Abstract
Glucose-6-phosphate dehydrogenase (G6PD) is a determinant in the antioxidant status of the red blood cell (RBC) and is also used as an indicator of cell age. However, it is unknown if the relationship between antioxidant status, cell age, and RBC-derived adenosine triphosphate (ATP) occurs immediately or over a period of time. Therefore, the development of a simultaneous determination of G6PD activity (via the determination of nicotinamide adenine dinucleotide phosphate (NADPH)) in RBCs and the determination of deformation-induced RBC-derived ATP is described. The NADPH and ATP were determined while undergoing a chemically-induced aging process via inhibition of G6PD with dehydroepiandroesterone (DHEA). Upon incubation with DHEA for 30 minutes, NADPH levels measured in a flow stream decreased to 7.96 ± 1.10 μM from an original value of 13.20 ± 1.80 μM in a 0.02% solution of RBCs. In order to demonstrate a direct relationship between G6PD activity and deformation-induced ATP release from RBCs, a simultaneous microflow determination of G6PD activity and ATP release was performed. Upon inhibition with DHEA, NADPH levels decreased to 8.62 ± 0.29 μM from its original value of 12.73 ± 0.50 μM while ATP release decreased from 0.21 ± 0.07 μM to 0.06 ± 0.02 μM. These values were validated by an examination of NADPH levels in, and ATP release from, RBC fractions containing younger and older cells (separated by cell density centrifugation). This determination provides evidence that antioxidant status in the RBC and its ability to release ATP, a known stimulus of nitric oxide production, are closely related.
Keywords: glucose-6-phosphate dehydrogenase, nicotinamide adenine dinucleotide phosphate, adenosine triphosphate, chemiluminescence, fluorescence
Introduction
Diabetes is one of the most common diseases found worldwide with an estimated 40 million people having been diagnosed with type 2 diabetes and its associated secondary complications such as retinopathy, neuropathy and cardiovascular diseases that include angina, heart attack and high blood pressure.1–4 A vast amount of research involving diabetes and complications is focused on areas such as insulin secretion and resistance, pancreatic beta cells, and maintenance of blood glucose levels.5,6
Reports suggesting a weakened antioxidant defense system in RBCs obtained from humans or animal models with type 2 diabetes were initially reported nearly a decade ago.7–9 As a result of this weakened antioxidant defense, the RBCs of these individuals are more prone to oxidant insult and are believed to be less deformable in comparison to RBCs obtained from healthy individuals. Indeed, it has been reported that the RBCs from people with type 2 diabetes are less deformable than those RBCs obtained from healthy controls.10–12
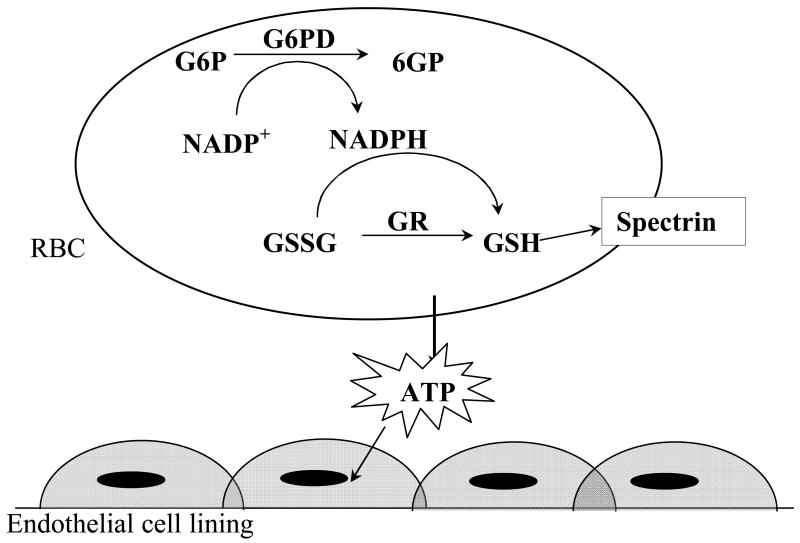
In its reduced form glutathione (GSH), a small tripeptide, is the main non-enzymatic antioxidant in the RBC and readily undergoes oxidation to the dimer (GSSG) to protect such important cellular components as proteins from oxidant insults (figure 1).13–15 Under normal conditions, cells are capable of regenerating GSH at the expense of the reducing factor nicotinamide adenine dinucleotide phosphate (NADPH). Though other cells have several methods to produce NADPH, RBCs rely solely on the enzyme glucose-6-phosphate dehydrogenase (G6PD) for regeneration of NADPH, thereby rendering G6PD activity important to the antioxidant status of the RBC.16
Fig. 1.
Schematic representation of an antioxidant defense mechanism in RBCs involving the pentose phosphate pathway.
It is well established that G6PD deficient individuals are subjected to high oxidative stress due to lack of NADPH production, and hence a weakened antioxidant defense system.7,17,18 G6PD, the key regulatory enzyme of the pentose phosphate pathway, regenerates NADPH during oxidation of glucose-6-phosphate (G6P) to 6-phosphogluconate (6GP). Though G6P levels appear to be normal in RBCs obtained from people with diabetes, it is established that G6PD activity in the RBCs from diabetes patients is deficient compared to healthy individuals,19–21 thus resulting in decreased NADPH levels in the RBCs from people with diabetes.
