Abstract
Purpose
To identify prognostic factors related to outcome in 255 patients with synovial sarcoma (SS) and to construct a preoperative nomogram to predict the risk of disease-specific death.
Design
Between July 1982 and June 2006, 301 patients underwent treatment at our institution for primary SS of all anatomic sites and 255 patients with localized disease at presentation were resected with curative intent. Data were collected prospectively and analyzed retrospectively.
Results
Five-year, 10-year, and 15-year disease-specific survival was 72%, 60%, and 53%, respectively. Multivariate analysis revealed size and primary tumor site as the only independent adverse predictors of disease-specific death. A nomogram based on preoperative data for surgical patients not receiving anthracycline-ifosfamide (AI) chemotherapy (N = 196) estimates three- and five-year DSS with a concordance index of 77.3%. For the first 3 years following diagnosis, the observed DSS for patients treated with AI chemotherapy (N = 59) was greater than that predicted by the preoperative nomogram based on patients not receiving AI chemotherapy. SYT-SSX fusion transcript data were available for 132 patients. Multivariate analysis of this subset showed that SYT-SSX1 fusion type was predictive of early, but not late, distant recurrence.
Conclusion
Size and location govern prognosis in primary SS resected with curative intent. A nomogram based on preoperative variables provides individualized patient survival estimates and demonstrates an early survival benefit to chemotherapy that may dissipate over time. This nomogram may improve decision-making with regards to selecting patients most likely to benefit from neoadjuvant/adjuvant chemotherapy.
Keywords: synovial sarcoma, preoperative nomogram, chemotherapy, SYT, SSX, SYT-SSX
Introduction
Synovial sarcomas comprise 5 to 10% of soft tissue sarcomas (STS) and contain unique chromosomal translocations forming the SYT-SSX1 or SYT-SSX2 genes.1, 2 Accumulating evidence suggests that synovial sarcoma is relatively chemosensitive3, 4 and that patient outcomes in appropriately selected cases are improved by the use of neoadjuvant/adjuvant chemotherapy.5, 6
Multiple studies have individually identified age7, size,5, 7–12 sex,13 margin status,11 location,8, 13 mitotic activity,11 bone or neurovascular invasion,10 grade,11 and possibly SYT-SSX fusion type12, 14 as significant prognostic factors in synovial sarcoma. Although larger tumor size has consistently emerged as an important predictor of worse survival across these studies, the relative prognostic value of the other factors remains equivocal.
The administration of adjuvant chemotherapy for adult patients with STS remains controversial.1 A minority of positive studies demonstrate a survival benefit to chemotherapy, while numerous other studies do not. Among patients with synovial sarcoma undergoing resection with curative intent, anthracycline/ifosfamide (AI) chemotherapy was associated with improved survival in a retrospective analysis.5 Since individual assessments of the risk of synovial sarcoma-specific death and the potential benefit of adjuvant/neoadjuvant AI chemotherapy are needed to guide treatment decisions, we chose to analyze the clinicopathologic predictors of distant recurrence and sarcoma-specific death in a large cohort of synovial sarcoma patients who presented with primary, localized disease of any site. We constructed a nomogram based on preoperative variables to more precisely define individual patient risk for disease-specific death for surgical patients not receiving AI chemotherapy and compared the predicted survival based on this nomogram to the observed survival for the subgroup of patients who received AI chemotherapy.
Methods
Between July 1982 and June 2006, 416 patients aged 16 years or greater underwent inpatient treatment for primary, locally recurrent, and metastatic synovial sarcoma at MSKCC. Of the 301 patients who presented with primary disease, 38 patients presented with synchronous primary and metastatic disease and were excluded from this analysis. An additional 8 patients did not undergo resection of the primary tumor because of poor performance status or progression on neoadjuvant chemotherapy. The remaining 255 patients were resected with curative intent and form the basis of this study.
Following approval for this retrospective study by the Institutional Review Board of MSKCC, clinical, pathologic, and treatment data were reviewed and analyzed with respect to distant recurrence-free (DRFS) and disease-specific survival (DSS). Histologic diagnosis of synovial sarcoma was assigned using published criteria15. Margin status was determined as part of the histopathologic assessment. Depth was categorized as either superficial or deep to the investing fascia.
Therapeutic decisions regarding the administration of radiotherapy and chemotherapy were based on the consensus judgment of the treating physicians and therefore represent aggregate institutional practice which has evolved over time. A proportion of the patients were administered radiotherapy as part of a clinical trial,16 whereas chemotherapy was not administered in the setting of a clinical protocol. Doxorubicin was typically given at 75 mg/m2/cycle as divided doses by intravenous bolus over 3 days, and ifosfamide was given at a dose of 6 to 9 g/m2/cycle in divided doses over 3 days.
For patients who received neoadjuvant chemotherapy prior to resection of the primary tumor, tumor size was defined as the maximum diameter measured by computed tomography or magnetic resonance imaging prior to treatment. For patients who received adjuvant chemotherapy or no chemotherapy following resection of the primary tumor, size was defined both by cross-sectional imaging as well as maximum diameter on pathologic analysis. The differences between these measurements were negligible. One hundred thirty-two patients (52%) had tumor tissue submitted for SYT-SSX fusion type analysis. SYT-SSX1 and SYT-SSX2 were distinguished by RT-PCR as described previously.14, 17
Fisher’s exact test and Wilcoxon rank-sum test were used to compare categorical and continuous variables, respectively, across groups. The associations of the examined clinical, pathologic, and treatment variables with DRFS, DSS, and OS were examined using the log-rank test for categorical variables and the score test for continuous variables. To examine the association of DRFS, DSS, and OS while adjusting for important prognostic factors, variables significant on univariate analysis at the 0.10 level were entered into a Cox proportional hazards model. An accelerated failure time analysis18, 19 with the Gehan estimator was used to examine the association of SYT-SSX fusion type with DRFS and DSS since the proportional hazard assumption does not hold for this variable. These analyses were performed with R software utilizing the Survival and Rankreg libraries.
