Summary
Epidemiological research suggests that both an individual’s genes and the environment underlie the pathophysiology of schizophrenia. Molecular mechanisms mediating the interplay between genes and the environment are likely to have a significant role in the onset of the disorder. Recent work indicates that epigenetic mechanisms, or the chemical markings of the DNA and the surrounding histone proteins, remain labile through the lifespan and can be altered by environmental factors. Thus, epigenetic mechanisms are an attractive molecular hypothesis for environmental contributions to schizophrenia. In this review, we first present an overview of schizophrenia and discuss the role of nature versus nurture in its pathology, where ‘nature’ is considered to be inherited or genetic vulnerability to schizophrenia, and ‘nurture’ is proposed to exert its effects through epigenetic mechanisms. Second, we define DNA methylation and discuss the evidence for its role in schizophrenia. Third, we define posttranslational histone modifications and discuss their place in schizophrenia. This research is likely to lead to the development of epigenetic therapy, which holds the promise of alleviating cognitive deficits associated with schizophrenia.
Keywords: schizophrenia, environment, epigenetic, DNA methylation, histone, HDAC, DNMT, cognition
1. Introduction
Schizophrenia is a severely debilitating, stigmatized disorder occurring in up to 1% of the population worldwide. The pathology observed in patients includes three core disabilities: 1) positive symptoms, which include hallucinations, delusions, and thought disorder; 2) negative symptoms, which include apathy, inappropriate mood, and poverty of speech; and, 3) cognitive dysfunction, which includes impaired working memory and conceptual disorganization. Together these symptoms render patients difficult to employ and socially isolated. Schizophrenia patients are therefore some of the most disadvantaged members of society. Antipsychotic drug treatments only alleviate symptoms of schizophrenia so elucidating the pathophysiological mechanisms causing schizophrenia is likely to facilitate the development of more effective treatments.
2. Nature, nurture and their roles in the pathophysiology of schizophrenia
Schizophrenia is predicted via path analyses to have a multifactorial mode of inheritance. This model includes several genetic and non-genetic factors acting in combination to produce the disorder. Epidemiological studies have detected several non-genetic risk factors for schizophrenia, including marijuana use and obstetric complications, but these risk factors rarely have odds ratios exceeding 2 [1–5]. Attempts to consider gene x environment interactions have led to the finding that some candidate genes interact with severe obstetric complications to increase the risk of developing schizophrenia. A recent study by Nicodemus and colleagues detected association between four candidate genes for schizophrenia which are likely to play a role in hypoxic events, AKT1, brain derived neurotrophic factor (BDNF), metabotropic glutamate receptor 3 (GRM3) and dystrobrevin binding protein-1 (DTNBP1) [6]. These genes showed significant evidence for gene x environment interaction in a study of schizophrenia patients with or without obstetric complications [6].
The environmental risks and heritability of schizophrenia have also been studied separately. The latter has been established after genetic epidemiological investigations, based on family, twin, and adoption studies, which we discuss in the following sections.
2.1 Studies of the heritability of schizophrenia
Evidence for the aggregation of a trait in families suggests a hereditary component. Indeed, there is considerable evidence for a familial component in schizophrenia. The risk of schizophrenia and related disorders to first degree relatives is approximately 9 times greater than the risk of schizophrenia in the general population [7, 8]. The degree of genetic identity to the proband is strongly associated with the degree of risk, such that first degree relatives have a greater risk of psychosis than more distant relatives of affected individuals [9].
Twin studies are typically used to estimate the heritability of a trait. Comprehensive studies of twins with schizophrenia have shown that the risk of schizophrenia in the co-twin of the proband is substantially higher for monozygotic (MZ, 53%) compared with dizygotic (DZ, 15%) twins, and that there is an overall heritability estimate of 68% for the underlying liability to schizophrenia [10, 11]. MZ discordance for schizophrenia may be due to reduced penetrance of a schizophrenia genotype, which has been supported by the observation that there is an increased risk of schizophrenia among the offspring of the unaffected twin of discordant MZ pairs [10, 11]. A possible explanation for these observations could lie in environmental factors altering the function of genes creating vulnerability to schizophrenia [12].
The cross-fostering adoption study design is a powerful approach to examine the joint contribution of genetic and environmental factors to psychiatric illness. This method has been employed to compare the rates of schizophrenia in two groups of adoptees: adoptees with schizophrenic biological parents but raised by normal adoptive parents, and adoptees with normal biological parents who have been raised by adoptive parents diagnosed with schizophrenia after the adoption process. In one report using this design, Wender and colleagues found that the rate of disorder in the adoptees in the former group was 18.8% compared with 10.7% in the latter group [13]. Therefore, this suggests that both genetic and environmental contacts with schizophrenia are risk factors, although environmental factors are likely to have a much lesser impact. In conclusion, family, twin, and adoption studies have confirmed the existence of a genetic component in the etiology of psychosis, but also highlight the importance of environmental factors.
2.2 Studies of environmental risks for schizophrenia
Genetic data demonstrate that even when identical twins are investigated, i.e. subjects with 100% genetic identity, there is not 100% concordance for schizophrenia. If one twin has schizophrenia, the other has approximately a 50% lifetime risk for developing the disease [10, 11]. Similar data have arisen from studies of twins with bipolar disorder, major depression and anxiety disorders.
The general epidemiological statistics of schizophrenia are summarized in Table 1 [14–28]. Differences between genders in the age of onset of schizophrenia have been noted, with the onset in male patients 2–3 years earlier than in females. The protective effect of estrogen may explain this difference [29], although alternative variables such as marital status and premorbid personality traits are likely confounding factors between the sexes. For example, patients who have never married appear to have a 50-fold higher risk of developing schizophrenia if male, compared with a 15-fold higher risk if female [30].
Table 1.
Summary of epidemiological studies of Schizophrenia
| Lifetime prevalence | 0.5–1.72%, broad diagnostic category 0.26–0.54%, narrow diagnostic category |
| Onset of illness | Male mean age of onset at 26 yrs Female mean age of onset at 31 yrs |
| Socio-economic risk factors | Inverse relationship between socio-economic status and incidence of schizophrenia Higher prevalence in urban environments, especially males Downward drift seen in patients, especially in males |
| Seasonal differences in onset | Some evidence for seasonal patterns in incidence of the disease |
| Ethnic differences | Higher incidence of schizophrenia among migrant and ethnic minority groups living in large cities |
Reports indicate that psychoses seem to aggregate in urban environments and in lower socioeconomic groups [14]. For example, Afro-Caribbean immigrants to the United Kingdom and especially their offspring have an approximate 10-fold increased risk of schizophrenia [23, 24], and ethnic minorities in Britain have at least a 3-fold increase in the incidence of schizophrenia [31]. These observations have led some to propose that schizophrenia might be a disease of epidemiological transition, or in other words, a disease that rises in incidence during the development of a society [32]. Many hypotheses have been proposed to explain the increased rate of schizophrenia in second generation immigrants from third world countries born in industrialized countries. These hypotheses include the possibilities that industrialization may cause changes in maternal nutrition, obstetric complications and perinatal survival, maternal exposure to novel infectious agents, psychosocial stressors on both first- and second-generation immigrants, and gene-environment interactions that influence the development of schizophrenia [33].
The largest effect of environmental risk factors for schizophrenia has been detected for obstetric complications, such as preeclampsia and perinatal brain damage, while factors such as Rhesus incompatibility, unwanted pregnancy, malnutrition of the mother (during the first trimester), season of birth and maternal influenza (during the second trimester) could also be disruptive [34–37]. Furthermore, an excess of problems with speech and education, social anxiety, preference for solitary play and below average mothering skills have been reported to be predictive of schizophrenia later in life [38, 39].
