Summary
A critical unresolved issue about the DNA damage/genotoxic stress response is how the resulting activation of the p53 tumor suppressor can lead either to cell cycle arrest and DNA repair, or to apoptosis. We show here that Hematopoietic Zinc Finger (Hzf), a zinc finger containing p53 target gene, modulates p53 transactivation functions in an autoregulatory feedback loop. Hzf is induced by p53 and binds to its DNA binding domain, resulting in preferential transactivation of pro-arrest p53 target genes over its pro-apoptotic target genes. Thus p53 activation results in cell cycle arrest in Hzf wt-MEFs while in Hzf−/− MEFs apoptosis is induced. Additionally, prolonged exposure to stress results in Hzf degradation concomitant with induction of apoptosis. Exposure of Hzf-null mice to IR resulted in enhanced apoptosis in several organs including skin and prostate, as compared to that of wt-mice. These findings provide novel insights into the regulation of p53 transactivation function that plays an important role in cell fate decisions in response to genotoxic stress.
Introduction
p53 is an important component of pathways mediating cellular response to genotoxic stress by inducing the transcription of a variety of genes that regulate diverse cellular processes including cell cycle progression, apoptosis and genomic stability (Harris and Levine, 2005; Vogelstein et al., 2000; Vousden and Lu, 2002). However little is known about the mechanism(s) that determines which sets of target genes i.e. cell cycle arrest genes like p21 (El-Deiry et al., 1993), 14-3-3σ (Hermeking et al., 1997) or pro-apoptotic genes such as Bax (Miyashita and Reed, 1995), Noxa (Oda et al., 2000; Villunger et al., 2003), Pidd (Lin et al., 2000), Puma (Nakano and Vousden, 2001; Villunger et al., 2003), Perp (Attardi et al., 2000) etc. are transactivated by p53 under a specific condition. p53 is a transcription factor that plays a central role in cellular responses to genotoxic stress like DNA damage, hypoxia, oncogene activation etc (Harris and Levine, 2005; Laptenko and Prives, 2006). In order to perform its cellular functions p53 must rapidly accumulate in response to these stressful conditions, as its basal level is very low. Activation of p53 has two major outcomes: cell cycle arrest or apoptosis. Cell cycle arrest allows DNA repair to take place before replication occurs thereby maintaining genomic integrity. On the other hand apoptosis results in elimination of irreparably damaged cells.
The regulation of p53 is usually achieved by post-translational modifications and through its interactions with various other proteins (Lavin and Gueven, 2006). p53 undergoes phosphorylations on numerous serine residues both in N-and C-terminal regions (Lavin and Gueven, 2006). The N-terminal phosphorylations inhibit its interactions with its negative regulator MDM2 (Canman et al., 1998; Chehab et al., 2000; Khosravi et al., 1999) while the C-terminal phosphorylations are thought to enhance the sequence specific DNA binding ability of p53 by inducing a conformational change (Hupp et al., 1992; Wang and Prives, 1995). Similarly, other modifications like ubiquitination, acetylation, and sumolation also affect its proteolytic turnover and sequence specific DNA binding ability (Brooks and Gu, 2006; Rodriguez et al., 1999). This can also be achieved by its interaction with cellular proteins such as Pin-1, ASPP family etc (Braithwaite et al., 2006). When Pin-1 binds to p53, it undergoes conformational change which enhances its transactivation ability (Zacchi et al., 2002; Zheng et al., 2002). Recently, a new family of proteins, known as ASPPs, were found to be potent activators of p53, providing an important insight into how p53 responds to apoptotic signals (Trigiante and Lu, 2006). The ASPP family consists of three members –ASPP1, ASPP2, and iASPP. ASPP1 and ASPP2 interact with p53 and specifically enhance p53-induced apoptosis but not cell cycle arrest while iASPP binds and inhibits p53-mediated apoptosis (Bergamaschi et al., 2006; Samuels-Lev et al., 2001).