In addition to its importance in the RBC as a major determinant in oxidative stress, G6PD activity is also employed as an indication of cell aging22 because cell age is often inversely related to G6PD activity.23 While other methods exist for estimating cellular aging, the G6PD activity in the RBC is of interest due to its reported relationship to diabetic complications through the increased activity of the aldose reductase pathway.24–26 It is reported that inhibition of this pathway with aldose reductase inhibitors such as tolrestat leads to an increase in the activity of the pentose phosphate pathway (and a subsequent increase in G6PD activity) and hence NADPH levels.27,28 Conversely, activation of the aldose reductase pathway leads to an apparent decrease in pentose phosphate pathway activity, which is associated with a decrease in G6PD activity and, hence, cellular NADPH levels.
Recently, we were able to demonstrate that deformation-derived ATP release from the RBC is clearly related to the cellular antioxidant defense status, and that the RBC’s ability to recover from oxidant insults depends upon G6PD activity.29 Importantly, ATP is a recognized stimulus of nitric oxide (NO) production in endothelial cells.30 Therefore, a link may exist between the oxidant status (and cell aging process) in the RBC and the potent vasodilator, NO.
Here we have developed a method to simultaneously monitor G6PD activity (via cellular NADPH levels) in RBCs, and the ability of the RBC to release ATP. In order to establish this relationship between G6PD activity and ATP release, RBCs were chemically “aged” by inhibiting G6PD activity with dehydroepiandroesterone (DHEA), a well known noncompetitive steroid inhibitor of G6PD.31–34 To further emphasize the relationship between G6PD activity and ATP release, we also confirm that the RBCs from patients with type 2 diabetes have reduced levels of NADPH.29 Moreover, studies were also performed to determine if aged cells, reported as G6PD activity (through measurement of NADPH), releases less ATP upon stimulation than their younger counterparts. To perform such experiments, the separation of young and old fractions of RBCs obtained from whole blood was performed, followed by the monitoring of cellular NADPH levels and deformation induced ATP release. Importantly, the method developed here is the first attempt to monitor cellular antioxidant status and its effect on RBC-derived ATP levels, simultaneously. Furthermore, this technique not only enables simultaneous monitoring of these important analytes, but also enables this analysis under conditions of continuous flow, an essential parameter of blood flow and deformation-induced ATP release.
Experimental
Collection of RBCs
All procedures involving animals or humans in this study were performed under protocols approved by the appropriate Animal Investigation Committee or Institutional Review Board. Rabbit RBCs were obtained from male New Zealand White rabbits (2.0–2.5 kg). Rabbits were anesthetized with ketamine (8.0 mg kg−1, im) and xylazine (1.0 mg kg−1, im) followed by pentobarbital sodium (15 mg kg−1 iv). After tracheotomy, the rabbits were mechanically ventilated (tidal volume 20 mL kg−1, rate 20 breaths min−1; Harvard ventilator). A catheter was placed into a carotid artery, heparin (500 units, iv) was given, and after 10 min, animals were exsanguinated. Human blood was obtained by venipuncture and collected in to a heparinized tube. To prepare the RBCs from the collected blood sample, whole blood was centrifuged at 500 × g at 4°C for 10 min. The plasma and buffy coat were removed and stored for other experiments within the laboratory. RBCs were resuspended and washed 3 times in a physiological salt solution (PSS). The PSS was prepared by combining TRIS buffer (25 mL, prepared by mixing 50.9 g of TRIS in 1 L of distilled and deionized water (DDW)) and Ringer’s (164.2 g NaCl, 7.0 g KCl, 5.9 g CaCl2.2H2O, and 2.5 g MgSO4 in 1 L DDW). After the addition of dextrose (0.50 g) and albumin bovine fraction IV (fatty acid free, 2.5 g) to the above mixture, the solution was diluted to 500 mL with DDW and the pH was adjusted to 7.35–7.45. The PSS was then triple filtered using a filter with 0.45 μM pores (Corning, Fisher Scientific). Cells were prepared on the day of use and studied within 8 h of removal from the animal or human subject. Human diabetes subjects were adults with hemoglobin A1c values of 7 % or higher.
Separation of RBCs into age-based fractions
RBCs were separated into fractions based on cell density centrifugation. In a 15 mL tube, 2 mL of a solution of Percoll (Sigma Chemical Co, St. Louis, MO) having a density of 1.115 g mL−1 were added to the bottom of the tube. Next, 2 mL of a similar solution of Percoll having a density of 1.105 g mL−1 were slowly added on to the top of the first 2 mL of the higher density solution. Finally, 1 mL of RBCs (70% hematocrit) was added to the top of the Percoll layers. The tube containing the Percoll and RBCs was then centrifuged at 3000 × g at 4 °C for 15 min. After centrifugation, two layers of RBCs are present; those having a density of less than the 1.105 g mL Percoll solution will appear at the top of the tube and represent those RBCs having the lowest density; these RBCs are considered the “younger” of the RBCs in the original RBC sample.35,36 Those below the higher density Percoll solution are the more dense, or aged, RBCs. These separated RBC fractions were removed by pipette and diluted to a hematocrit of 7% using the PSS described above. ATP release and NADPH levels measurements were made with 7% and 0.02% hematocrit RBCs, respectively using the methods described below.
Measurement of NADPH levels
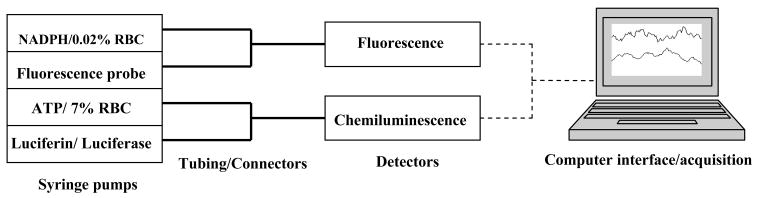
The general schematic for all flow-based measurements is shown in figure 2. The activity of the G6PD enzyme was monitored by measuring the cellular NADPH levels. A 200 μM NADPH stock solution was prepared by dissolving NADPH (0.0043 g) in 25 mL of DDW. NADPH standards (0.0– 20.0 μM) were prepared by diluting the stock solution of NADPH in DDW, accordingly.