It is probable that examination of each environmental risk separately will not identify reliable predictors for developing schizophrenia. Instead, it may be more biologically relevant to classify each of these risks as an environmental stressor and consider these in combination with an individual’s genotype. Indeed, an example of this strategy has been employed in a study of cannabis use in schizophrenia patients. Presence of the catecholamine-o-methyltransferase (COMT) valine158 allele was a predictor of psychotic symptoms and schizophreniform disorder only in subjects using cannabis, while subjects with the COMT 158 methionine homozygous genotype had no such adverse influence. Therefore the genetic vulnerability to psychosis produced by possession of the COMT valine158 allele was exacerbated by an environmental factor, cannabis use [40].
In summary, extensive epidemiological research does imply a supporting role of an individual’s environment in schizophrenia. How then might the environment render an individual susceptible to schizophrenia? The answer may lie in the influence of environmental factors on epigenetic mechanisms, such as DNA methylation and histone modifications, which contribute to the regulation of gene activity in the CNS.
3. The role of epigenetic mechanisms in schizophrenia
Epigenetics refers to the covalent modifications of chromatin, which is the DNA-histone protein complex present in the cell nucleus. Epigenetic mechanisms not only perpetuate lasting variation in gene activity states in the CNS but also influence transient modulation in gene transcription that support activity-dependent changes in gene expression necessary for cognition [41–44]. Thus, the possibility of an epigenetic contribution to schizophrenia is an attractive molecular hypothesis, and indeed has been the focus of many studies which we will discuss in the following sections.
4. DNA Methylation
DNA methylation is a direct covalent modification of DNA, where at least three encoded enzymes known as DNA methyltransferases (DNMTs) catalyze the addition of a -CH3 group to cytosine residues at the 5-position of the pyrimidine ring [45, 46]. DNMT1 is responsible for perpetuating methylation marks after cell division, regenerating the methyl-cytosine marks on the newly synthesized complementary DNA strand that arises from DNA replication [45, 46]. Thus, DNMT1 is typically viewed as a “maintenance” DNMT. By contrast, DNMT3a and 3b place new methylation marks on DNA, for example, when genes are turned off as part of cell fate determination [45, 46]. Consequently, DNMT3a and 3b are considered “de novo” DNMTs.
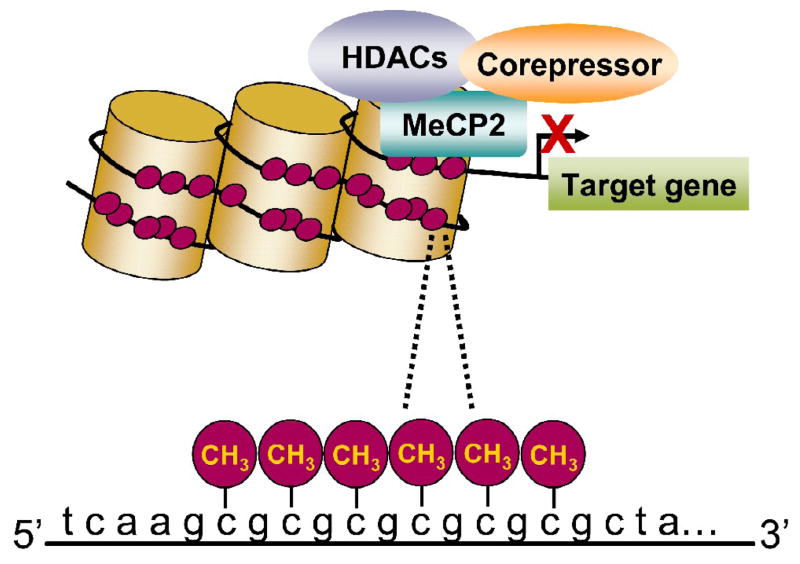
Cytosines that are followed by a guanine can be methylated, and these CpG dinucleotide sequences are typically found in and around gene regulatory regions in clusters known as CpG islands [45, 46]. DNA methylation within regulatory regions is usually associated with the suppression of gene transcription [45, 46]. In brief, methylated cytosines recruit methyl-DNA binding proteins, for example MeCP2, which help recruit histone deacetylases (HDACs). HDACS are enzymes that will remove acetyl groups from histone proteins. Together, these events compact chromatin structure, limit accessibility of transcriptional machinery, and in turn suppress gene transcription (Figure 1). However, it is important to note that while DNA methylation around gene promoters is typically associated with transcriptional suppression, recent work has indicated that DNA methylation can also be associated with transcriptional activation [47, 48].
Figure 1.
Schematic of transcriptional suppression by DNA methylation. Methylated cytosines bind, for example, methyl-CpG-binding protein (MeCP2), which recruits co-repressors and histone deacetylases (HDACs). Together, this leads to compaction of chromatin structure, which hinders gene transcription.
4.1 The stable vs. dynamic nature of DNA methylation
DNA methylation has been implicated in a number of developmental processes which are associated with long-lasting phenotypic changes, including genomic imprinting, cell differentiation, and X-chromosome inactivation [49]. Moreover, dysregulation of DNA methylation contributes to a number of neurodevelopmental disorders, including Rett syndrome and Fragile X mental retardation [50, 51]. In these cases, the phenotypic outcomes are due to the programming of DNA methylation very early in development, and these patterns of DNA methylation remain very stable through the lifespan. Such cases fit the historic view of epigenetic regulation of gene expression, in that it is a static process following neural development and cell differentiation.
Unlike these disorders, however, the onset of schizophrenia occurs later in life, usually during or after adolescence, and the environment might have some role in this onset. Then, if DNA methylation is a mechanism contributing to the onset of the disorder, DNA methylation would also have to be a dynamic process that can be influenced by environmental factors. Data continue to highlight that DNA methylation is indeed dynamic and susceptible to environmental influences. For example, while DNMT activity is generally restricted to dividing cells during mitosis with the highest level of expression occurring early during development [52–56], there are robust levels of DNMT enzymatic activity observable in adult CNS neurons [52, 57, 58]. Furthermore, methylation of DNA has been shown to be plastic in the adult CNS, and also appears to be important for the induction of synaptic plasticity and associative memory formation [59–62]. Finally, studies continue to highlight that the postnatal caregiving environment has a direct influence on DNA methylation patterns of several genes [63–65].
As a final note, since there is a high level of heritability of schizophrenia, one might ask whether DNA methylation patterns can also be transmitted from one generation to the next. This also appears to be the case, because reports continue to indicate that the epigenetic status of particular genes in the previous generation influences the next generation [64, 66–71]. For example, the transmission of positive aspects of maternal behavior in rats, as well as adult stress responses, appears to be attributable to the methylation status of the promoter of the glucocorticoid receptor gene in the hippocampus of the mother, as well as the methylation status of the promoter of the estrogen receptor alpha gene in the medial preoptic area [42, 66, 70]. Furthermore, changes in the methylation status of the BDNF gene due to early stress (abuse and neglect) can be transmitted to the next generation [64].
Then, it is reasonable to hypothesize that DNA methylation plays a role in schizophrenia. Indeed it has been previously postulated that the discordance observed in twin studies could be explained by epigenetic DNA modifications [72]. In the following sections, we discuss evidence for the role of DNA methylation in schizophrenia. To date, DNA methylation has been examined for only a handful of candidate genes, which are highlighted in Table 2 [73–85]. In the following sections we limit our discussion to the reelin gene (RELN), the glutamic acid decarboxylase 67 gene (GAD1), the serotonin-2A receptor gene (HTR2A), the catechol-O-methyltransferase gene (COMT), and BDNF.
Table 2.