While studying the genome-wide transcriptional response to p53 induction we found that one of the genes upregulated was the hematopoietic zinc finger gene (Hzf). Hzf was originally identified as a gene induced in hematopoietic progenitor cells derived from differentiating embryonic stem cells (Hidaka et al., 2000). It encodes a zinc finger protein of 366 amino acids. It has three C2H2-type zinc finger domains. The zinc finger domains in Hzf are widely spaced with long linker regions connecting the fingers as a consequence of which it cannot form any stable nucleic acid-protein complex (Sharma et al., 2004). Recently, it was reported that Hzf is a direct transcriptional target of p53, which plays a role in p53-mediated cell cycle arrest in response to DNA damage in NIH 3T3 cells (Sugimoto et al., 2006). We found that Hzf is unique among the different p53 transcriptional targets in that upon induction by p53 or DNA damage, it binds to the p53 DNA binding domain and modulates its transactivation function in an autoregulatory feedback loop. We further investigated the role of Hzf in the p53-mediated DNA damage response. Here, we show that when Hzf binds to p53, it is preferentially recruited to the promoters of its pro-cell cycle arrest target genes rather than its pro-apoptotic target genes. Thus, in presence of Hzf, p53-mediated cell cycle arrest is promoted while apoptosis is repressed in response to genotoxic stress.
Results
Inhibition of Hzf induction in response to DNA damage represses p21 expression but enhances Bax levels
Through microarray analysis of cDNA expression, we identified Hzf as one of the upregulated genes whose transcript levels were elevated in response to p53 induction (Han et al., 2002). To confirm the array result, we carried out several Northern and Western blot analyses to examine Hzf expression in response to different types of stresses in several cell lines of diverse tissue origin and different p53 background. Under various stress conditions including DNA damage and oxidative stress, Hzf was induced in a p53-dependent manner (Figure 1A). Moreover, promoter analysis including the reporter gene assay and ChIP analysis supported that Hzf is a direct p53 transcriptional target (Figure S1).
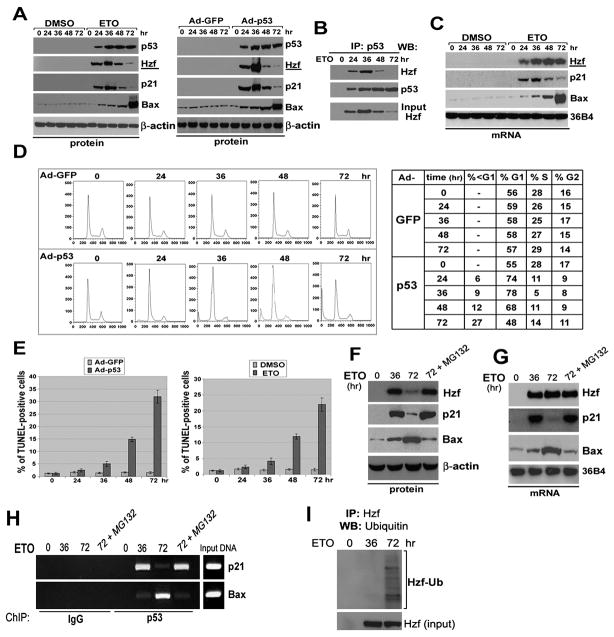
Figure 1. Hzf induction in response to various stresses modulates p53 transcriptional function.
(A) Hzf is induced by genotoxic and oxidative stress in a p53-dependent manner. HCT116, HCT116 p53−/−, U2OS and LNCaP cells were treated with etoposide (ETO, 40 μM), IR (4 Gy) or H2O2 (0.4 mM) for the indicated time points. The cells were then harvested, and northern blots or western blots were carried out.
(B) Loss of Hzf represses p21 induction but enhances Bax expression in response to DNA damage or ectopic p53. U2OS and EJ cells were treated with etoposide (ETO, 40 μM) or infected with Ad-p53 respectively for 24 hours. The cells were then harvested and Western blots carried out for the indicated proteins.
(C) Wt and Hzf−/− MEFs were treated with etoposide (ETO, 40 μM) for 24 hours. The cells were then harvested and Northern blots carried out as indicated.