Fig. 2.
Schematic representation of the flow-based system employed to quantitatively determine the concentration of NADPH and ATP release from the RBCs obtained from either rabbits or humans. For NADPH determinations alone, only the NADPH and probe streams were employed.
A 0.02 % hematocrit of RBCs was used in all the experiments where NADPH was quantitatively measured. Specifically, NADPH was determined using the Vybrant Cytotoxicity Assay Kit (Invitrogen Corp., Carlsbad, CA). In this assay, NADPH reduces resazurin to generate the fluorescence product resorufin. To determine NADPH, an aliquot of the reaction mixture was placed in a 500 μL syringe (Hamilton, Fisher Scientific). NADPH standards, or the RBCs, were placed in another syringe and both solutions were pumped through 30 cm sections of microbore tubing, having an internal diameter of 50 μm (Polymicro Technologies, Phoenix, AZ), at a rate of 1.0 μL min−1 using a dual syringe pump (Harvard Apparatus, Boston, MA). The two streams were combined at a mixing T-junction having an internal volume of 560 nL (Upchurch Scientific, Oak Harbor, WA). The now combined content was allowed to flow through a 90 cm long segment of microbore tubing having an internal diameter of 150 μm. The larger bore tubing resulted in a slower linear rate of the stream, thus enabling the required incubation period prior to detection of the resultant fluorescence using a flow-through fluorescence detector (Jasco, FP-2020 fluorescence detector). The total time taken for a single measurement was 30 min. The fluorescence emission intensity was measured at 587 nm (excitation at 563 nm).
In order to determine the effect of a G6PD inhibitor (DHEA) on the cellular NADPH concentrations, a 1 mM DHEA solution was prepared by dissolving DHEA (0.0043 g) in the aforementioned PSS. The required volume of RBCs was placed in a 10 ml volumetric flask containing DHEA and diluted up to the mark with PSS creating a 7% RBC solution in 100 μM DHEA. This mixture was incubated for 30 min at room temperature prior to diluting the RBC mixture to a measurable hematocrit of 0.02 %. This hematocrit enabled a quantitative signal to be generated without excessive spectral interference from the complex matrix, especially the hemoglobin in the RBC. The diluted RBCs containing the DHEA were then allowed to react with the resazurin reaction mixture.
Measurement of ATP release
A 100 μM ATP stock solution was prepared by dissolving ATP (0.0619 g) in DDW and diluting to 1.00 L in DDW. ATP standards (0.0 – 1.5 μM) were prepared by diluting aliquots of the stock ATP solution in PSS. To prepare the luciferin/luciferase mixture required for chemiluminescence determination of ATP, luciferin (2 mg, Sigma) was dissolved in 20 mL of DDW. A 5 mL aliquot of this luciferin was added to a vial containing a solid mixture of luciferin/luciferase (FLE-50, Sigma).
To measure the ATP release, the luciferin/luciferase mixture was placed in a 500 μL syringe. ATP standards, or RBCs, were placed in the second syringe and both solutions were pumped through 30 cm sections of microbore tubing having an internal diameter of 50 μm at a rate of 6.7 μL min−1 using the dual syringe pump mentioned above. The streams containing the luciferin/luciferase mixture and ATP standard/RBCs were combined at the mixing T-junction. The combined stream flowed through a segment of microbore tubing having an internal diameter of 75 μm, allowing the detection of the resultant chemiluminescence from the reaction of the ATP (either in standard form or that released from RBCs) using a photomultiplier tube (PMT; Hamamatsu Corporation, Hamamatsu, Japan) placed in a light excluding box. The polyimide coating was removed from the microbore tubing on the segment over the PMT to facilitate light transport through the tubing and to the PMT.
Simultaneous detection of ATP and NADPH
A required volume of RBCs was placed in a 10 mL volumetric flask and diluted with PSS to prepare a 7 % hematocrit of RBCs. Next, approximately 285 μl of this solution was diluted up to 100 ml in DDW to create a solution of RBCs with hematocrit of 0.02 %.
For the simultaneous determination of ATP and NADPH, two dual syringe pumps were employed as shown in figure 2. NADPH standards or the 0.02 % RBCs were placed in one syringe and the resazurin reaction mixture was placed in another and both were pumped at a rate of 1.00 μL min−1 through 50 μm internal diameter tubing.
The content of the two streams were combined at a mixing-T and incubated for 30 min by passing through the 90 cm long, 150 μm internal diameter microbore tubing. The resultant fluorescence was detected online with the flow-through fluorescence detector mentioned above. Simultaneous to the NADPH measurements, the ATP release from a 7% hematocrit of RBCs was measured as described above. These measurements were repeated with the RBCs in the presence of the DHEA.
Results and Discussion
It is well known that the antioxidant defense system of the RBCs obtained from people with type 2 diabetes is deficient in comparison to those RBCs obtained from healthy individuals.7,17 This is believed to be the result of decreased levels of cofactor NADPH due to a reduced activity of G6PD.19–21 NADPH acts as a substrate for glutathione reductase (GSHred), an enzyme that helps maintain GSH in its reduced, antioxidant form. In its reduced form, GSH is the most abundant non-enzymatic antioxidant in RBCs.37 Upon oxidant insult, GSH is oxidized to its dimeric form GSSG, thereby protecting important cellular components and membrane proteins from being subjected to oxidant stress. Upon decrease of NADPH, GSHred activity decreases, thereby resulting in lower levels of reduced GSH and subsequent weakening of cellular antioxidant defense.