Candidate genes for schizophrenia which are epigenetically modified.
| Gene | Locus | Linkage | Association | References |
|---|---|---|---|---|
| Glutamic acid decarboxylase (GAD1) | 2q31 | 2q37 [73] 2q33.3 [74] |
Several SNPs and haplotypes associated with SCZ | Discussed in section 4.2 |
| Reelin (RELN) | 7q22 | - | Association in Caucasians [75] | Discussed in section 4.2 |
| Serotonin2A receptor (HTR2A) | 13q14-21 | - | HTR2A -1438 G/A associated with SCZ | Discussed in section 4.3 |
| Monoamine oxidase A (MAOA) | Xp11.3 | - | No association* | [76] |
| Tyrosine hydroxylase (TH) | 11p15.5 | - | 11p14.1 associated with SCZ in GWA [77] | [76] |
| Catechol-o-methyltransferase (COMT) | 22q11 | Strong linkage with SCZ | 158 val/met associated with SCZ | Discussed in section 4.4 |
| Dopamine D2 receptor (DRD2) | 11q23 | - | rs6277 associated with SCZ* | Discussed in section 4.4 |
| Dopamine D3 receptor (DRD3) | 3q13.3 | - | No association* | [78] |
| Dopamine transporter (SLC6A3) | 5p15.3 | - | No association* | [76] |
| Brain derived neurotrophic factor (BDNF) | 11p13 | - | BDNF 66 val/met associated with SCZ | Discussed in section 4.5 |
| Glucocorticoid receptor (NR3C1) | 5q31.3 | Within region of strong linkage [79] | No association* | [80,81] |
| Potassium-chloride Co-Transporter 3 (SLC12A6) | 15q13-14 | Linkage of 15q14- 15 with BPD and SCZ | Association with BPD and SCZ | [82] |
| Sex-determining region Y-box containing gene 10 (SOX10) | 22q13.1 | Linkage with SCZ | Association of rs139887 with SCZ in Japan [83] | [84] |
| Synapsin III (SYN III) | 22q12.3 | Linkage with SCZ | No association* | [85] |
SZGene at www.schizophreniaforum.org
Abbreviations: SCZ=schizophrenia, BPD=bipolar affective disorder, GWA=genome-wide association
4.2 Genes influencing the GABAergic system
There is growing evidence that DNA methylation has a role in the dysfunction of GABAergic neurons in schizophrenia. In 2000, seminal observations were made that both RELN and the GAD1 genes were down-regulated in cortical [86] and hippocampal [87] GABAergic neurons in post-mortem samples from individuals with schizophrenia. Since then, reductions of RELN and GAD1 expression (both the mRNA and protein) have been one the most consistent findings in the postmortem studies [88–94].
RELN encodes an extracellular matrix protein that is not only important for neural development and synapse integrity, but plays a necessary role in the long-term potentiation that supports synaptic and behavioral plasticity in the adult [95]. RELN is expressed predominately by GABAergic interneurons which regulate neighboring glutamatergic neurons [96]. The GAD1 gene encodes one of the enzymes, GAD67, which synthesizes GABA from glutamate [97]. Down-regulation of either of these genes can certainly disrupt GABAergic neurotransmission, and altered GABA activity appears responsible for at least some of the clinical features of schizophrenia [98, 99]. Moreover there are risk associated SNPs in GAD1 which are also associated with decreased expression in the hippocampus and dorsal prefrontal cortex of patients with schizophrenia [94]. However, this does not exclude the possibility that methylation of DNA may also contribute to the down-regulation of RELN and GAD67 activity observed in schziophrenia.
Indeed there is mounting evidence to suggest that this may be the case. In adult cortical GABAergic interneurons, RELN, GAD1, and DNMT1 mRNAs co-localize [100, 101]. Reports have shown that both DNMT1 mRNA and protein levels are significantly increased in the cortex of individuals with schizophrenia [100–102], and that these increases parallel deficits in both RELN and GAD1 [101, 102]. Similar results have been documented in GABAergic neurons located in the basal ganglia [103].
To examine whether changes in gene expression could explain altered methylation status of CpG sites within the regulatory regions of RELN and GAD1 in cortical structures, investigators have used a variety of approaches. Data suggest that the down-regulation of these transcripts is most likely due to hypermethylation of their gene promoters [104–106]. However it is important to note that one study has documented decreased methylation of the GAD1 promoter in schizophrenia patients with both repressive chromatin and lower levels of GAD1 mRNA [107]. A possible explanation for this apparent paradox is that down-regulation of GAD1 in the hippocampus also appears attributable to the up-regulation of an HDAC enzyme [108].
Using a genome-wide epigenetic approach, investigators have recently found there to be as many as 100 loci with altered CpG methylation in schizophrenia, including several other gene families related to the GABAergic system: glutamate receptor genes (NR3B and GRIA2), glutamate transporters (VGLUT1 and 2), and a protein that regulates production of GABA receptors (MARLIN-1) [109, 110]. Together, these studies and those reviewed above indicate that there are epigenetic changes associated with schizophrenia. But do these epigenetic changes contribute to the genetic dysfunction observed in the disorder?
The brief answer is we do not know. However, basic research results help address this question, by demonstrating that DNA methylation and the associated chromatin remodeling could indeed play a pivotal role in the down-regulation of RELN and GAD1 documented in schizophrenia patients. Chronic treatment of mice with L-methionine (MET), a precursor necessary for DNMT catalytic activity, produces a schizophrenic-like phenotype [111, 112]. This treatment also replicates some of the molecular aspects of schizophrenia, including an increase in methylation of the RELN promoter and the constituent down-regulation of RELN and GAD1 in GABAergic neurons [111, 112]. Using these mice, investigators have also been able to show that both RELN and GAD1 promoters show increased recruitment of methyl-CpG binding proteins, such as MeCP2 [112]. Finally, using neuronal progenitor cells to further understand the epigenetic regulation of these genes, investigator have also shown that both DNMT and HDAC inhibitors activate RELN and GAD1 [113].
Though there is strong evidence for epigenetic changes in RELN and GAD1 in schizophirenia, it is not likely the case that their epigenetic dysfunction alone confers susceptibility to schizophrenia. Rather, it is likely that many genes are affected, as indicated by a study utilizing genome-wide epigenetic approaches [109, 110].
4.3 Genes influencing the serotonergic system
Altered serotonergic function is hypothesized to increase vulnerability to psychiatric diseases, including schizophrenia, anxiety disorders and affective disorders. To date, fourteen serotonin receptors have been identified within the serotonin system, of which the serotonin-2A (5-HT2A) receptor is one of three subtypes within the 5-HT2 receptor group [114]. The involvement of serotonin in schizophrenia first emerged from the observation that lysergic acid diethylamide (LSD), a potent 5-HT2 receptor agonist, had hallucinogenic properties [115, 116]. The LSD-induced psychosis includes both the hallucinations and delusions observed in schizophrenia patients, but not the negative symptoms (such as withdrawal, blunted affect and apathy). The affinity of many hallucinogenic drugs for 5-HT2 receptor sites has been found to be closely related to their potency as hallucinogens in humans [117, 118]. The atypical antipsychotic drugs and many antidepressants have high affinities for 5-HT2 receptors [119].
Variation of the gene encoding the 5-HT2A receptor (HTR2A) has been associated with schizophrenia in case control studies. The most extensively studied HTR2A polymorphism occurs in exon 1 and is a synonymous T/C change at position 102 (102 T/C). There are several findings of association between the HTR2A C102 allele and schizophrenia in Caucasian and Japanese studies [120–122]. The genetic association of this SNP with schizophrenia appears to vary with ethnicity because studies of Chinese postmortem samples show increased frequencies of the HTR2A T102 allele in schizophrenia, which may indicate that a different SNP in linkage disequilibrium with 102 T/C is likely to be causative, or that epigenetic modification at this site could be confounding these analyses.