(D) Effects of Hzf inhibition on p53-mediated transcription. Reporter gene constructs containing the p53-responsive elements (pGL3-p21-Luc or pGL3-Bax-Luc) or control vector alone (pGL3-Basic) were co-transfected into U2OS and Saos2 cells with either Hzf shRNA or control scrambled shRNA, and 24 hours later cells were treated with etoposide or infected with Ad-p53 for another 24 hours. At the end of the time point Luciferase assay was carried out. Error bars are means ± SD of three independent experiments with duplicate samples.
To investigate the role of Hzf in p53-mediated stress response, we examined whether Hzf induction contributes directly to the ability of p53 to transactivate p21 and Bax. To do so, we used shRNA against Hzf to knockdown the induction of endogenous Hzf in response to p53-dependent DNA damage response or ectopic p53 expression. We transiently transfected U2OS (wt p53) and EJ (nonfunctional p53) cell lines with Hzf shRNA or scrambled shRNA constructs followed by treatment with chemotherapeutic agent etoposide or infection with Ad-p53 to induce p53 expression (Figure 1B). Transfection of Hzf shRNA resulted in the suppression of endogenous Hzf induction by etoposide treatment or Ad-p53 infection, while control scrambled shRNA had no effect on induced Hzf levels. In U2OS cells transfected with the scrambled shRNA construct, upon etoposide treatment or Ad-p53 infection, concomitant with p53 induction, Hzf and p21 induction was seen while Bax levels was minimally increased. However, depletion of Hzf expression resulted in enhanced Bax induction while p21 induction was significantly reduced in response to DNA damage (Figure 1B, left panel). In EJ cells containing nonfunctional p53, similar results were obtained with Ad-p53 infection (Figure 1B, right panel). To exclude off-target effects of shRNA, two other Hzf shRNAs were used, and similar results were obtained (data not shown).
We next examined the levels of p21 and Bax transcripts in response to DNA damage (etoposide treatment) in Hzf+/+ and Hzf−/− MEFs. As shown Figure 1C, Hzf−/− MEFs showed drastically lower the induction levels of p21 mRNA than Hzf+/+ MEFs, while Bax induction was significantly enhanced in Hzf−/− MEFs. To further investigate the role of Hzf in p53-mediated transcription of these target genes, we used reporter assays with either the p21 or Bax promoter fused to a luciferase reporter (pGL3-basic, p21-Luc, and Bax-Luc). U2OS and EJ cells were co-transfected with Hzf shRNA or scrambled shRNA constructs together with pGL3 basic, p21-Luc or Bax-Luc constructs followed by treatment with a DNA damaging agent etoposide or infection with Ad-p53 (Figure 1D). We found that the ability of p53 to transactivate the p21 promoter was severely compromised upon the knockdown of Hzf while its ability to transactivate the Bax promoter was significantly enhanced in response to etoposide treatment or Ad-p53 infection in U2OS cells containing wt-p53 (Figure 1D, upper panel). In the EJ cells, this effect was also observed upon ectopic p53 expression (Figure 1D, lower panel). All of these results suggested that Hzf affected p53-mediated transcription in a positive way for p21 transcription and in a negative way for Bax transcription.
Hzf is induced by p53 and binds directly to its DNA binding domain
Since Hzf was identified as a novel zinc finger protein (Hidaka et al., 2000), is induced by p53 and modulates p53 mediated transactivation, we next tested whether Hzf physically interacts with p53. For this purpose, we used extracts made from U2OS cells treated with etoposide or EJ cells infected with Ad-p53 for immunoprecipitation using the p53-specific monoclonal antibody (PAb1801). In both cell lines we found that Hzf co-immunoprecipitated with p53 (Figure 2A, top panels). We also performed the reverse co-IP experiment where immunoprecipitation was carried out using anti-Hzf polyclonal antibody (Figure 2A, bottom panels). As shown in Figure 2A, p53 also co-immunoprecipitated with Hzf. As a control an IP was carried out in etoposide treated Hzf−/− MEF using a Hzf antibody. As shown in Figure S2, p53 was not in the precipitated complex, and no Hzf was detected, confirming the specificity of the Hzf antibody used.
Figure 2. Hzf physically interacts with p53.
(A) U2OS and EJ cells were treated with ETO (40 μM) or infected with Ad-p53 respectively for 24 hours. The cells were then harvested and subjected to immunoprecipitations using anti-p53 antibody (top panels) or anti-Hzf antibody (bottom panels) and Western blots carried out for the indicated proteins.