Recently, we hypothesized that a decrease in the pentose phosphate pathway, or an increase in the aldose reductase pathway, would lead to a more stiffened RBC membrane and an accompanying decrease in ATP release from these RBCs when subjected to deformation. Although a relationship between the pentose phosphate pathway and RBC-derived ATP was established29, the simultaneous measurement of G6PD activity and ATP release was not performed.
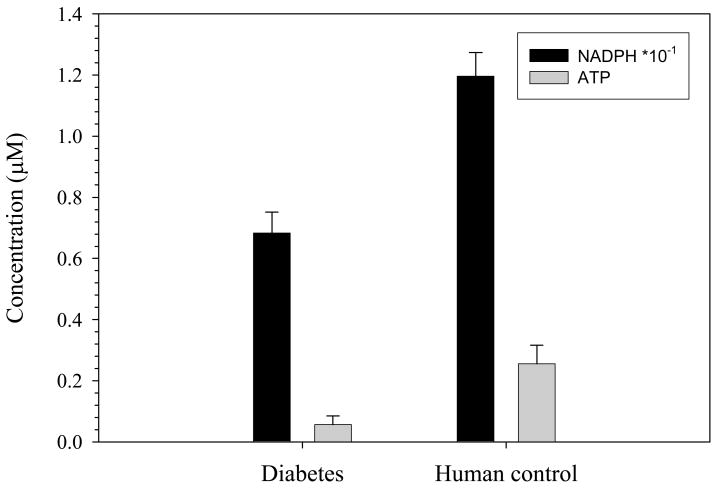
It has been reported in the literature that people with diabetes have RBCs with lower concentrations of NADPH in comparison to the RBCs obtained from healthy controls. As shown in the data in figure 3, the NADPH level in the RBCs obtained from the patients with diabetes was about 50% less in comparison to healthy human controls. This result is in agreement with previously reported non-flow based NADPH measurements in RBCs obtained from people with type 2 diabetes.28,29 In addition, a determination of ATP release from the RBCs obtained from the type 2 diabetes group (and a control group who were not diagnosed with type 2 diabetes) was also performed. The data in figure 3 provide evidence that G6PD activity (measured as NADPH concentrations in the RBC) is related to the ability of the cell to release ATP.
Fig. 3.
Measurement of cellular NADPH levels and ATP release from RBCs obtained from people with type 2 diabetes and healthy controls. The black bars represent NADPH (n = 5 individuals) and the gray bars represent ATP release (n = 8 individuals). There is an approximate 50% decrease in NADPH concentrations and a 78% decrease in ATP release from the RBCs from diabetic patients as compared to healthy individuals. The values between the two groups are significantly different (p < 0.01).
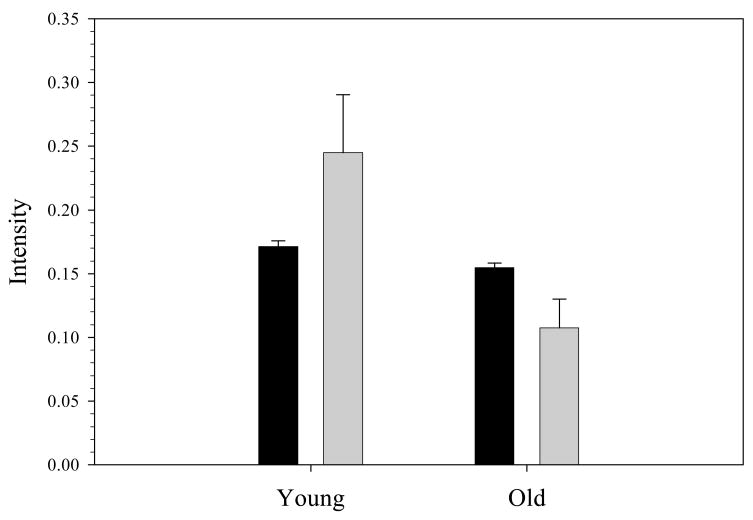
The data in figure 3 suggest that G6PD activity is related to the cell’s ability to release NO-stimulating ATP. It is well established that as cells age, the G6PD activity within the cell decreases resulting in a smaller RBC that is more dense and, importantly, less deformable. Therefore, to further verify the relationship between G6PD activity and ATP release, RBCs from healthy rabbits were separated based on cell density centrifugation as described above. The cellular NADPH levels and deformation induced ATP release were measured in these young and old fractions obtained from the whole sample of RBCs. As shown in figure 4, both NADPH concentration in, and deformation-induced ATP release from, the RBCs are significantly different in the younger and older fractions. As expected, both NADPH levels and ATP release from the RBCs obtained from the less dense, “younger” fraction are greater than the more dense RBCs with less G6PD activity. In the younger fraction, the NADPH level is 11 % greater compared to the older fraction, while the ATP release was 56 % greater in the younger RBC fraction compared to the older portion of the cells.
Fig. 4.
NADPH and ATP levels in young and old fractions of RBCs. The black bars represent the fluorescence intensity corresponding to NADPH while the gray bars represent the chemiluminescence intensity corresponding to ATP release from the RBCs (n= 3 rabbits). The values between the two groups are significantly different (p < 0.01).