The variables considered for the creation of the nomogram were anatomic site (upper extremity, lower extremity, other), size, depth (superficial, deep), and histologic variant (monophasic, biphasic). The nomogram modeling and validation procedure were similar to that used previously.20, 21 An individual patient’s predicted probability of DSS was assumed to be a function of two components: the baseline hazard function shared by all patients and a linear combination of an individual patient’s predictor variable values. For validation, the nomogram was subjected to bootstrapping in order to calculate an unbiased measure of its ability to discriminate among patients. This effect is quantified by the concordance index. The concordance index is similar to the area under the receiver operating characteristic curve and ranges from 0.5 (no discrimination) to 1.0 (perfect discrimination). Given a randomly selected pair of patients, the concordance index is the probability that the patient who dies first had the higher predicted probability of death. These analyses were performed with R software using the Design and Hmisc libraries.
Results
Clinicopathologic and Treatment Characteristics
Among this cohort of 255 patients, there was an equal gender distribution, the median age was 34, and 81% of the tumors were located on the extremity (58% lower, 23% upper). There were a comparable number of primary tumors from the thorax, trunk, and head and neck locations. Only 2% of tumors were located intraabdominally or within the retroperitoneum. Given the sample size, all non-extremity sites (48 patients, 19% of the cohort) were grouped together for purposes of statistical analysis.
Ninety-nine percent of the tumors were high grade, 93% were deep, and the median maximal dimension was 6 cm (range 0.5 – 30 cm, with 44% of the tumors less than 5 cm). The monophasic subtype was more prevalent than the biphasic one (66% compared to 34%). SYT-SSX fusion type was available for 52% of patients with SYT-SSX1 and SYT-SSX2 fusions detected in 73 and 59 tumors, respectively. As depicted in Table 1, the median age for patients with the SYT-SSX1 and SYT-SSX2 fusion type was 41 and 35, respectively (p = 0.07). The gender distribution for SYT-SSX1 patients was 26 females and 47 males compared to 37 females and 22 males for patients with the SYT-SSX2 fusion transcript (p=0.005). Fusion transcript distribution was significantly different for the 91 monophasic tumors (41 SYT-SSX1 and 50 SYT-SSX2) compared to the 41 biphasic tumors (32 SYT-SSX1 and 9 SYT-SSX2) (p=0.001). Fusion transcript type was not significantly associated with tumor size, depth or location, although there was a trend toward a higher frequency of distal extremity tumors, defined as tumors at or distal to the wrist or ankle, among the SYT-SSX1 subtype (21% versus 11% for SYT-SSX2, p = 0.16). This may explain, in part, the statistically significant increase in the rate of amputation among patients with the SYT-SSX1 subtype (18% versus 3% for SYT-SSX2, p = 0.01).
Table 1.
Clinicopathologic Characteristics of 132 Patients with Known Fusion Transcript Data
| Characteristic | SYT-SSX1 N=73 (%) | SYT-SSX2 N=59 (%) | Univariate P value | |
|---|---|---|---|---|
| Gender* | Male | 47 (64%) | 22 (37%) | 0.005 |
| Female | 26 (36%) | 37 (63%) | ||
| Age at Diagnosis | Median 41 (range 16 – 80) | Median 35 (range 18 – 78) | 0.07 | |
| Site | Lower Extremity | 43 (59%) | 33 (56%) | 0.56 |
| Upper Extremity | 15 (20%) | 11 (19%) | ||
| Trunk | 5 (7%) | 3 (5%) | ||
| Head and Neck | 4 (6%) | 2 (3%) | ||
| Thoracic | 4 (6%) | 9 (15%) | ||
| Retroperitoneal/Intraabdominal | 2 (3%) | 1 (2%) | ||
| Grade | High | 71 (97%) | 59 (100%) | 0.50 |
| Low | 2 (3%) | 0 (0%) | ||
| Depth | Deep | 70 (96%) | 57 (97%) | 1.00 |
| Superficial | 3 (4%) | 2 (3%) | ||
| Primary Tumor Size | ≤ 5 cm | 21 (29%) | 16 (27%) | 0.86 |
| 5–10 cm | 34 (47%) | 26 (44%) | ||
| > 10 cm | 18 (25%) | 17 (29%) | ||
| Margin Status | R0 | 66 (90%) | 49 (83%) | 0.36 |
| R1 | 5 (7%) | 9 (15%) | ||
| R2 | 1 (1%) | 1 (2%) | ||
| Unknown | 1 (1%) | 0 (0%) | ||
| Subtype* | Monophasic | 41 (56%) | 50 (85%) | 0.001 |
| Biphasic | 32 (44%) | 9 (15%) | ||
| Extent of Resection* | Limb-Sparing | 60 (82%) | 57 (97%) | 0.01 |
| Amputation | 13 (18%) | 2 (3%) | ||
| Adjuvant | Yes | 48 (66%) | 44 (75%) | 0.34 |
| Radiotherapy | No | 25 (34%) | 15 (25%) | |
| Perioperative | Ifosfamide-Based | 24 (33%) | 18 (30%) | 0.68 |
| Chemotherapy | Non-ifosfamide-Based | 10 (14%) | 6 (10%) | |
| None | 38 (52%) | 35 (59%) | ||
| Unknown | 1 (1%) | 0 (0%) | ||
Because of rounding, not all percentages sum to 100.
Indicates statistically significant differences.
An R0 resection was achieved in 86% of patients, and limb salvage was possible in 88% of patients (85% of patients with extremity tumors). Sixty-three percent of patients received radiotherapy, and the vast majority (151 patients) were treated with postoperative radiation. External beam radiation predominated over brachytherapy by an approximate 3:2 ratio. In this non-randomized cohort of patients, radiotherapy was not statistically associated with DSS, OS, or local recurrence-free survival (data not shown). Thirty-nine percent (99 patients) were administered perioperative chemotherapy of whom 50 received adjuvant and 49 received neoadjuvant therapy. After 1990, chemotherapy was almost exclusively AI-based (59 of 65 patients, 91%).