Initial evidence from analyses of postmortem brain indicated that there may be polymorphic differential expression of HTR2A alleles because 4 out of 18 heterozygous (T/C) subjects tested expressed only one allele at the 102 T/C site [123]. Another study has shown that the HTR2A is paternally imprinted in human fibroblasts and transcribed from the maternal allele only [124]. However, Polesskaya and Sokoloff [125] were not able to replicate this ‘on or off’ polymorphic imprinting, and a more recent study also found no evidence of imprinting of HTR2A [126]. Regardless, expression levels of HTR2A C102 have been shown to be reduced in the temporal cortex [125], and ligand binding studies have shown that subjects with the HTR2A C102 allele have reduced receptor binding in postmortem brain [127] and in platelets [128]. But it is important to note that there have been conflicting data [129–131], suggesting that these effects are not universal and could vary with brain region, ethnicity, and/or environmental influences.
Two polymorphic sites in HTR2A have been detected to have methylated CpG sites in recent studies by Polesskaya et al [132]. The first site is at the 102 T/C SNP and the second is at the -1438 A/G SNP in the promoter of the gene. The methylation of the HTR2A C102 allele was found to correlate with DNMT1 expression levels. Furthermore, methylation of the promoter correlated with HTR2A expression levels. However, De Luca et al. reported no differences in HTR2A methylation between controls and schizophrenia cases in a postmortem study [133]. These methylation events could provide explanations for the conflicting data generated by different research groups investigating HTR2A expression and genetic association with schizophrenia.
Epigenetic variation and imprinting of HTR2A in schizophrenia have yet to be extensively tested. The finding of differential DNA methylation within HTR2A could indicate variation in the activity of alleles 102 C and 102 T and thus confound genetic association studies of HTR2A, especially because the gene has not been intensively screened for methylated sites.
4.4 Genes influencing the dopaminergic system
Dysfunction of the limbic circuitry in schizophrenia and its influence on dopamine release is considered to be the primary pathophysiological mechanism of schizophrenia. Dopamine is involved in several brain functions, including attention, executive function (including working memory) and reward mechanisms. Therefore understanding the mechanisms of regulation of dopamine levels, particularly by catecholamine-o-methyltransferase enzyme or COMT, and the signal transduction of dopamine receptors within the limbic system is fundamental to the research of schizophrenia, addiction and related disorders [134].
The gene encoding the COMT enzyme (COMT) has been intensively investigated in genetic studies of schizophrenia. COMT is of particular interest because it is located in the schizophrenia ‘linkage hotspot’, 22q11, which is deleted in velocardio facial syndrome or VCFS (also called Di George syndrome or 22q11 deletion syndrome). VCFS patients have a range of symptoms, including psychosis, and together with replicated findings of linkage with schizophrenia in the 22q11 region, genes within this locus are strong candidates for investigation in schizophrenia research. Not only is COMT a strong positional candidate, it also has an important role in the regulation of monoamine metabolism and therefore is a strong physiological candidate gene for schizophrenia research [135].
COMT is an S-adenosylmethionine dependent methyltransferase enzyme which methylates catecholamines (including dopamine and norepinepherine) and catechol estrogens. Two isoforms of COMT enzyme have been reported; a membrane-bound COMT (MB-COMT) and soluble COMT (S-COMT), each with its own promoter. COMT is solely responsible for the metabolism of dopamine in the dorsolateral prefrontal cortex, a region important for working memory performance, which is dysfunctional in schizophrenia. The focus of genetic studies of COMT has been the valine to methionine substitution, which occurs at codon 158 in the MB-COMT isoform and at codon 108 in S-COMT. This polymorphism has been shown to alter the activity and thermal stability of the enzyme, so that subjects with the methionine homozygous genotype are estimated to have up to 50% reduction of COMT activity [136]. Dopamine signaling is therefore likely to be enhanced in subjects with the met 158 allele in comparison with subjects homozygous for the val 158 allele. A groundbreaking study by Egan et al. demonstrated that COMT val 158 homozygous subjects exhibited reduced prefrontal cognitive performance and efficiency, in comparison with met 158 homozygous individuals [137]. COMT SNPs in untranslated, promoter and intronic regions are thought to impact COMT function through altered gene expression [138]. While there have been many reports of association between the COMT val allele and schizophrenia, the data have not always been consistent. Combined genetic and epigenetic data are likely to produce more accurate predictors of psychiatric phenotypes than genetic variation alone and could be more correlated with gene x environment interactions in an integrated model.
DNA methylation analyses of COMT have been conducted in postmortem brain series. The promoter of MB-COMT has been found to be methylated and this isoform of COMT is predominantly responsible for the metabolism of dopamine in the brain. One study indicated that methylation of the MB-COMT gene promoter was reduced by approximately 50% in schizophrenia and bipolar disorder subjects compared with controls, especially in the left frontal lobe [139]. Furthermore, MB-COMT gene expression was raised in schizophrenia and bipolar disorder compared with the controls, and subjects with the 158 val allele had lower levels of MB-COMT promoter methylation. MB-COMT hypomethylation was also correlated with dopamine D2 receptor gene (DRD2) promoter hypomethylation in schizophrenia and bipolar disorder compared with controls [139]. Another study has indicated that the 158 val/val homozygote subjects had a greater degree of exonic DNA methylation in the frontal cortex, but there was no association between the level of methylation and psychosis in this region [110]. However, these studies did not specifically measure methylation within the dorsolateral prefrontal cortex, so whether methylation of COMT could be related to working memory in schizophrenia has not been established.
4.5 BDNF
BDNF is another gene known to play an important role in cognition, and aberrant regulation of this gene has been implicated in the etiology and pathogenesis of several cognitive and mental disorders, including schizophrenia [140–144]. The BDNF protein is synthesized from a gene that has a rather complex structure. The BDNF gene contains nine 5’ non-coding exons (I-IX) linked to the common 3’ coding exon (IX), which encodes for BDNF pre-protein [145, 146]. Despite the known importance of BDNF function and gene expression in normal neural processes and CNS disorders, there has been little investigation into the molecular mechanisms responsible for complex BDNF transcriptional readout in the brain.
Recently, several studies have begun to implicate DNA methylation as a provocative molecular mechanism contributing to ongoing regulation of BDNF transcription in the CNS to mediate synaptic plasticity and memory formation [59, 60, 62,146]. For example, Sun’s group has shown that DNA methylation plays a role in activity-dependent BDNF gene regulation [147]. In addition, it has been shown that alterations in DNA methylation levels at the BDNF promoter or intragenic regions occurs in response to fear learning [60]. Specifically, a Pavlovian learning paradigm (contextual fear conditioning) elicits changes in hippocampal DNA methylation across the BDNF gene, and that this mechanism is involved in differential BDNF transcript read-out necessary for long-term memory formation. Furthermore, pharmacologically inhibiting DNMT activity sufficiently alters basal BDNF transcript levels in hippocampus [60]. Together, these studies shed light on the potential role of epigenetic mechanisms, namely DNA methylation, in dynamic regulation of the BDNF gene in the adult CNS, and highlight the fact that altered BDNF regulation could contribute to schizophrenia [141].
In addition to regulation of gene expression changes supporting synaptic plasticity and memory formation, BDNF DNA methylation has also been shown to play a role in altered gene expression in response to environmental influences, such as social experiences [64]. In fact, stressful social experiences early in life have long-lasting effects on behavior, including increased anxiety, increased drug-seeking behavior, cognitive deficits, and altered affiliative behaviors. In relation to BDNF, depriving an infant of social interaction for example yields a reduction in hippocampal and cortical BDNF mRNA and protein levels that persists well into adulthood [148–150]. Finally, it was recently shown that social experiences early during the first postnatal week trigger lasting changes in DNA methylation across the BDNF gene that is associated with decreases in BDNF gene expression in the adult prefrontal cortex [64].