(B) Yeast two hybrid assay to show direct interaction between Hzf and p53 was carried out as described in methods.
(C) The region of p53 involved in binding to Hzf was analyzed in GST-pull down assays. U2OS cells were transfected with the indicated GST-p53 plasmids and then infected with Ad-GFP or Ad-Hzf. 24 hours post-infection the cells were lysed and subjected to GST pull down. Western blotting was then carried out for p53, and Flag epitope.
(D) Mutations in DNA binding domain of p53 affect the Hzf-p53 interaction. EJ cells containing unfunctional p53 were transfected with the indicated p53 plasmids and then infected with Ad-GFP or Ad-Hzf. 24 hours post-infection the cells were lysed and subjected to immunoprecipitations using anti-p53 antibody. Western blotting was carried out for p53, and Flag epitope.
To determine whether the interaction between Hzf and p53 was a direct one, a yeast two hybrid assay was performed. Expression constructs in which p53 was fused to the Gal4 DNA binding domain (pGBKT7-p53), and Hzf was fused to Gal4 transactivation domain to generate pGADT7-Hzf, and then transformed into yeast strains AH109 and Y187, respectively. Blue colored Ade+/His+ colonies were obtained when yeast transformants containing the pGBKT7-p53 and pGADT7-Hzf were mated, as observed in a positive control mating experiment between p53 (pGBKT7-p53) and SV40 T antigen (pGADT7-T Ag) (Figure 2B) (Iwabuchi et al., 1993; Li and Fields, 1993).
To map the domain of p53 to which Hzf bound, we carried out GST-pull-down experiments where constructs expressing different domains of p53 fused to GST were transfected into EJ cells followed by infection with control Ad-GFP or Ad-Hzf. As shown in Figure 2C, Hzf bound to GST-p53 (full length) but not to GST alone supporting our immunoprecipitation and yeast two hybrid assay results. The GST-p53 segment containing aa 100–300 specifically bound to the Hzf protein, which represents the DNA binding domain of p53 (Figure 2C). To study the Hzf-p53 interaction more closely, we determined whether Hzf could bind to tumor-derived p53 DNA binding domain mutants including p53/175Pro, p53/175His and p53/143Ala. It was reported that p53/175His and p53/143Ala had no DNA binding ability while p53/175Pro was capable of some DNA binding activity but could trigger only arrest but not apoptosis (Kern et al., 1991; Rowan et al., 1996; Zhang et al., 1994). As shown in Figure 2D, Hzf bound to wt-p53 robustly but reduced binding was observed with p53/175Pro and p53/175His. The binding of Hzf with p53/143Ala was severely repressed. Nevertheless, none of these p53 mutations was able to abolish the Hzf-p53 interaction completely. This suggests that p53-Hzf interaction involves several key residues in DNA binding domain of p53 and mutations in any one of them weaken the binding but cannot fully abolish it. Together, these results indicate that Hzf is induced by p53 and directly binds to the DNA binding domain of p53.
Hzf influences the specificity of p53-mediated transactivation
Since our results demonstrated that Hzf binds to p53 and differentially modulates expression levels of p53 targets p21 and Bax, we next determined whether this regulation of p53 transactivation function by Hzf is a wide-ranging phenomenon encompassing other p53 pro-arrest and pro-apoptotic targets. Thus we infected Hzf+/+ and Hzf−/− MEFs with adenovirus expressing p53 (Ad-p53) or treated the cells with etoposide, and analyzed the expression of p53 target genes including both pro-arrest and pro-apoptotic targets. In the presence of Hzf, ectopic p53 or etoposide treatment preferentially induced expression of pro-arrest p53 targets p21 and 14-3-3σ, whereas in the absence of Hzf, the expression of pro-apoptotic targets Bax, Perp, Puma and Noxa was selectively induced (Figure 3A and 3B). Mdm2 is a p53 target involved in p53 protein regulation but does not specifically influence neither arrest nor apoptosis, Mdm2 levels were not affected by Hzf inactivation (Figure 3A and 3B). In addition, exogenous expression of Hzf in Hzf−/− MEFs restored preferential induction of cell cycle arrest targets p21 and 14-3-3σ and lowered the levels of Perp, Puma, Noxa and Bax expression, but caused no change seen in p53 induction of Mdm2 (Figure 3C).