Predictors of Disease-Specific and Distant Recurrence-Free Survival
With a median follow-up of 72 months for survivors (range 0 – 287), median DSS was not reached. Five, 10, and 15-year DSS were 72% (95% CI 66 – 78%), 60% (95% CI 52 – 68%), and 53% (95% CI 45 – 63%), respectively. Median DRFS was 103 months, and 5, 10, and 15-year DRFS were 55% (95% CI 549 – 62%), 47% (95% CI 40 – 55), and 44% (95% CI 36 – 54), respectively.
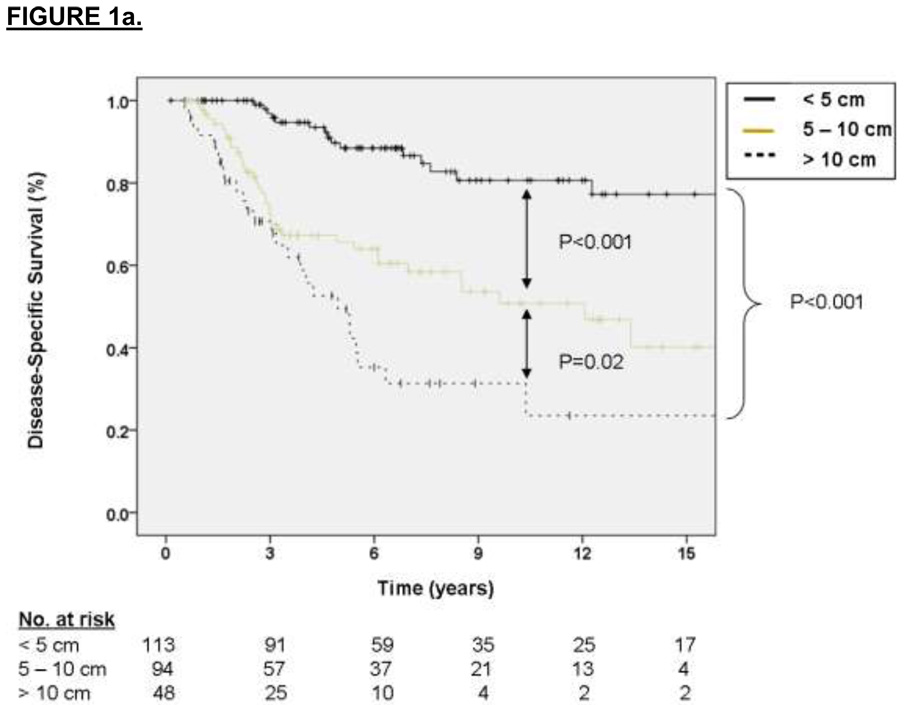
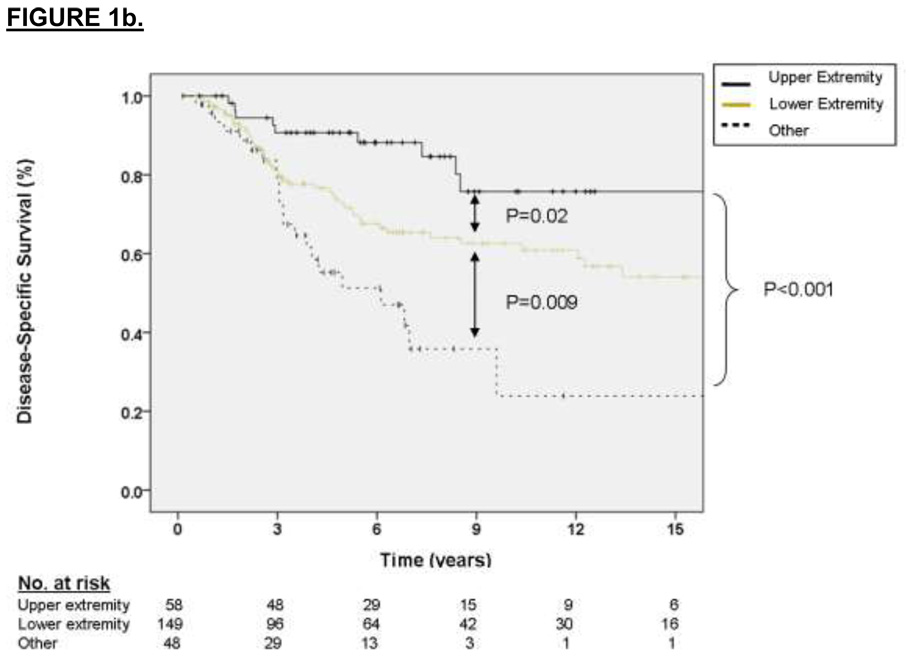
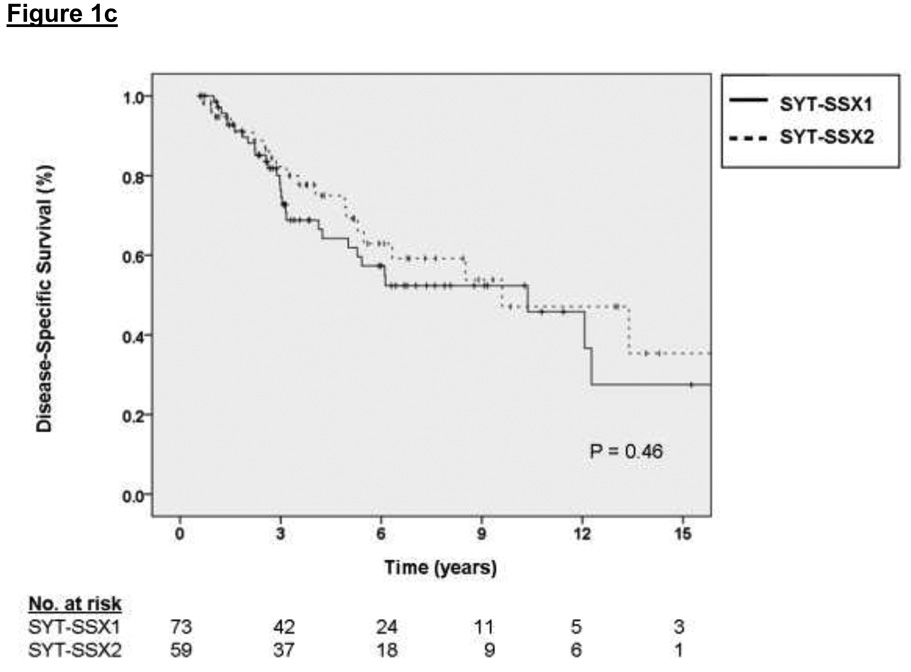
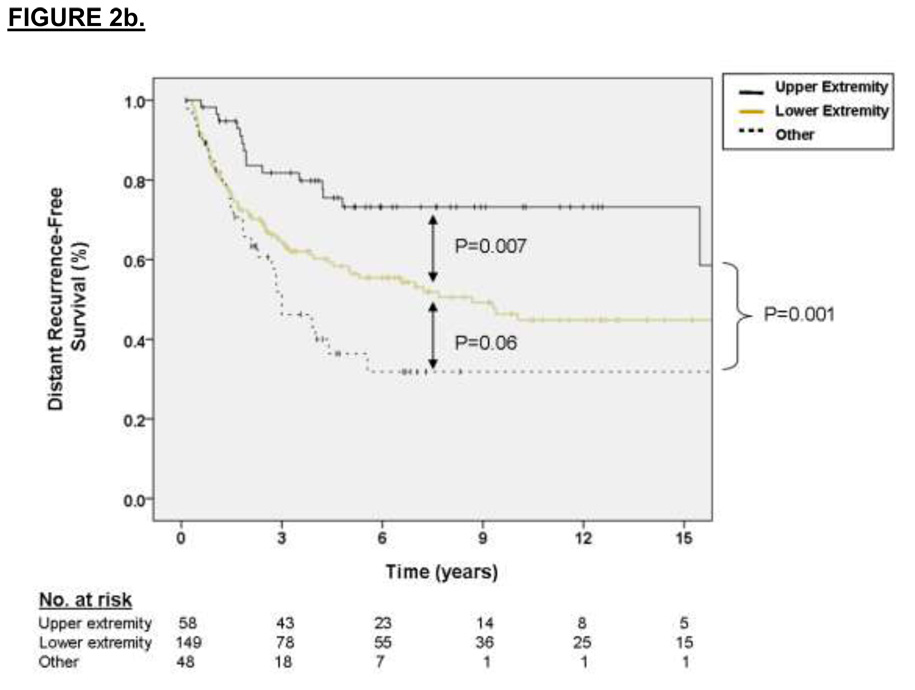
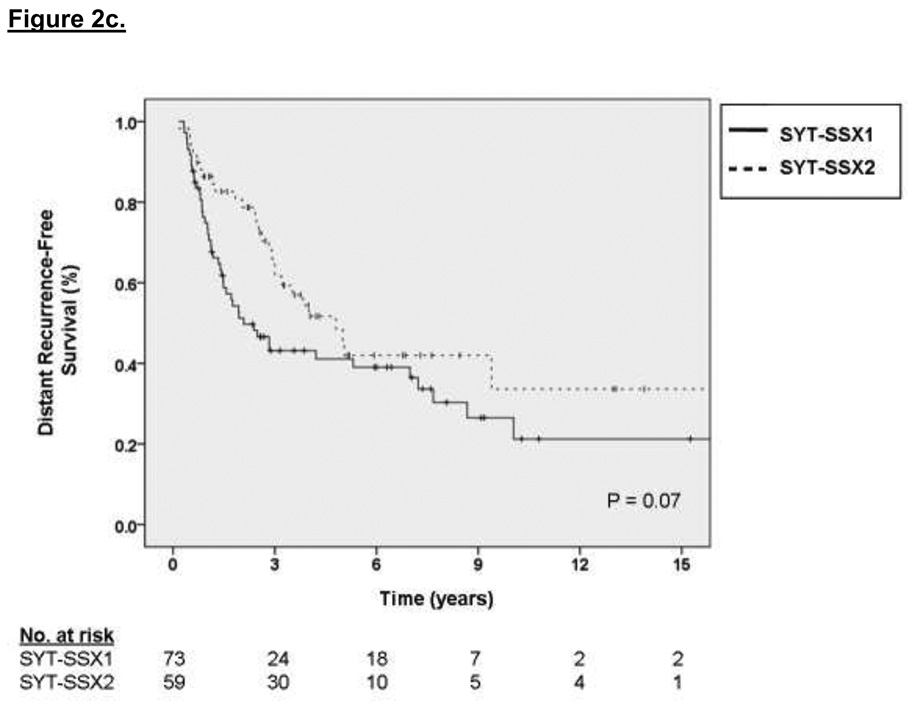
The factors associated with DSS on multivariate analysis were primary tumor size (2.75 HR for sarcoma-specific death for tumors 5 to 10 cm relative to ≤ 5 cm, p = 0.01, and 5.17 HR for tumors > 10 cm relative to ≤ 5 cm, p < 0.001) and primary tumor site (2.01 HR for sarcoma-specific death for lower extremity location relative to upper extremity, p = 0.06, and 3.63 HR for death for other sites relative to upper extremity, p = 0.01). These results are depicted graphically in Figure 1a and 1b, respectively. For the subset of patients with data available on SYT-SSX fusion type, the median and 3-year DSS for the SYT-SSX1 and SYT-SSX2 was 123 months and 71% versus 115 months and 78%, respectively (see Figure 1c, p=0.7 using an accelerated failure time analysis).