The results in figure 4 provide further evidence that NADPH concentrations in the RBC, an indicator of G6PD activity, are decreased in the aged cell. While this has been reported previously by other groups,38,39 the results here expand on previous results to show the relationship between this G6PD activity and ATP release. However, neither the data in figure 3, nor figure 4 demonstrates whether this relationship between G6PD activity and ATP release is a phenomenon that forms over a lengthy period of time (e.g., hours to days) or if it occurs immediately. To gain a more clear understanding of this relationship, a measurement scheme would need to be developed to measure NADPH in the presence of RBCs in a flow stream simultaneously with the deformation-induced ATP release from the RBC. Flow based measurements provide a closer mimic to the actual circulation and stimulate flow-induced ATP release from the RBC. Without the flow, the relationship between the NADPH concentration and ATP release from the RBC could not be determined.
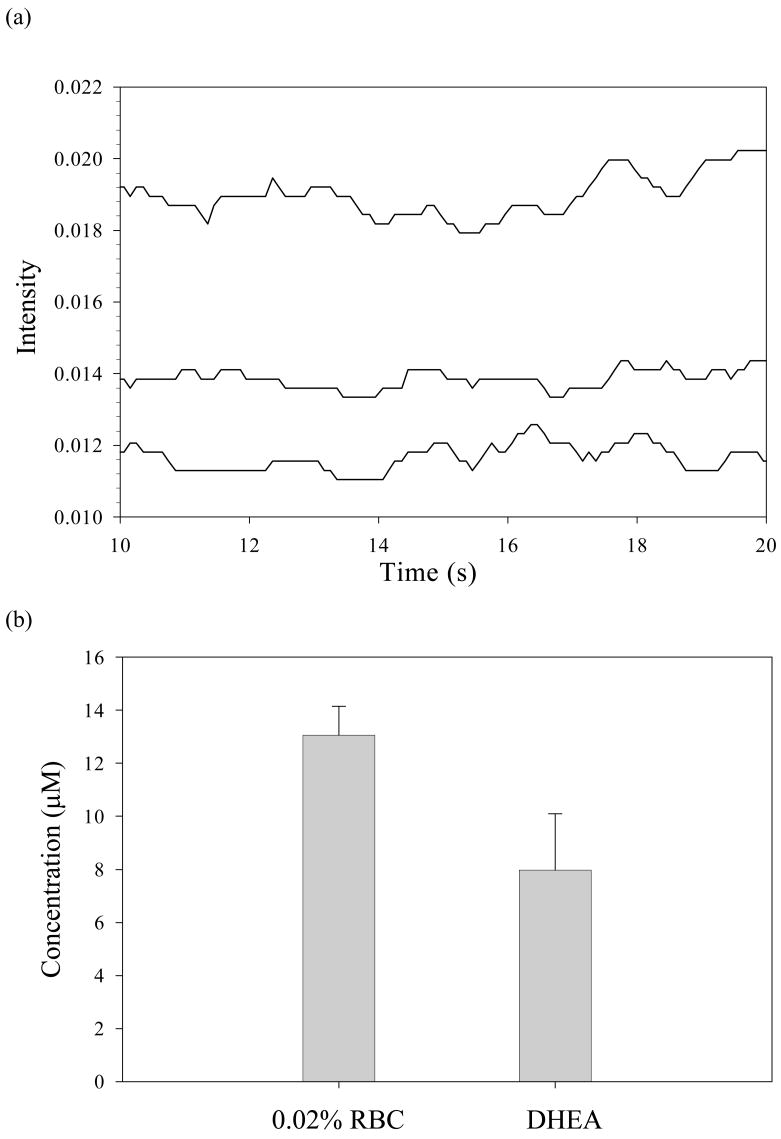
As a first step towards developing such a simultaneous detection method, a qualitative and quantitative measurement of NADPH under conditions of continuous flow was performed using NADPH standards. The schematic for this measurement is shown in figure 2 and the obtained data are shown in figure 5a. Once the method was established using NADPH standards, the measurement of NADPH levels in normal rabbit RBCs and chemically aged RBCs (by inhibiting G6PD with DHEA) was performed. As summarized in figure 5b, the DHEA-induced aging (via inhibition of the G6PD and the pentose phosphate pathway) resulted a decrease in NADPH level from original value of 13.2 ± 1.8 μM (in normal RBCs) to 8.0 ± 1.1 μM (in RBCs incubated in DHEA) in a 0.02% solution of RBCs, a 39% decrease in NADPH concentrations.
Fig. 5.
Quantitative measurement of cellular NADPH levels using flow through fluorescence detection. (a) An NADPH standard (lower trace) in comparison with NADPH levels in 0.02 % RBCs (top trace) and chemically aged RBCs (middle trace). (b) Quantitative representation of NADPH levels in 0.02 % rabbit RBCs and DHEA inhibited rabbit RBCs (n= 6 rabbits). There is a 39 % decrease in NADPH concentration upon incubation with the G6PD inhibitor DHEA for 30 min. The values in the absence and presence of DHEA are significantly different (p < 0.01).