There has been little investigation into whether BDNF DNA methylation is altered in schizophrenia. Postmortem reports have indicated that in the prefrontal cortex and hippocampus of schizophrenic patients there is both decreased BDNF protein [143, 151, 152] and BDNF mRNA levels [140, 143, 144]. Whether DNA methylation is a mechanism responsible for the abnormal regulation of the BDNF gene is certainly not clear. However, one study to date by Mill and colleagues suggests this may indeed be the case [110]. They found modest evidence for an association between DNA methylation and the BDNF genotype at a nonsynonymous SNP (rs6265 or val66met) which affects exonic CpG sites.
Overall, data suggest that DNA methylation may indeed be an epigenetic mechanism that contributes to the aberrant regulation of genes associated with schizophrenia. Another epigenetic mechanism we will now consider is the posttranslational modification of histone tails, as the functional significance of histone modifications in the regulation of genes involved in schizophrenia is increasingly becoming the focus of vigorous research.
5. Histone Modifications
In the nucleus, DNA is wrapped around an octamer of 8 different histone proteins (H2A, H2B, H3, and H4), with H1 serving as a linker protein. Histone tail amino acid residues are subject to covalent modifications, including lysine acetylation, methylation, SUMOylation, and ubiquitinylation; arginine methylation; serine phosphorylation; and proline isomerization [153]. Depending on the amino acid residue modified there can be different affects on gene transcription. For example, acetylation is linked with gene activation, while SUMOylation is associated with gene repression. However, some histone modifications are more complex, such as methylation, and can be associated with either gene activation or repression dependent upon the nature of the modifications.
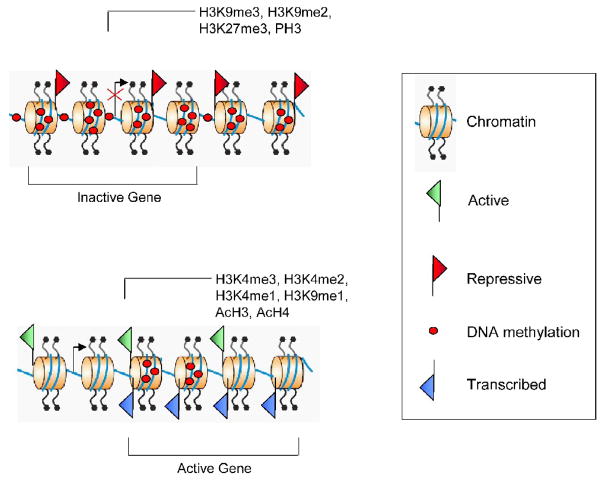
Histone methylation at lysine residues can be up to three forms: mono-, di-, and tri-methylated. For example, tri-methylation of lysine 4 of histone H3 is localized at gene promoter regions, and is linked with active gene transcription. Additional examples of the complexity of lysine methylation include methylation of lysine 9 and 27 of histone H3 and methylation of lysine 20 of histone H4. Mono-methylation is associated with active transcription while di- and tri-methylation at these lysine residues are associated with transcriptional repression [154]. Figure 2 illustrates some specific histone modifications that are associated with gene activation or gene suppression.
Figure 2.
The nucleosome core histones (H2A, H2B, H3, and H4) are subject to a variety of covalent modifications including: lysine acetylation, methylation, SUMOylation, and ubiquitinylation; arginine methylation; and serine phosphorylation. Chromatin can be divided into accessible regions named euchromatin or inaccessible regions called heterochromatin. Some histone marks are associated with gene activation and others with gene repression. For example, histone H3, H4 acetylation (AcH3, AcH4), and phosphorylation (PH3) are associated with activation. However, other histone marks, such histone methylation, have different effects depending on the lysine residue. The H3K4me3, H3K4me2, H3K4me1, and H3K9me1 mark the active transcription start site regions of a gene, while H3K27me3 is found in regions that encompass inactive genes. Histone lysine 9 mono- and dimethylated (H3K9me2 and H3K9me3) is also associated with the heterochromatic regions.
5.1 Histone Modifications in schizophrenia
To date, far less is known regarding histone modifications in comparison to DNA methylation in schizophrenia. However, histone modifications are beginning to receive attention for their likely contribution [155, 156]. Importantly, histone marks are relatively preserved in human postmortem tissue [157].
In 2005, Akbarian’s group provided the first demonstration that there are histone modifications associated with schizophrenia [158]. Specifically, they found in a small subset of prefrontal cortex samples that there was significantly higher open chromatin-associated H3-methylation (at arginine 17) in schizophrenic patients than controls [158]. Since their seminal study, they and others have provided further evidence for histone modifications in schizophrenia. For example, there is altered H3-lysine 4 and 27 trimethylation [107, 159] and increased HDAC1 expression in the prefrontal cortex of schizophrenic patients [160].
Most of what we know regarding histone regulation of gene activity comes by way of studies in the learning and memory field indicating that specific histone modifications at gene promoters are an important control mechanism for transcriptional regulation in the adult CNS [60,161–166]. To date, this has been studied for the BDNF gene. Specifically, histone H3 acetylation and phosphorylation is increased at BDNF gene promoters in association with exon-specific BDNF gene expression in an electroconvulsive seizure model [166]. Other forms of histone modifications at BDNF promoters, such as histone H3 dimethylation, have also been described in transcriptionally repressed BDNF transcripts in a mouse model of depression [165]. Thus, an overall emerging hypothesis is that post-translational modification of histones can alter chromatin structure at gene promoter regions and subsequently control transcription of the genes in response to environmental cues. These data along with postmortem analyses suggest that histone modifications might also contribute to the aberrant regulation of genes associated with schizophrenia.
6. Conclusions and future directions
It is becoming increasingly clear that epigenetic mechanisms not only provide a molecular mechanism responsible for stable changes in brain function and behavior, but also have some necessary role in the dynamic nature of the adult CNS in response to the environment. Thus, an epigenetic contribution to schizophrenia has become an attractive molecular hypothesis, and evidence gathered to date appears to support this hypothesis. Unfortunately, postmortem studies make it difficult to address whether epigenetic mechanisms are causally related to the pathogenesis of schizophrenia. Thus, rodent models of neuropsychiatric disorders will likely have to fill this void in future studies.
One of the most important questions that future studies will likely address is whether epigenetic drugs can alleviate the cognitive deficits associated with schizophrenia. Encouragingly, studies on abnormal gene expression in postmortem brain have revealed that histone methylation may be a viable avenue for early detection for some cases of schizophrenia [155]. To date there is limited clinical and basic research data examining the role of DNMT and HDAC inhibitors in alleviating cognitive deficits [167–170]. However, beneficial effects of DNMT or HDAC inhibition have been observed in both alleviating some of the molecular and behavioral manifestations of cognitive dysfunction in rodents [156].
Such observations hold promise that drugs that can modify chromatin will be a viable therapy for treating schizophrenia. The continued study of epigenetic marks in normal brain development, in the regulation of normal cognition, and in postmortem tissue promises a future where we will be able to unravel the molecular mysteries underlying schizophrenia. Elucidating these pathophysiological mechanisms will certainly facilitate the development of more effective therapeutic strategies.