Figure 1.
A. Kaplan-Meier curve depicting disease-specific survival grouped by primary tumor size. Univariate P values are shown. B. Kaplan-Meier curve depicting disease-specific survival grouped by site of primary tumor. Univariate P values are shown. C. Kaplan-Meier curve depicting disease-specific survival grouped by type of fusion transcript. Univariate P values are shown.
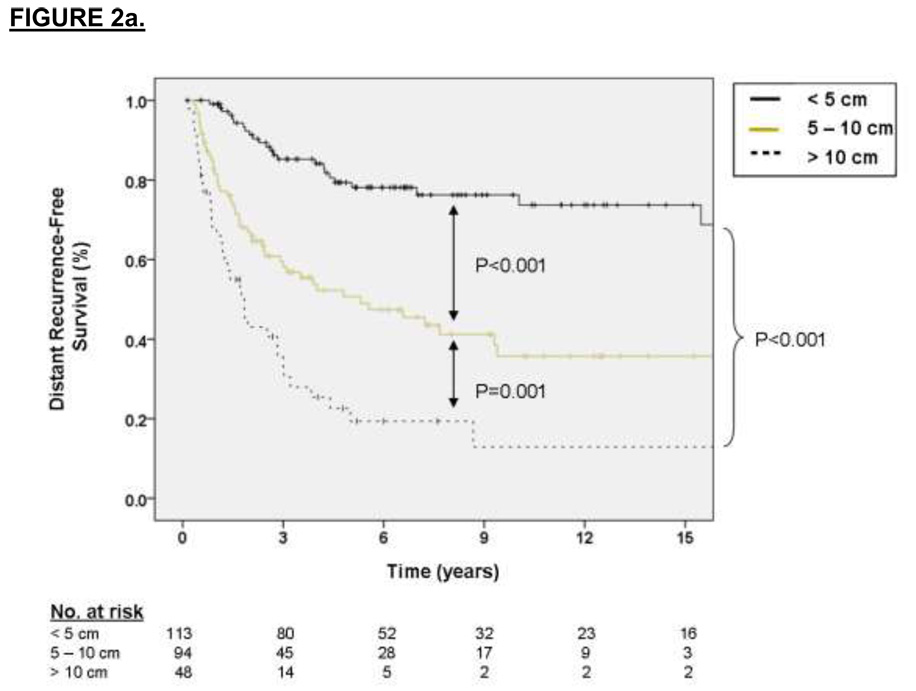
Multivariate analysis of independent variables associated with DRFS revealed that primary tumor size was strongly predictive of DRFS (2.72 HR for distant recurrence for tumors 5 to 10 cm relative to ≤ 5 cm, p < 0.001, 5.72 HR for distant recurrence for tumors > 10 cm relative to ≤ 5 cm, p < 0.001) as was primary tumor site (1.77 HR for distant recurrence for lower extremity location relative to upper extremity, p = 0.05, 2.51 HR for distant recurrence for other sites relative to upper extremity, p = 0.005). Kaplan-Meier curves of DRFS grouped by primary tumor size, site and fusion type are shown in Figures 2a, 2b, and 2c, respectively. For the subset of patients with data available on SYT-SSX fusion type, the median and 3-year DRFS for the SYT-SSX1 and SYT-SSX2 was 29 months and 44% versus 45 months and 56%, respectively (p=0.07 using an accelerated failure time univariate analysis). Multivariate analysis of this 132 patient subset using an accelerated failure time analysis showed that SYT-SSX1 fusion type was independently predictive of early distant recurrence (p=0.02, AR =−1.15, see Table 2)
Figure 2.
A. Kaplan-Meier curve depicting distant recurrence-free survival grouped by primary tumor size. Univariate P values are shown. B. Kaplan-Meier curve depicting distant recurrence-free survival grouped by site of primary tumor. Univariate P values are shown. C. Kaplan-Meier curve depicting distant recurrence-free survival grouped by type of fusion transcript. Univariate P values are shown.
Table 2.
Association of SYT-SSX fusion type with Distant Recurrence-Free and Disease-Specific Survival (n=132) using accelerated failure time analysis
| Characteristic | Distant Recurrence-Free Survival | Disease-Specific Survival | ||
|---|---|---|---|---|
| Univariate P value | Multivariate P value [AR*, 95% CI] | Univariate P value | Multivariate P value [AR*, 95% CI] | |
| SYT-SSX fusion type | 0.066 | 0.722 | ||
| SYT-SSX2 | ||||
| SYT-SSX1 | 0.019 [−1.15, −2.12 – −0.19] | 0.794 [−0.22, −1.87 – 1.43] | ||
| Primary Tumor Size | ||||
| ≥ 5 cm | ||||
| 5 – 10 cm | 0.039 [−2.19, −4.27 – −0.12] | 0.058 [−3.58, −7.29 – 0.13] | ||
| > 10 cm | 0.004 [−3.11, −5.21 – −1.01] | 0.046 [−3.93, −7.78 – −0.07 | ||
| Primary Tumor Site | ||||
| Upper | ||||
| Extremity | ||||
| Lower | ||||
| Extremity | 0.041 [−1.63, −3.20 – −0.07] | 0.014 [−3.90, −7.02 – −0.78] | ||
| Other Sites¶ | 0.048 [−1.68, −3.35 – −0.01] | 0.020 [−3.93, −7.24 – −0.63] | ||
Indicates acceleration rate
Includes truncal, thoracic, retroperitoneal/intraabdominal, and head and neck locations.