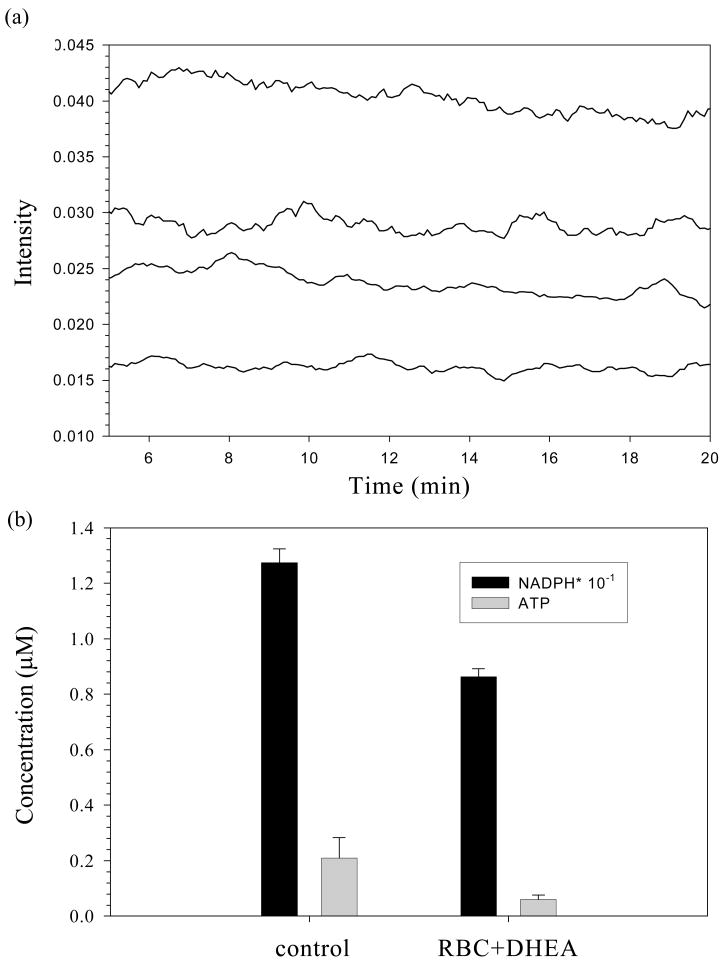
Although there are reports suggesting a relation between decreased G6PD activity and increased RBC stiffness, this is the first attempt to monitor the consequences of these characteristics simultaneously and quantitatively while permitting the forces required (flow) to induce the ATP release. The experimental setup shown in figure 2 was used to measure both G6PD activity and deformation induced RBC-derived ATP release. As expected, a direct relationship between decreased NADPH levels and the decreased values of deformation-induced RBC-derived ATP is measured (figure 6a). Figure 6b shows that the decrease in ATP release is proportional to the decrease in the cellular NADPH levels. Upon inhibition with the DHEA, the ATP release decreased to 0.06 ± 0.02 μM, down from an initial value of 0.21 ± 0.07 μM in 7 % RBCs. In a similar manner, the initial concentration of NADPH (12.73 ± 0.50 μM) decreased to 8.62 ± 0.29 μM upon addition of DHEA.
Fig. 6.
(a) Qualitative representation of simultaneous detection of NADPH levels and deformation induced ATP release from rabbit RBCs. NADPH levels were measured using a fluorescence based G6PD assay, while ATP release was measured using chemiluminescence. The bottom two traces represent ATP release from normal RBCs (higher of the pair) and G6PD inhibited (lower of the pair) RBCs. The top two traces represent NADPH levels in 0.02% RBCs in the absence (higher of the pair) and presence (lower trace of the pair) of DHEA. (b) Quantitative representation of simultaneously detected NADPH levels and deformation derived ATP release. The black bars represent NADPH concentration (n= 5 rabbits), while the gray bars represent ATP release (n= 5 rabbits). The values between the two groups are significantly different (p < 0.01).
Conclusions
It is well established that G6PD deficient individuals are subjected to high oxidative stress and, hence, stiffening of the cell membrane due to oxidation of important membrane proteins.13,15 This effect is pronounced in RBCs where G6PD is the sole producer of NADPH, an essential cofactor in the antioxidant defense mechanism. In type 2 diabetes, RBCs are under high oxidative stress and are believed to be less deformable, leading to lowered levels of deformation-induced ATP release. Also G6PD activity is an indication of cell aging and upon aging cell membranes become more stiffened due to a weakened oxidative defense system.
Here we were able to measure cellular NADPH levels in the RBC (via a fluorescence based assay) and deformation-derived ATP release from the RBC (via chemiluminescence assay) simultaneously, under continuous flow conditions. Results are consistent with the expected pattern of a direct relationship between NADPH concentrations in the RBC and deformation-derived ATP release from these cells. With a decrease in NADPH concentration, there is a decrease in deformation derived ATP release from the RBC. A similar trend was observed with chemically aged (via inhibition of G6PD) RBCs, as well as young and old fractions of RBCs separated from whole blood. RBCs obtained from people with type 2 diabetes showed the same trend confirming that oxidative stress leads to a decrease in deformation derived ATP release. Due to the ability of ATP to stimulate NO production in other cells (e.g., endothelial cells and platelets), the RBC thus becomes a potential determinant of blood flow in the diabetic circulation. Perhaps more evident from the work reported here, however, is the potential use of NADPH and ATP as biomarkers of oxidative stress in the RBC.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL073942 and DK071088).