Figure 3. p53 bound to Hzf is preferentially recruited to p21 and 14-3-3σ promoter.
(A, B) Loss of Hzf enhances expression of proapoptotic targets of p53, but it suppresses the expression of p21 and 14-3-3σ. Western blotting of p53 targets in Hzf+/+ and Hzf−/−MEFs. Hzf+/+ and Hzf−/− MEFs were infected with Ad-GFP or Ad-p53 (A) or treated with ETO (40 μM) (B). 24 hours later, Western blot analysis was carried out for the indicated proteins.
(C) Re-expression of Hzf restores preferential induction of p21 and 14-3-3σ and represses proapoptotic targets in response to p53. Hzf−/− MEFs were infected with Ad-GFP or Ad-Hzf 6 hours prior to infection with Ad-GFP or Ad-p53. 24 hours post infection, Western blots were carried out for the indicated proteins.
(D) Multiple ChIP analysis on the promoters of p53 targets. Wt and Hzf−/− MEFs were treated with ETO (40 μM). 24 hours post drug treatment ChIP was carried out using control mouse IgG or anti-p53 antibody and PCR was done for the indicate promoters.
(E) Part of the chromatin immunoprecipitated with p53 antibody was again subjected to ChIP using control rabbit IgG or Hzf antibody. Input represents 2% of total chromatin from untreated Hzf+/+ MEFs used for immunoprecipitation.
(F, G) EMSA showing the DNA binding activity of p53 to oligonucleotides containing the p53 binding site in p21 promoter, Bax first intron, and Mdm2 promoter in wt-MEF (F) and Hzf-null MEF (G). Wt and Hzf−/− MEFs were treated with ETO (40 μM). 24 hours post drug treatment, nuclear extracts were prepared, and EMSA carried out as described in supplementary methods. The arrows indicate the position of the p53-DNA complex (lower) and supershifted complex containing anti-p53 antibody (pAb421).
To further evaluate the effect of Hzf on the transactivation function of p53, we measured DNA binding activity of p53 in response to DNA damage on the promoters of p53 targets including p21, 14-3-3σ, Bax, Noxa, Perp and Mdm2 in Hzf+/+ and Hzf−/−MEFs by using chromatin immunoprecipitation assay (ChIP). Hzf+/+ and Hzf−/− MEFs were exposed to a DNA damaging agent, etoposide, for 24 hours, and ChIP assays were performed using control IgG or p53 antibody for several of p53 target genes. Upon DNA damage treatment of Hzf+/+ MEFs p53 predominantly bound to the promoter of pro-arrest targets such as p21 and 14-3-3σ but DNA binding activity of p53 on the promoters of pro-apoptotic target genes such as Bax, Perp and Noxa was significantly lower (Figure 3D). In contrast, in Hzf-null MEFs upon DNA damage DNA binding activity of p53 was significantly enhanced for the promoters of its pro-apoptotic target genes such as Bax, Perp and Noxa, as compared to that of p21 and 14-3-3σ (Figure 3D). The binding ability of p53 to the Mdm2 and Hzf promoter remained unchanged irrespective of Hzf status. Quantitative ChIP assays by real-time PCR also confirmed the above results with wt- and Hzf−/− MEFs (Figure S3). These results strongly suggest that upon Hzf binding to p53, p53 is preferentially recruited to the promoters of its cell cycle arrest mediating target genes. To further verify this, we performed “ReChIP” experiments using Hzf antibody. As shown in Figure 3E, Hzf was detected only at the promoters of p21, 14-3-3σ Hzf and Mdm2 but not at the promoters of Bax, Noxa and Perp. Moreover, quantitative ChIP assays by real-time PCR were carried out with etoposide treated p53+/+ and p53−/− MEFs using IgG control antibody and Hzf antibody (Figure S4). Our results indicate that Hzf is at the cell cycle arrest promoters only in presence of p53. To further strengthen the differential effects of Hzf binding on the DNA binding activities of p53 to its targets, EMSA was carried out in etoposide treated Hzf+/+ and Hzf−/− MEFs using oligonucleotides specific for p53 binding site in p21 promoter (El-Deiry et al., 1993), mdm2 promoter (Wu et al., 1993) and Bax first intron (Thornborrow et al., 2002). As shown in Figure 3F, the DNA binding activity of p53 on p21 promoter was significantly increased in etoposide-treated wt-MEF, whereas there was no strong DNA binding activity of p53 on Bax promoter. However, in the absence of Hzf no significant binding activity of p53 was observed on the p21 promoter but significantly higher DNA binding activities seen on the Bax first intron (Figure 3G). The binding of p53 to Mdm2 promoter remained unchanged irrespective of Hzf status. Taken together, these data demonstrate that Hzf preferentially enhances the DNA binding and transactivation functions of p53 on cell cycle arrest targets such as p21 and 14-3-3σ.