Acknowledgments
This work was funded by grants from the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression, Civitan International, and the Evelyn F. McKnight Brain Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boog G. Obstetrical complications and further schizophrenia of the infant: A new medicolegal threat to the obstertrician? J Gynecol Obstet Biol Reprod. 2003;32:720–727. [PubMed] [Google Scholar]
- 2.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: Historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 3.Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M. Obstetric complications and the risk of schizophrenia: A longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- 4.Geddes JR, et al. Schizophrenia and complications of pregnancy and labor: An individual patient data meta-analysis. Schizophr Bull. 1999;25:413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 5.Preti A. Obstetric complications, genetics and psychosis. Eur J Obstet Gynecol Reproduc Biol. 2006;126:127–128. doi: 10.1016/j.ejogrb.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Nicodemus KK, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13:873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 7.McGue M, Gottesman I. The genetic epidemiology of schizophrenia and the design of linkage studies. Eur Arch Psychiatry Clin Neurosci. 1991;240:174–181. doi: 10.1007/BF02190760. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Diehl SR. The genetics of schizophrenia: A current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19:261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: New findings promise new insights. Mol Psychiatry. 2003;9:14–27. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- 10.Sham P. Genetic epidemiology. Br Med Bull. 1996;52:408–433. doi: 10.1093/oxfordjournals.bmb.a011557. [DOI] [PubMed] [Google Scholar]
- 11.Tsuang M. Schizophrenia: Genes and environment. Biol Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 12.Oh G, Petronis A. Environmental studies of schizophrenia through the prism of epigenetics. Schizophr Bull. 2008;34:1122–1129. doi: 10.1093/schbul/sbn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wender PH, Rosenthal D, Kety SS, Schulsinger F, Welner J. Crossfostering. A research strategy for clarifying the role of genetic and experimental factors in the etiology of schizophrenia. Arch Gen Psychiatry. 1974;30:121–128. doi: 10.1001/archpsyc.1974.01760070097016. [DOI] [PubMed] [Google Scholar]
- 14.Aro S, Aro H, Keskimaki I. Socio-economic mobility among patients with schizophrenia or major affective disorder. A 17-year retrospective follow-up. Br J Psychiatry. 1995;166:759–767. doi: 10.1192/bjp.166.6.759. [DOI] [PubMed] [Google Scholar]
- 15.Castle DJ, Scott K, Wessely S, Murray RM. Does social deprivation during gestation and early life predispose to later schizophrenia? Soc Psychiatry Psychiatr Epidemiol. 1993;28:1–4. doi: 10.1007/BF00797825. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood MR, Peter AM. Epidemiology and seasonal affective disorder. Psychol Med. 1988;18:799–806. doi: 10.1017/s0033291700009727. [DOI] [PubMed] [Google Scholar]
- 17.Faraone SV, Chen WJ, Goldstein JM, Tsuang MT. Gender differences in age at onset of schizophrenia. Br J Psychiatry. 1994;164:625–629. doi: 10.1192/bjp.164.5.625. [DOI] [PubMed] [Google Scholar]
- 18.Farris RE, Dunham HW. Mental disorders in urban areas. University of Chicago Press; Chicago: 1939. [Google Scholar]
- 19.Goldberg EM, Morrison SL. Schizophrenia and social class. Br J Psychiatry. 1963;109:785–802. doi: 10.1192/bjp.109.463.785. [DOI] [PubMed] [Google Scholar]
- 20.Jablensky A, et al. Schizophrenia: Recent epidemiologic issues. Epidemiol Rev. 1995;17:10–20. doi: 10.1093/oxfordjournals.epirev.a036164. [DOI] [PubMed] [Google Scholar]
- 21.Jablensky A, et al. Schizophrenia: Manifestations, incidence and course in different cultures. A world health organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 22.Hare EH, Walter SD. Seasonal variation in admissions of psychiatric patients and its relation to seasonal variation in their births. J Epidemiol Comm Health. 1978;32:47–52. doi: 10.1136/jech.32.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison G, Owens D, Holton A, Neilson D, Boot D. A prospective study of severe mental disorder in afro-caribbean patients. Psychol Med. 1988;18:643–657. doi: 10.1017/s0033291700008321. [DOI] [PubMed] [Google Scholar]
- 24.Harvey M, Williams P, McGuffin BK, Toone The functional psychoses in afro-caribbeans. Br J Psychiatry. 1990;157:515–522. doi: 10.1192/bjp.157.4.515. [DOI] [PubMed] [Google Scholar]
- 25.Kohn ML. Social class and schizophrenia: A critical review and a reformulation. Schizophr Bull. 1973;1:60–79. [Google Scholar]
- 26.Hollingshead AB, Redlich FC. Social class and mental illness: A community study. John Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis G, David A, Andreasson S, Allenbeck P. Schizophrenia and city life. Lancet. 1992;340:137–140. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- 28.Suhail K, Cochrane R. Seasonal variations in hospital admissions for affective disorders by gender and ethnicity. Soc Psychiatry Psychiatr Epidemiol. 1998;33:211–217. doi: 10.1007/s001270050045. [DOI] [PubMed] [Google Scholar]
- 29.Hafner H, et al. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol Med. 1993;23:925–940. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- 30.Tien AY, Eaton WW. Psychopathologic precursors and sociodemographic risk factors for the schizophrenia syndrome. Arch Gen Psychiatry. 1992;49:37–46. doi: 10.1001/archpsyc.1992.01820010037005. [DOI] [PubMed] [Google Scholar]
- 31.King M, Coker E, Leavey G, Hoare A, Johnson-Sabine E. Incidence of psychotic illness in London: Comparison of ethnic groups. BMJ. 1994;309:1115–1119. doi: 10.1136/bmj.309.6962.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner R. Time trends in schizophrenia: Changes in obstetric risk factors with industrialization. Schizophr Bull. 1995;21:483–500. doi: 10.1093/schbul/21.3.483. [DOI] [PubMed] [Google Scholar]
- 33.Eagles JM. The relationship between schizophrenia and immigration. Are there alternatives to psychosocial hypotheses? Br J Psychiatry. 1991;159:783–789. doi: 10.1192/bjp.159.6.783. [DOI] [PubMed] [Google Scholar]
- 34.Dassa D, et al. Relationship of birth season to clinical features, family history, and obstetric complication in schizophrenia. Psychiatry Res. 1996;64:11–17. doi: 10.1016/0165-1781(96)02868-5. [DOI] [PubMed] [Google Scholar]
- 35.Kinney DK, et al. Season of birth and obstetrical complications in schizophrenics. J Psychiatr Res. 1994;28:499–509. doi: 10.1016/0022-3956(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 36.Maki P, et al. Predictors of schizophrenia--a review. Br Med Bull. 2005;73–74:1–15. doi: 10.1093/bmb/ldh046. [DOI] [PubMed] [Google Scholar]
- 37.Patterson PH. Neuroscience: Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 38.Helgeland MI, Torgersen S. Stability and prediction of schizophrenia from adolescence to adulthood. Eur Child Adolesc Psychiatry. 2005;14:83–94. doi: 10.1007/s00787-005-0436-0. [DOI] [PubMed] [Google Scholar]
- 39.Malaspina D, et al. Harlap, Acute maternal stress in pregnancy and schizophrenia in offspring: A cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caspi A, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: Longitudinal evidence of a gene x environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 41.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development, Neurosci. Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Graff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 46.Miranda TB, Jones PA. DNA methylation: The nuts and bolts of repression. J Cell Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 47.Chahrour M, et al. Mecp2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen S, Zhou Z, Greenberg ME. Activating a repressor. Science. 2008;320:1172–1173. doi: 10.1126/science.1159146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang PK, Kuroda MI. Noncoding rnas and intranuclear positioning in monoallelic gene expression. Cell. 2007;128:777–786. doi: 10.1016/j.cell.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Amir RE, et al. Rett syndrome is caused by mutations in x-linked mecp2, encoding methyl- cpg-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 51.Das S, et al. Methylation analysis of the fragile x syndrome by pcr. Genet Test. 1997;1:151–155. doi: 10.1089/gte.1997.1.151. [DOI] [PubMed] [Google Scholar]
- 52.Goto K, et al. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56:39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- 53.Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development. 1987;99:371–382. doi: 10.1242/dev.99.3.371. [DOI] [PubMed] [Google Scholar]