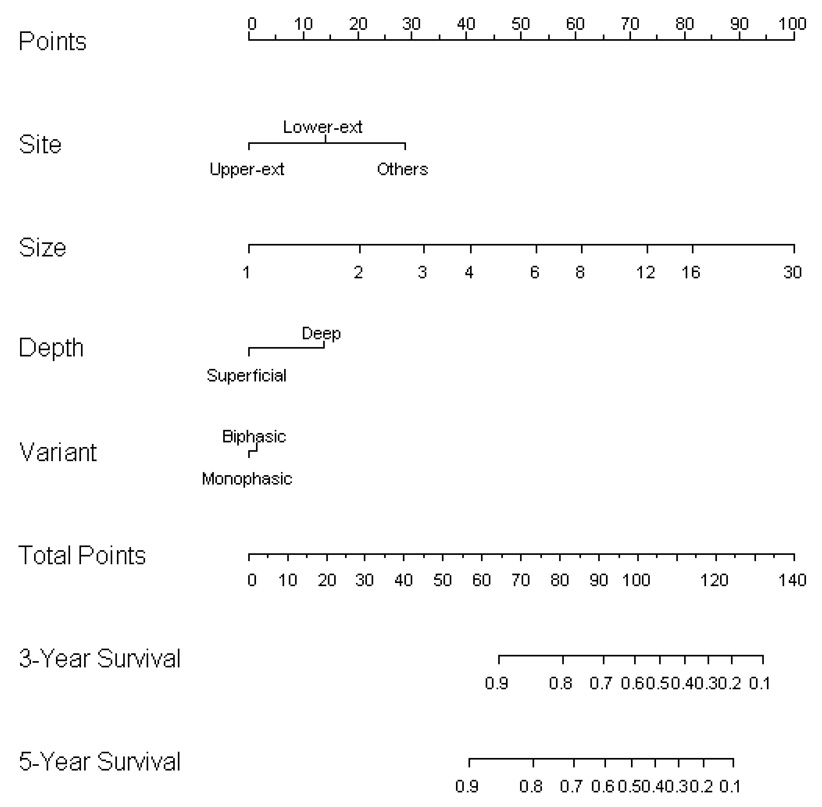
Preoperative Nomogram
A nomogram to calculate synovial sarcoma-specific 3- and 5-year DSS for surgical patients not receiving AI chemotherapy (149 patients received no chemotherapy, 47 patients received non-ifosfamide chemotherapy) based on preoperative variables is depicted in Figure 3. We choose to focus on variables readily available to the clinician to enable improved prognostication for an individual patient prior to any definitive therapy. We purposely avoided variables such as mitotic activity and necrosis because, on a core biopsy, these parameters are often difficult to quantify, not representative of the entire tumor, and not reproducible among different pathologists.22 The fusion transcript type was not included in the nomogram since it had no significant association with DSS using an accelerated failure time analysis.
Figure 3.
Nomogram based on preoperative variables used to calculate synovial sarcoma-specific 3 and 5-year survival.
Although the data were mature enough to reliably predict DSS up to 10 years with reasonably narrow confidence intervals, we chose to focus on the earlier time points since decisions regarding neoadjuvant/adjuvant therapy seem better informed by the risk of early/intermediate recurrence and death. The nomogram predicts the probability that a patient will be alive 3 and 5 years following the diagnosis and surgical resection of synovial sarcoma, excluding death from another cause. The bootstrapping concordance index was 77.3 %.
Predicted versus Observed Survival based on Type of Chemotherapy
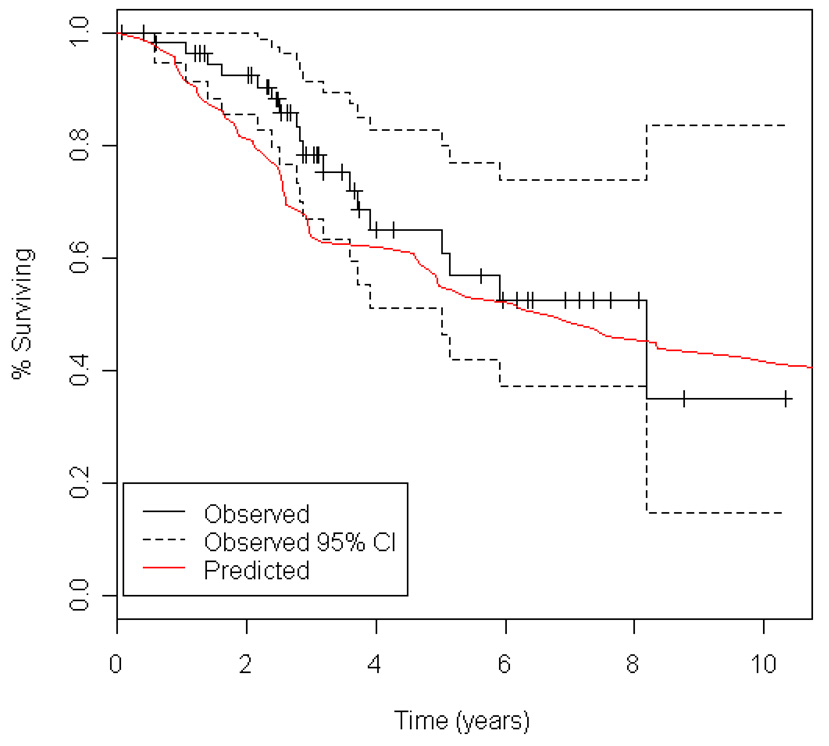
Using our preoperative nomogram, we plotted the predicted survival curve of DSS for resected patients receiving ifosfamide-containing chemotherapy (Figure 4), and compared this to the observed survival (with 95% confidence intervals) for these patients. As depicted in Figure 4, during the first 3 years following diagnosis, the observed DSS for patients treated with AI chemotherapy (N = 59) was greater than that predicted by the preoperative nomogram. After 3 years, the nomogram-predicted survival and the observed survival for AI-treated patients then converge. Median follow-up for AI-treated patients was 37 months (range 1 – 124) for survivors.
Figure 4.