Hzf promotes the cell cycle arrest function of p53
We next examined the effects of Hzf on p53-mediated cell cycle arrest or apoptosis in Hzf+/+ and Hzf−/− MEFs. In Hzf+/+ MEFs, the ectopic expression of p53 by Ad-p53 infection induced prominent G1 arrest but little apoptosis (Figure 4). In Hzf−/− MEFs, the apoptotic function of p53 was significantly enhanced, as measured by TUNEL assay, sub-G1 population and DNA fragmentation (Figures 4A and 4B). To further verify if indeed Hzf deficiency modulates p53 functions under physiological conditions, Hzf+/+ and Hzf−/− MEFs were exposed to etoposide, and then TUNEL positive apoptotic cells were measured. The loss of Hzf led to enhanced apoptosis sensitivity to etoposide (Figure 4C, left panel). Re-expression of Hzf in Hzf −/− MEFs through Ad-Hzf infection reversed this effect of Hzf deficiency and resulted in a decrease in apoptosis induced by etoposide treatment or overexpression of p53 similar to that seen in etoposide-treated or Ad-p53 infected Hzf+/+ MEF (Figure 4C) by modulating p53-mediated transactivation of its target genes (Figure 3C) that was due to altered DNA binding activity of p53 (Figure S5). These results indicate that Hzf modulates the p53 mediated stress response favoring cell cycle arrest over apoptosis.
Figure 4. Hzf promotes cell cycle arrest function of p53.
(A) Hzf+/+ and Hzf−/− MEFs were infected with Ad-GFP or Ad-p53. 24 hours post-infection FACS analysis was carried out.
(B) Hzf+/+ and Hzf−/− MEFs were similarly treated as in (A) and TUNEL staining and DNA fragmentation assays were carried out. Error bars are means ± SD of three independent experiments.
(C) Re-expression of Hzf decreases apoptosis in response to ETO or p53 overexpression in Hzf−/− MEFs. Wt-MEFs and Hzf−/− MEFs were treated with ETO (40 μM). In cases where the cells were infected with Ad-GFP or Ad-Hzf prior to drug treatment, there was a difference of 6 hours between viral infection and drug treatment. 24 hours post drug treatment the cells were subjected to TUNEL assay. Error bars are means ± SD of three independent experiments.
Prolonged p53 expression/activation or extended exposure to stress results in Hzf protein downregulation/degradation leading to apoptosis
To examine whether levels of Hzf expression are correlated with levels of genotoxic stress, we investigated the kinetics of Hzf induction at extended time points during stress response in U2OS cells. The results shown in Figure 5A suggest that Hzf protein expression is rapidly induced in response to etoposide treatment or ectopic p53 up to 36 hours, but beyond 36 hours Hzf protein levels start declining and are markedly decreased at the 72 hours. p21 expression also followed a similar pattern to that observed with Hzf. In contrast, Bax protein levels rose very slowly up to 36 hours but beyond that time exhibited a sharp increase (Figure 5A). We also immunoprecipitated p53 over the time courses of etoposide treatment to examine the amounts of Hzf bound to p53. We found that there was a sharp decline in the amounts of p53-bound Hzf beyond the 36-hour time point to the undetectable levels at 72 hours (Figure 5B). However, Hzf mRNA levels remained steady throughout the time course while mRNA levels of p21 and Bax were similar to their protein levels (Figure 5C), suggesting that Hzf down-regulation is due to the degradation of the Hzf protein. To determine the implication for these differential responses in Hzf protein levels in response to genotoxic stress or p53, we next tested the cell cycle profile under these conditions by flow cytometry. Ad-p53 overexpression induced a pronounced arrest in G1 phase of the cell cycle at 24–36 hours (Figure 5D), after which there was a marked increase in the apoptotic population. TUNEL staining confirmed these results (Figure 5E). These results indicate that extended exposure to stress and the induction of a p53-mediated apoptotic response correlated with a reduction of Hzf, suggesting that Hzf may play a role in controlling the switch in the p53-dependent stress response.