- 54.Singer-Sam J, Robinson MO, Bellve AR, Simon Ml, Riggs AD. Measurement by quantitative pcr of changes in hprt, pgk-1, pgk-2, aprt, mtase, and zfy gene transcripts during mouse spermatogenesis. Nucl Acids Res. 1990;18:1255–1259. doi: 10.1093/nar/18.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szyf M, Bozovic V, Tanigawa G. Growth regulation of mouse DNA methyltransferase gene expression. J Biol Chem. 1991;266:10027–10030. [PubMed] [Google Scholar]
- 56.Szyf M, et al. Cell cycle-dependent regulation of eukaryotic DNA methylase level. J Biol Chem. 1985;260:8653–8656. [PubMed] [Google Scholar]
- 57.Brooks PJ, Marietta C, Goldman D. DNA mismatch repair and DNA methylation in adult brain neurons. J Neurosci. 1996;16:939–945. doi: 10.1523/JNEUROSCI.16-03-00939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravindran CRM, Ticku MK. Methylation of nmda receptor nr2b gene as a function of age in the mouse brain. Neurosci Letters. 2005;380:223–228. doi: 10.1016/j.neulet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 59.Levenson JM, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 60.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of bdnf gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 62.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early- life adversity on the bdnf gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weaver ICG, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 66.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McLeod J, Sinal CJ, Perrot-Sinal TS. Evidence for non-genomic transmission of ecological information via maternal behavior in female rats. Genes Brain Behav. 2007;6:19–29. doi: 10.1111/j.1601-183X.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 69.Richards EJ. Inherited epigenetic variation - revisiting soft inheritance. Nat Rev Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 70.Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reproductive Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Arai JA, Li S, Hartley DM, Feig LA. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci. 2009;29:1496–1502. doi: 10.1523/JNEUROSCI.5057-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petronis A. The origin of schizophrenia: Genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004;55:965–970. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Wijsman EM, et al. Genome-wide scan in a large complex pedigree with predominantly male schizophrenics from the island of kosrae: Evidence for linkage to chromosome 2q. Mol Psychiatry. 2003;8:695–705. doi: 10.1038/sj.mp.4001356. [DOI] [PubMed] [Google Scholar]
- 74.Åberg K, et al. Support for schizophrenia susceptibility locus on chromosome 2q detected in a swedish isolate using a dense map of microsatellites and snps. Am J Med Genet. 2008;147B:1238–1244. doi: 10.1002/ajmg.b.30762. [DOI] [PubMed] [Google Scholar]
- 75.Shifman S, et al. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdolmaleky HM, Smith CL, Zhou JR, Thiagalingam S. Epigenetic alterations of the dopaminergic system in major psychiatric disorders. Methods Mol Biol. 2008:187–212. doi: 10.1007/978-1-59745-205-2_9. [DOI] [PubMed] [Google Scholar]
- 77.O'Donovan MC, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 78.Petronis A. The genes for major psychosis: Aberrant sequence or regulation? Neuropsychopharmacol. 2000;23:1–12. doi: 10.1016/S0893-133X(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 79.Schwab S, et al. A genome-wide autosomal screen for schizophrenia susceptibility loci in 71 families with affected siblings: Support for loci on chromosome 10p and 6. Mol Psychiatry. 2000;5:638–649. doi: 10.1038/sj.mp.4000791. [DOI] [PubMed] [Google Scholar]
- 80.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oberlander T, Weinberg J, Papsdorf M, Grunau R, S M, AM D. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (nr3c1) and infant cortisol stress responses. Epigenetics. 2008;4 doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 82.Moser D, et al. Functional analysis of a potassium-chloride co-transporter 3 (slc12a6) promoter polymorphism leading to an additional DNA methylation site. Neuropsychopharmacol. 2008;34:458–467. doi: 10.1038/npp.2008.77. [DOI] [PubMed] [Google Scholar]
- 83.Maeno N, et al. Association of sox10 with schizophrenia in the Japanese population. Psychiatric Genet. 2007;17:227–231. doi: 10.1097/YPG.0b013e3280ae6cd8. [DOI] [PubMed] [Google Scholar]
- 84.Iwamoto K, et al. DNA methylation status of sox10 correlates with its downregulation and oligodendrocyte dysfunction in schizophrenia. J Neurosci. 2005;25:5376–5381. doi: 10.1523/JNEUROSCI.0766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy B, O'Reilly R, Singh S. DNA methylation and mrna expression of syn III, a candidate gene for schizophrenia. BMC Med Genet. 2008;9:115. doi: 10.1186/1471-2350-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guidotti A, et al. Decrease in reelin and glutamic acid decarboxylase67 (gad67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 87.Fatemi S, Earle J, McMenomy T. Reduction in reelin in immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–663. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 88.Abdolmaleky HM, et al. Methylomics in psychiatry: Modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet. 2004;127B:51–59. doi: 10.1002/ajmg.b.20142. [DOI] [PubMed] [Google Scholar]
- 89.Abdolmaleky HM, Thiagalingam S, Wilcox M. Genetics and epigenetics in major psychiatric disorders: Dilemmas, achievements, applications, and future scope. Am J Pharmacogenomics. 2005;5:149–160. doi: 10.2165/00129785-200505030-00002. [DOI] [PubMed] [Google Scholar]
- 90.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered gad1/gad67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. Gad67 and gad65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76:581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Burgos G, Lewis DA. Gaba neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grayson DR, et al. The human reelin gene: Transcription factors (+),repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther. 2006;111:272–86. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Straub RE, et al. Allelic variation in gad1 (gad67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 95.Costa E, et al. Reelin and schizophrenia: A disease at the interface of the genome and the epigenome. Mol Intervent. 2002;2:47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- 96.Pesold A, et al. Reelin is preferentially expressed in neurons synthesizing γ-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinal CS, Tobin AJ. Uniqueness and redundancy in gaba production. Perspective Dev Neurobiol. 1998;5:109–118. [PubMed] [Google Scholar]
- 98.Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Res Rev. 2007;56:427–442. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 99.Wassef A, Baker J, Kochan LD. Gaba and schizophrenia: A review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- 100.Veldic M, et al. DNA-methyltransferase 1 mrna is selectively overexpressed in telencephalic gabaergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci USA. 2005;102:2152–7. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruzicka WB, et al. Selective epigenetic alteration of layer I gabaergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 103.Veldic M, et al. Epigenetic mechanisms expressed in basal ganglia gabaergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdolmaleky HM, et al. Hypermethylation of the reelin (reln) promoter in the brain of schizophrenic patients: A preliminary report. Am J Med Genet. 2005;134:60–6. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- 105.Costa E, et al. Gabaergic promoter hypermethylation as a model to study the neurochemistry of schizophrenia vulnerability. Expert Rev Neurotherapeutics. 2009;9:87–98. doi: 10.1586/14737175.9.1.87. [DOI] [PubMed] [Google Scholar]
- 106.Grayson A, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang HS, Akbarian S. Gad1 mrna expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS ONE. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benes FM, et al. Regulation of the gaba cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Connor CM, Akbarian S. DNA methylation changes in schizophrenia and bipolar disorder. Epigenetics. 2008;3:55–58. doi: 10.4161/epi.3.2.5938. [DOI] [PubMed] [Google Scholar]