Kaplan-Meier curve depicting observed disease-specific survival (with 95% confidence intervals) for patients treated with ifosfamide-containing chemotherapy compared to disease-specific survival predicted by the preoperative nomogram for otherwise similar patients treated without ifosfamide-based chemotherapy.
Discussion
Since synovial sarcoma constitutes a minority of cases of an already rare disease (STS), it has been difficult to definitively characterize the unique behavior, prognostic factors, and outcome with multimodality therapy among patients with this histology. Of the retrospective studies that have examined the natural history of this disease, size has clearly emerged as the dominant predictor of outcome.5, 7–12 However, controversy exists regarding the relative influence of other prognostic variables, including age7, neurovascular invasion10, mitotic rate11, sex13, grade23, and type of fusion transcript14, 23.
Our study identified size and site as significant predictors of DSS and DRFS on multivariate analysis (although there was only a trend toward statistical significance for lower extremity tumors relative to upper extremity for both DSS and DRFS). Although Trassard13 found truncal location to be adversely associated with DSS but not metastasis-free survival, the current study is the first study to demonstrate that primary tumor location is associated with DSS and DRFS differences even after adjusting for size.
In the current series, SYT-SSX fusion transcript data were available for 52% of patients, and largely reflects the patients who were diagnosed later in the study period and/or those who had their definitive resection performed at our institution, providing sufficient material for molecular analysis. In the subset of patients with available fusion transcript data, SYT-SSX1 was independently associated with an increased risk of early distant recurrence after adjusting for tumor size and location (p=0.02) but was not associated with an increased risk of DSS (p=0.8). In the present study we confirmed a significant association between fusion type and patient sex with SYT-SSX1 and SYT-SSX2 cases having a male to female ratio of 1.7:1 and 0.6:1, respectively. Most large studies have observed that synovial sarcoma among women is twice as likely to contain the SYT-SSX2 fusion type compared to those arising in men.12, 23, 24
Histologic subtype was also associated with fusion type. Biphasic tumors predominantly demonstrated the SYT-SSX1 fusion type. In a prior study from our institution of 45 synovial sarcoma patients (39 localized, 6 metastatic), patients with the SYT-SSX1 fusion type had a significantly shorter DRFS than patients with SYT-SSX2.14 These findings were subsequently confirmed in a large multi-institutional analysis of 243 patients.12 The present study suggests that fusion type is more important in early (<3 year) distant recurrence and that late recurrence is largely dependent on tumor size and location. Other investigators have made different observations. A study of 141 patients with localized synovial sarcoma employing the 3-tier Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system found histologic grade, and not fusion type, to be a significant predictor of DSS and DRFS on multivariate analysis.23 Using a grading system similar to the FNCLCC, Takenaka et al. observed tumor size but not fusion transcript type to be prognostic for both OS and DRFS in 91 patients with localized disease.24
Although retrospective analyses are important to identify factors independently associated with prognosis, they are limited in their ability to provide survival estimates for individual patients. Nomograms are increasingly accepted as models in which identified prognostic factors can be incorporated into a scoring system and used to predict likelihood of DSS. These statistically based tools not only use the factors included in a clinical staging system, but may also incorporate additional factors suspected to have an impact on outcome. A postoperative nomogram calculating 12-year DSS for all patients with resected STS has previously been published with the goal of providing more individualized patient counseling for follow-up scheduling and consideration for additional therapy or clinical trials.20
We provide a synovial sarcoma specific nomogram based on preoperatively known clinical and pathologic variables. Although the only independent determinants of outcome were size and location, we also included depth and histologic variant in the nomogram because of their ability to incrementally enhance the prognostic value of the nomogram. This nomogram demonstrates the magnitude of the risk of sarcoma death as well as the variability in survival estimates that exist among individual patients with synovial sarcoma. For example, with a deep, monophasic tumor of the lower extremity, a 5 cm tumor has a favorable predicted 3-year survival of 85% and 5-year survival of 82%. However, this predicted survival drops significantly to 73% at 3 years and to 64% at 5 years if the tumor increases to 8 cm in size. For a 10 cm tumor, the predicted survival is 65% at 3 years and 55% at 5 years. The generic postoperative sarcoma nomogram provides the same 12-year estimate for DSS of 71% for all these hypothetical patients since tumor size is grouped by increments of 5 cm. Similarly, for a 15 cm deep, biphasic synovial sarcoma of the thorax, the synovial specific nomogram estimates a 3 and 5-year survival of 25% and 15%, while the generic sarcoma nomogram estimates a 12-year DSS of 28%.
We chose to focus on 3- and 5-year time points for this synovial specific nomogram since decisions regarding adjuvant/neoadjuvant chemotherapy are typically based on shorter term estimations of recurrence and death. This makes direct comparisons between the synovial specific and generic sarcoma nomograms difficult. Given the confidence intervals for the respective nomograms, the differences between nomograms may represent less pronounced true differences. Nevertheless, it is noteworthy that the synovial specific nomogram predicts a more favorable survival than the generic sarcoma nomogram for the low risk patient and a worse survival for the higher risk patients. Ultimately, the synovial sarcoma specific nomogram may prove to be a more sensitive tool for predicting outcome, consistent with the idea that sarcomas exhibit unique patterns of recurrence and mortality depending on histologic type.
The synovial sarcoma specific nomogram may also allow more informed decision-making with regard to risk, particularly for the typical size cutpoints clinicians use for consideration of neoadjuvant chemotherapy. For example, most clinicians would consider a young patient with a 5 cm synovial sarcoma a potential candidate for systemic chemotherapy (www.nccn.org/professionals/sarcoma). From the synovial sarcoma nomogram (Figure 3), it is evident that the 3-year DSS can vary significantly for a 5 cm tumor. A hypothetical patient with a deep, 5 cm, biphasic synovial sarcoma of the trunk has a total point score of 93. According to the synovial sarcoma nomogram, this corresponds to a 3-year sarcoma-specific survival of 68%. In contrast, another hypothetical patient with a superficial, 5 cm, monophasic synovial sarcoma of the upper extremity has a total point score of 46. This corresponds to an estimated 3-year sarcoma-specific survival that exceeds 90%.