References
- 1.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic b-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55:1074–1079. doi: 10.2337/diabetes.55.04.06.db05-1109. [DOI] [PubMed] [Google Scholar]
- 2.Hammes HP. Diabetic retinopathy, Internal medical aspects of an ophthalmologic topic. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2004;101:1159–1164. doi: 10.1007/s00347-004-1133-y. [DOI] [PubMed] [Google Scholar]
- 3.Icks A, Trautner C, Haastert B, Berger M, Giani G. Blindness due to diabetes: population-based age- and sex-specific incidence rates. Diabetic medicine : a journal of the British Diabetic Association. 1997;14:571–575. doi: 10.1002/(SICI)1096-9136(199707)14:7<571::AID-DIA384>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa T, Sato Y, Komatsu M, Hashizume K. ATP-sensitive potassium channel-independent, insulinotropic action of glucose in the B-cells. Endocrine Regulations. 1992;26:159–162. [PubMed] [Google Scholar]
- 5.Welters HJ, Senkel S, Klein-Hitpass L, Erdmann S, Thomas H, Harries LW, Pearson ER, Bingham C, Hattersley AT, Ryffel GU, Morgan NG. Conditional expression of hepatocyte nuclear factor-1b, the maturity-onset diabetes of the young-5 gene product, influences the viability and functional competence of pancreatic b-cells. Journal of Endocrinology. 2006;190:171–181. doi: 10.1677/joe.1.06768. [DOI] [PubMed] [Google Scholar]
- 6.MacRury SM, Small M, Anderson J, MacCuish AC, Lowe GD. Evaluation of red cell deformability by a filtration method in type 1 and type 2 diabetes mellitus with and without vascular complications. Diabetes research (Edinburgh, Lothian) 1990;13:61–65. [PubMed] [Google Scholar]
- 7.Gumieniczek A. Modification of oxidative stress by pioglitazone in the heart of alloxan-induced diabetic rabbits. Journal of Biomedical Science (Dordrecht, Netherlands) 2005;12:531–537. doi: 10.1007/s11373-005-6733-2. [DOI] [PubMed] [Google Scholar]
- 8.Winiarska K, Drozak J, Wegrzynowicz M, Fraczyk T, Bryla J. Diabetes-induced changes in glucose synthesis, intracellular glutathione status and hydroxyl free radical generation in rabbit kidney-cortex tubules. Molecular and Cellular Biochemistry. 2004;261:91–98. doi: 10.1023/b:mcbi.0000028742.83086.43. [DOI] [PubMed] [Google Scholar]
- 9.Cicha I, Tateishi N, Suzuki Y, Maeda N. Rheological changes in human red blood cells under oxidative stress: effects of thiol-containing antioxidants. Pathophysiology. 1999;6:121–128. [Google Scholar]
- 10.Srour MA, Bilto YY, Juma M. Susceptibility of erythrocytes from non-insulin-dependent diabetes mellitus and hemodialysis patients, cigarette smokers and normal subjects to in vitro oxidative stress and loss of deformability. Clinical Hemorheology and Microcirculation. 2000;22:173–180. [PubMed] [Google Scholar]
- 11.Cignarelli M, Blonda M, Cospite MR, Damato A, Nardelli G, Giorgino R. Alterations of erythrocyte lipid pattern and of some membrane related functions as a consequence of plasma lipid disorder in diabetes mellitus. Diabete Metab FIELD Full Journal Title:Diabete & metabolisme. 1983;9:272–276. [PubMed] [Google Scholar]
- 12.Spolarics Z, Condon MR, Siddiqi M, Machiedo GW, Deitch EA. Red blood cell dysfunction in septic glucose-6-phosphate dehydrogenase-deficient mice. American Journal of Physiology. 2004;286:H2118–H2126. doi: 10.1152/ajpheart.01085.2003. [DOI] [PubMed] [Google Scholar]
- 13.Costagliola C. Oxidative state of glutathione in red blood cells and plasma of diabetic patients: in vivo and in vitro study. Clinical Physiology and Biochemistry. 1991;8:204–210. [PubMed] [Google Scholar]
- 14.Wan GH, Tsai SC, Chiu DTY. Decreased blood activity of glucose-6-phosphate dehydrogenase associates with increased risk for diabetes mellitus. Endocrine. 2002;19:191–195. doi: 10.1385/ENDO:19:2:191. [DOI] [PubMed] [Google Scholar]
- 15.Fischer TM, Haest CWM, Stoehr M, Kamp D, Deuticke B. Selective alteration of erythrocyte deformability by sulfhydryl reagents. Evidence for an involvement of spectrin in membrane shear elasticity. Biochimica et Biophysica Acta, Biomembranes. 1978;510:270–282. doi: 10.1016/0005-2736(78)90027-5. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. American Journal of Physiology. 2005;289:F1040–F1047. doi: 10.1152/ajprenal.00076.2005. [DOI] [PubMed] [Google Scholar]
- 17.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. American Journal of Physiology. 1997;272:H1886–H1891. doi: 10.1152/ajpheart.1997.272.4.H1886. [DOI] [PubMed] [Google Scholar]
- 18.Sailaja YR, Baskar R, Saralakumari D. The antioxidant status during maturation of reticulocytes to erythrocytes in type 2 diabetics. Free Radical Biology & Medicine. 2003;35:133–139. doi: 10.1016/s0891-5849(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 19.Edwards J, Sprung R, Spence D, Sprague R. Chemiluminescence detection of ATP release from red blood cells upon passage through microbore tubing. Analyst (Cambridge, United Kingdom) 2001;126:1257–1260. doi: 10.1039/b100519g. [DOI] [PubMed] [Google Scholar]
- 20.Leverve XM, Guigas B, Detaille D, Batandier C, Koceir EA, Chauvin C, Fontaine E, Wiernsperger NF. Mitochondrial metabolism and type-2 diabetes: a specific target of metformin. Diabetes & Metabolism. 2003;29:6S88–86S94. doi: 10.1016/s1262-3636(03)72792-x. [DOI] [PubMed] [Google Scholar]