- 110.Mill J, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tremolizzo L, et al. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci USA. 2002;99:17095–100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong A, et al. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci USA. 2005;102:12578–83. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and gad67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hannon J, Hoyer D. Molecular biology of 5-ht receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 115.Gaddum JH, Hameed KA. Drugs which antagonize 5-hydroxytryptamine. Br J Pharmacol Chemother. 1954;9:240–248. doi: 10.1111/j.1476-5381.1954.tb00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci U S A. 1954;40:228–231. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glennon RA, Titeler M, McKenney JD. Evidence for 5-ht2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 118.Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-ht2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacol. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- 119.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacol. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 120.Inayama Y, et al. Positive association between a DNA sequence variant in the serotonin 2a receptor gene and schizophrenia. Am J Med Genet. 1996;67:103–105. doi: 10.1002/(SICI)1096-8628(19960216)67:1<103::AID-AJMG18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 121.Williams J, et al. Association between schizophrenia and t102c polymorphism of the 5-hydroxytryptamine type 2a-receptor gene. European multicentre association study of schizophrenia (emass) group. Lancet. 1996;347:1294–1296. doi: 10.1016/s0140-6736(96)90939-3. [DOI] [PubMed] [Google Scholar]
- 122.Williams J, et al. Meta-analysis of association between the 5-ht2a receptor t102c polymorphism and schizophrenia. Emass collaborative group. European multicentre association study of schizophrenia. Lancet. 1997;349:1221. doi: 10.1016/s0140-6736(05)62413-0. [DOI] [PubMed] [Google Scholar]
- 123.Bunzel R, et al. Polymorphic imprinting of the serotonin-2a (5-ht2a) receptor gene in human adult brain. Brain Res Mol Brain Res. 1998;59:90–92. doi: 10.1016/s0169-328x(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 124.Kato MV, et al. Paternal imprinting of mouse serotonin receptor 2a gene htr2 in embryonic eye: A conserved imprinting regulation on the rb/rb locus. Genomics. 1998;47:146–148. doi: 10.1006/geno.1997.5089. [DOI] [PubMed] [Google Scholar]
- 125.Polesskaya OO, Sokolov BP. Differential expression of the "C" And "T" Alleles of the 5-ht2a receptor gene in the temporal cortex of normal individuals and schizophrenics. J Neurosci Res. 2002;67:812–822. doi: 10.1002/jnr.10173. [DOI] [PubMed] [Google Scholar]
- 126.De Luca V, Likhodi O, Kennedy JL, Wong AHC. Parent-of-origin effect and genomic imprinting of the htr2a receptor gene t102c polymorphism in psychosis. Psychiatry Res. 2007;151:243–248. doi: 10.1016/j.psychres.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 127.Turecki G, et al. Prediction of level of serotonin 2a receptor binding by serotonin receptor 2a genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 128.Khait VD, et al. Association of serotonin 5-ht2a receptor binding and the t102c polymorphism in depressed and healthy Caucasian subjects. Neuropsychopharmacol. 2005;30:166–172. doi: 10.1038/sj.npp.1300578. [DOI] [PubMed] [Google Scholar]
- 129.Bray NJ, et al. The serotonin-2a receptor gene locus does not contain common polymorphism affecting mrna levels in adult brain. Mol Psychiatry. 2004;9:109–114. doi: 10.1038/sj.mp.4001366. [DOI] [PubMed] [Google Scholar]
- 130.Kouzmenko AP, et al. No correlation between a(−1438)g polymorphism in 5-ht2a receptor gene promoter and the density of frontal cortical 5-ht2a receptors in schizophrenia. Hum Hered. 1999;49:103–105. doi: 10.1159/000022853. [DOI] [PubMed] [Google Scholar]
- 131.Kouzmenko AP, et al. 5-ht2a receptor polymorphism and steady state receptor expression in schizophrenia. Lancet. 1997;349:1815–1815. doi: 10.1016/S0140-6736(05)61695-9. [DOI] [PubMed] [Google Scholar]
- 132.Polesskaya OO, Aston C, Sokolov BP. Allele c-specific methylation of the 5-ht2a receptor gene: Evidence for correlation with its expression and expression of DNA methylase dnmt1. J Neurosci Res. 2006;83:362–373. doi: 10.1002/jnr.20732. [DOI] [PubMed] [Google Scholar]
- 133.De Luca V, Viggiano E, Dhoot R, Kennedy JL, Wong AHC. Methylation and qtdt analysis of the 5-ht2a receptor 102c allele: Analysis of suicidality in major psychosis. J Psychiatric Res. 2009;43:532–537. doi: 10.1016/j.jpsychires.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 134.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 135.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 136.Chen J, et al. Functional analysis of genetic variation in catechol-o-methyltransferase (comt): Effects on mrna, protein, and enzyme activity in postmortem human brain. Am J Human Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Egan MF, et al. Effect of comt val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tunbridge EM, Lane TA, Harrison PJ. Expression of multiple catechol-o-methyltransferase (comt) mrna variants in human brain. Am J Med Genet Part B Neuropsychiatric Genet. 2007;144B:834–839. doi: 10.1002/ajmg.b.30539. [DOI] [PubMed] [Google Scholar]
- 139.Abdolmaleky HM, et al. Hypomethylation of mb-comt promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Angelucci A, Brene S, Mathe AA. Bdnf in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 141.Lu B, Martinowich K. Cell biology of bdnf and its relevance to schizophrenia. Novartis Found Symp. 2008;289:119–129. doi: 10.1002/9780470751251.ch10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 143.Weickert CS, et al. Reductions in neurotrophin receptor mrnas in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–50. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- 144.Weickert CS, et al. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- 145.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat bdnf gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu QR, et al. Rodent bdnf genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 147.Martinowich K, et al. DNA methylation-related chromatin remodeling in activity- dependent bdnf gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 148.Branchi I, Francia N, Alleva E. Epigenetic control of neurobehavioural plasticity: The role of neurotrophins. Behav Pharmacol. 2004;15:353–362. doi: 10.1097/00008877-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 149.Fumagalli A, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 150.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 151.Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: Postmortem findings from the Stanley neuropathology consortium. Mol Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 152.Torrey EF, et al. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–60. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 153.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 154.Barski A, et al. High-resolution profiling of histone methylation in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 155.Akbarian S, Huang HS. Epigenetic regulation in human brain--focus on histone lysine methylation. Biol Psychiatry. 2009;65:198–203. doi: 10.1016/j.biopsych.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Deutsch S, Rosse R, Mastropaolo J, Long K, Gaskins B. Epigenetic therapeutic strategies for the treatment of neuropsychiatric disorders: Ready for prime time? Clin Neuropharmacol. 2008;31:104–119. doi: 10.1097/WNF.0b013e318067e255. [DOI] [PubMed] [Google Scholar]
- 157.Stadler A, et al. Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. J Neurochem. 2005;94:324–336. doi: 10.1111/j.1471-4159.2005.03190.x. [DOI] [PubMed] [Google Scholar]
- 158.Akbarian S, et al. Chromatin alterations associated with down-regulated metabolic gene expression in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2005;62:829–840. doi: 10.1001/archpsyc.62.8.829. [DOI] [PubMed] [Google Scholar]
- 159.Huang HS, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at gabaergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: Analysis of the national brain databank microarray collection. Schizophrenia Res. 2008;98:111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bredy TW, et al. Histone modifications around individual bdnf gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 164.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine- induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 165.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 166.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Abel T, Zukin RS. Epigenetic targets of hdac inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bowden CL. Spectrum of effectiveness of valproate in neuropsychiatry. Expert Rev Neurotherapeutics. 2007;7:9–16. doi: 10.1586/14737175.7.1.9. [DOI] [PubMed] [Google Scholar]
- 169.Deutsch S, Rosse R, Mastropaolo J, Long K, Gaskins B. Epigenetic therapeutic strategies for the treatment of neuropsychiatric disorders: Ready for prime time? Clin Neuropharmacol. 2008;31:104–119. doi: 10.1097/WNF.0b013e318067e255. [DOI] [PubMed] [Google Scholar]
- 170.Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Ann Rev Pharmacol Toxicol. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]