Based on these risk assessments, although both hypothetical patients have 5 cm tumors, it would be reasonable to consider neoadjuvant or adjuvant ifosfamide-based chemotherapy for the first patient, while it would be difficult to justify it for the second since it is difficult to improve significantly on the excellent predicted outcome for this patient by the addition of chemotherapy. Thus, this synovial sarcoma specific nomogram enables the patient and physician to make more informed decisions regarding neoadjuvant/adjuvant chemotherapy based on assessment of predicted risk for an individual patient rather than employing traditional size cutpoints alone.
Our non-randomized data also lend support to the premise that AI-based chemotherapy is associated with improved survival in synovial sarcoma patients resected with curative intent.5 When comparing the DSS predicted by the nomogram constructed from non-AI treated patients to the observed survival for AI-treated patients, controlling for other factors, the DSS for patients treated with AI chemotherapy was greater than that predicted by the preoperative nomogram for the first 3 years following diagnosis (Figure 4). After 3 years, the nomogram-predicted survival was no longer statistically different from untreated patients, and after 4 years the curves converge and are essentially superimposable. It is conceivable that with longer follow-up of the AI-treated patients (median 40 months, range 8 to 140) statistically significant differences in survival may be extended. However, it may be that there is a time-varying effect to AI-chemotherapy with a loss of DSS benefit over time, as has been observed in other randomized and non-randomized studies of heterogeneous cohorts of STS patients.25, 26 Ultimately, the hypothesis that chemotherapy provides a survival advantage for patients with synovial sarcoma requires confirmation in randomized trials.
Acknowledgement
This work was supported by Soft Tissue Sarcoma Program Project grant P01 CA 047179 (SS, ML, LXQ, RGM, MFB).
Footnotes
Statement of translational relevance: A nomogram was developed based on preoperative variables to more precisely define individual patient risk for disease-specific death for surgical patients presenting with synovial sarcoma. This synovial sarcoma specific nomogram enables the patient and physician to make more informed decisions regarding neoadjuvant/adjuvant chemotherapy based on assessment of predicted risk for an individual patient rather than employing traditional size cutpoints alone.
REFERENCES
- 1.Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the Soft Tissues and Bone. In: Vincent DeVita, Samuel Hellman, Steven Rosenberg., editors. Cancer: Principles and Practice of Oncology. 7th Edition. Lippincott, Williams, and Wilkins; 2005. pp. 1581–1637. [Google Scholar]
- 2.Singer S, Demetri GD, Baldini EH, Fletcher CD. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000;1:75–85. doi: 10.1016/s1470-2045(00)00016-4. [DOI] [PubMed] [Google Scholar]
- 3.Rosen G, Forscher C, Lowenbraun S, et al. Synovial sarcoma. Uniform response of metastases to high dose ifosfamide. Cancer. 1994;73(10):2506–2511. doi: 10.1002/1097-0142(19940515)73:10<2506::aid-cncr2820731009>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Kampe CE, Rosen G, Eilber F, et al. Synovial sarcoma. A study of intensive chemotherapy in 14 patients with localized disease. Cancer. 1993;72(7):2161–2169. doi: 10.1002/1097-0142(19931001)72:7<2161::aid-cncr2820720716>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246(1):105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okcu MF, Munsell M, Treuner J, et al. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol. 2003;21(8):1602–1611. doi: 10.1200/JCO.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Spillane AJ, A'Hern R, Judson IR, et al. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol. 2000;18(22):3794–3803. doi: 10.1200/JCO.2000.18.22.3794. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res. 2004;419:155–161. [PubMed] [Google Scholar]
- 9.Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627–634. doi: 10.1002/cncr.20386. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JJ, Antonescu CR, Leung DH, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18(10):2087–2094. doi: 10.1200/JCO.2000.18.10.2087. [DOI] [PubMed] [Google Scholar]
- 11.Singer S, Baldini EH, Demetri GD, et al. Synovial sarcoma: prognostic significance of tumor size, margin of resection, and mitotic activity for survival. J Clin Oncol. 1996;14(4):1201–1208. doi: 10.1200/JCO.1996.14.4.1201. [DOI] [PubMed] [Google Scholar]
- 12.Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62(1):135–140. [PubMed] [Google Scholar]
- 13.Trassard M, Le Doussal V, Hacene K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19(2):525–534. doi: 10.1200/JCO.2001.19.2.525. [DOI] [PubMed] [Google Scholar]
- 14.Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338(3):153–160. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 16.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14(3):859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CR, Kawai A, Leung DH, et al. Strong association of SYT-SSX fusion type and morphologic epithelial differentiation in synovial sarcoma. Diagn Mol Pathol. 2000;9(1):1–8. doi: 10.1097/00019606-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Jin Z, Lin DY, Wei LJ, et al. Rank-based inference for the accelerated failure time model. Biometrika. 90:341–353. [Google Scholar]
- 19.Kalbfleisch JG, Prentice R. The Statistical Analysis of Failure Time Data. 2nd Edition. New York: Wiley; 2002. [Google Scholar]
- 20.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 21.Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101(10):2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 22.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12(3):326–331. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 23.Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol. 2004;22(20):4040–4050. doi: 10.1200/JCO.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 24.Takenaka S, Ueda T, Naka N, et al. Prognostic implication of SYT-SSX fusion type in synovial sarcoma: A multi-institutional retrospective analysis in Japan. Oncol Rep. 2008;19(2):467–476. [PubMed] [Google Scholar]
- 25.Cormier JN, Huang X, Xing Y, et al. Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol. 2004;22(22):4567–4574. doi: 10.1200/JCO.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 26.Frustaci S, De Paoli A, Bidoli E, et al. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65 Suppl 2:80–84. doi: 10.1159/000073366. [DOI] [PubMed] [Google Scholar]