- 21.Alba-Loureiro TC, Hirabara SM, Mendonca JR, Curi R, Pithon-Curi TC. Diabetes causes marked changes in function and metabolism of rat neutrophils. Journal of Endocrinology. 2006;188:295–303. doi: 10.1677/joe.1.06438. [DOI] [PubMed] [Google Scholar]
- 22.De Schepper GG, Van Noorden CJF, Houtkooper JM. Age-related changes of glucose-6-phosphate dehydrogenase activity in mouse oocytes. Histochemical Journal. 1987;19:467–470. doi: 10.1007/BF01675415. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers GP, Lichtman HC, Sheff MF. Red blood cell glucose-6-phosphate dehydrogenase activity in aged humans. Journal of the American Geriatrics Society. 1983;31:8–11. doi: 10.1111/j.1532-5415.1983.tb06281.x. [DOI] [PubMed] [Google Scholar]
- 24.Mishra J, Pandeya SN. Aldose reductase inhibitors in diabetic complications. Oriental Journal of Chemistry. 2006;22:571–592. [Google Scholar]
- 25.Demiot C, Tartas M, Fromy B, Abraham P, Saumet JL, Sigaudo-Roussel D. Aldose reductase pathway inhibition improved vascular and C-fiber functions, allowing for pressure-induced vasodilation restoration during severe diabetic neuropathy. Diabetes. 2006;55:1478–1483. doi: 10.2337/db05-1433. [DOI] [PubMed] [Google Scholar]
- 26.Setter SM, Campbell RK, Cahoon CJ. Biochemical pathways for microvascular complications of diabetes mellitus. Annals of Pharmacotherapy. 2003;37:1858–1866. doi: 10.1345/aph.1D002. [DOI] [PubMed] [Google Scholar]
- 27.Stribling D, Armstrong FM, Harrison HE. Aldose reductase in the etiology of diabetic complications: 2 Nephropathy. J Diabet Complications FIELD Full Journal Title:The Journal of diabetic complications. 1989;3:70–76. doi: 10.1016/0891-6632(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 28.Bravi MC, Pietrangeli P, Laurenti O, Basili S, Cassone-Faldetta M, Ferri C, De Mattia G. Polyol pathway activation and glutathione redox status in non-insulin-dependent diabetic patients. Metabolism, Clinical and Experimental. 1997;46:1194–1198. doi: 10.1016/s0026-0495(97)90216-x. [DOI] [PubMed] [Google Scholar]
- 29.Carroll J, Raththagala M, Subasinghe W, Baguzis S, Oblak TDa, Root P, Spence D. An altered oxidant defense system in red blood cells affects their ability to release nitric oxide-stimulating ATP. Molecular BioSystems. 2006;2:305–311. doi: 10.1039/b604362n. [DOI] [PubMed] [Google Scholar]
- 30.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature (London, United Kingdom) 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 31.Shantz LM, Talalay P, Gordon GB. Mechanism of inhibition of growth of 3T3-L1 fibroblasts and their differentiation to adipocytes by dehydroepiandrosterone and related steroids: role of glucose-6-phosphate dehydrogenase. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3852–3856. doi: 10.1073/pnas.86.10.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabrese EJ, Horton HM, Leonard DA. The in vivo effects of four steroids on glucose-6-phosphate dehydrogenase activity of C57L/J mouse erythrocytes. Journal of Environmental Science and Health, Part A (Environmental Science and Engineering. 1987;A22:563–574. [Google Scholar]
- 33.Kimura K, Spate LD, Green MP, Roberts RM. Effects of D-glucose concentration, D-fructose, and inhibitors of enzymes of the pentose phosphate pathway on the development and sex ratio of bovine blastocysts. Molecular Reproduction and Development. 2005;72:201–207. doi: 10.1002/mrd.20342. [DOI] [PubMed] [Google Scholar]
- 34.Yang NC, Jeng KCG, Ho WM, Chou SJ, Hu ML. DHEA inhibits cell growth and induces apoptosis in BV-2 cells and the effects are inversely associated with glucose concentration in the medium. Journal of Steroid Biochemistry and Molecular Biology. 2000;75:159–166. doi: 10.1016/s0960-0760(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 35.Risso A, Turello M, Biffoni F, Antonutto G. Red blood cell senescence and neocytolysis in humans after high altitude acclimatization. Blood Cells, Molecules, & Diseases. 2007;38:83–92. doi: 10.1016/j.bcmd.2006.10.161. [DOI] [PubMed] [Google Scholar]
- 36.Omodeo-Sale F, Motti A, Basilico N, Parapini S, Olliaro P, Taramelli D. Accelerated senescence of human erythrocytes cultured with Plasmodium falciparum. Blood FIELD Full Journal Title:Blood. 2003;102:705–711. doi: 10.1182/blood-2002-08-2437. [DOI] [PubMed] [Google Scholar]
- 37.Raththagala M, Root PD, Spence DM. Dynamic Monitoring of Glutathione in Erythrocytes, without a Separation Step, in the Presence of an Oxidant Insult. Analytical Chemistry. 2006;78:8556–8560. doi: 10.1021/ac061163u. [DOI] [PubMed] [Google Scholar]
- 38.Gupta BL, Nehal M, Baquer NZ. Effect of experimental diabetes on the activities of hexokinase, glucose-6-phosphate dehydrogenase and catecholamines in rat erythrocytes of different ages. Indian Journal of Experimental Biology. 1997;35:792–795. [PubMed] [Google Scholar]
- 39.Kil IS, Lee JH, Shin AH, Park JW. Glycation-induced inactivation of NADP+-dependent isocitrate dehydrogenase: implications for diabetes and aging. Free Radical Biology & Medicine. 2004;37:1765–1778. doi: 10.1016/j.freeradbiomed.2004.08.025. [DOI] [PubMed] [Google Scholar]