.1007/s10886-009-9599-3. [DOI] [PubMed] [Google Scholar]
- 34.Golden JW, Riddle DL. The Caenorhabditis-Elegans Dauer Larva - Developmental Effects of Pheromone, Food, and Temperature. Developmental Biology. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 35.Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci U S A. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butcher RA, Ragains JR, Clardy J. An Indole-Containing Dauer Pheromone Component with Unusual Dauer Inhibitory Activity at Higher Concentrations. Org Lett. 2009 doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder FC, Gibson DM, Churchill AC, Sojikul P, Wursthorn EJ, Krasnoff SB, Clardy J. Differential analysis of 2D NMR spectra: new natural products from a pilot-scale fungal extract library. Angew Chem Int Ed Engl. 2007;46:901–904. doi: 10.1002/anie.200603821. [DOI] [PubMed] [Google Scholar]
- 39.Butcher RA, Ragains JR, Li W, Ruvkun G, Clardy J, Mak HY. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci U S A. 2009;106:1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumlinson JH, Brennan MM, Doolittle RE, Mitchell ER, Brabham A, Mazomenos BE, Baumhover AH, Jackson DM. Identification of a Pheromone Blend Attractive to Manduca-Sexta (L) Males in a Wind-Tunnel. Archives of Insect Biochemistry and Physiology. 1989;10:255–271. [Google Scholar]
- 41.Hildebrand JG. Analysis of Chemical Signals by Nervous Systems. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golden JW, Riddle DL. A Gene Affecting Production of the Caenorhabditis-Elegans Dauer-Inducing Pheromone. Molecular & General Genetics. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- 43.Fouquey C, Polonsky J, Lederer E. [Chemical structure of ascarylic alcohol isolated from Parascaris equorum.] Bull Soc Chim Biol (Paris) 1957;39:101–132. [PubMed] [Google Scholar]
- 44.Jezyk PF, Fairbairn D. Ascarosides and ascaroside esters in Ascaris lumbricoides (Nematoda) Comp Biochem Physiol. 1967;23:691–705. doi: 10.1016/0010-406x(67)90334-9. [DOI] [PubMed] [Google Scholar]
- 45.Tarr GE, Fairbairn D. Conversion of ascaroside esters to free ascarosides in fertilized eggs of Ascaris suum (nematoda) J Parasitol. 1973;59:428–433. [PubMed] [Google Scholar]
- 46.Bartley JP, Bennett EA, Darben PA. Structure of the ascarosides from Ascaris suum. J. Nat Prod. 1996;59:921–926. doi: 10.1021/np960236+. [DOI] [PubMed] [Google Scholar]
- 47.Lapage G. Veterinary Parasitology. Oliver and Boyd; Edinburgh: 1956. [Google Scholar]
- 48.Barrett J. Studies on the induction of permeability in Ascaris lumbricoides eggs. Parasitology. 1976;73:109–121. doi: 10.1017/s0031182000051374. [DOI] [PubMed] [Google Scholar]
- 49.Fagerholm HP, Nansen P, Roepstorff A, Frandsen F, Eriksen L. Differentiation of cuticular structures during the growth of the third-state larva of Ascaris suum (Nematoda, Ascaridoidea) after emerging from the egg. Journal of Parasitology. 2000;86:421–427. doi: 10.1645/0022-3395(2000)086[0421:DOCSDT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 50.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 51.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morran LT, Cappy BJ, Anderson JL, Phillips PC. Sexual Partners for the Stressed: Facultative Outcrossing in the Self-Fertilizing Nematode C. Elegans. Evolution. 2009 doi: 10.1111/j.1558-5646.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zwaal RR, Mendel JE, Sternberg PW, Plasterk RHA. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riddle DL, Blumenthal T, Meyer BJ, Priess JR. Introduction to C. elegans III. Life history and evolution. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. Cold Spring Harbor Laboratory Press; Plainview, New York: 1997. [Google Scholar]