Figure 5. Sustained p53 activation promotes ubiquitination and degradation of Hzf.
(A) Prolonged p53 activation induces Hzf protein down-regulation. U2OS cells were exposed to ETO (40 μM) or were infected with Ad-GFP or Ad-p53 for the indicated time points. The cells were lysed and Western blots were carried out for the indicated proteins.
(B) U2OS cells exposed to ETO (40 μM) for the indicated time points were immunoprecipitated with p53 Ab, and the precipitates were immunoblotted with Hzf Ab.
(C) Hzf mRNA levels in response to ETO treatment remain steady. U2OS cells were exposed to ETO (40 μM) for the indicated time points and Northern blots were carried out as indicated.
(D) U2OS cells were infected with Ad-GFP or Ad-p53 for the indicated time points and FACS analysis was carried out.
(E) U2OS cells were similarly treated as in (A) and TUNEL assays were carried out for the indicated time points. Error bars are means ± SD of three independent experiments.
(F, G, H) Proteasome-dependent Hzf degradation. U2OS cells were treated for 36 and 72 hours with ETO (40 μM), and MG132 (10 μM) was added during the last 10 hours prior to the end of the 72 hours time point. The cells were then harvested, and Western blotting, Northern blotting or ChIP was carried out with the indicated antibodies. Input represents 2% of total chromatin from untreated U2OS cells used for immunoprecipitation.
(I) Genotoxic stress-induced ubiquitination of Hzf. U2OS cells were treated with ETO (40 μM) for 36 and 72 hours, and with MG132 for the last 10 hours. Cell extracts were immunoprecipitated with anti-Hzf Ab, and immunoblotted with anti-ubiquitin Ab.
Since ubiquitin-mediated proteasome degradation pathway is a frequently engaged mechanism for protein downregulation, we investigated the potential role of the proteasome in stress-induced Hzf protein degradation in cells undergoing p53-dependent apoptotic response. We treated U2OS cells with etoposide for 0, 36 and 72 hours. At 10 hours prior to the end of the 72-hour time point, the cells were treated with the proteasome inhibitor, MG132. Loss of Hzf triggered by genotoxic stress at 72 hours time point was significantly attenuated by the proteasome inhibitor MG132 resulting in elevated p21 levels (Figure 5F). However, Bax levels were diminished similar to those at 36 hours in MG132 treated U2OS cells (Figure 5F). Furthermore, northern blot analysis showed that the proteosome inhibitor treatment elevated the levels of p21 mRNA, but decreased Bax mRNA (Figure 5G), supporting the hypothesis that the preferential DNA binding activities of p53 to p21 promoter results from stabilization of Hzf protein. This was confirmed by chromatin IP as well as quantitative ChIP assays by real-time PCR (Figure 5H; Figure S6A). We next determined whether prolonged stress in cells triggered the polyubiquitination of Hzf prior to Hzf degradation. Treatment of U2OS cells with etoposide in the presence of MG132 revealed that endogenous Hzf undergoes extensive ubiquitination after prolonged exposure (72 hours treatment) to genotoxic stress, while no such ubiquitinated forms were observed at 36 hours treatment (Figure 5I). Moreover, proteosome inhibitor MG132-mediated stabilization of Hzf could indeed prevent the p53-mediated apoptosis upon prolonged exposure to genotoxic stress (Figures S6B and S6C). Thus, the appearance of ubiquitinated forms of Hzf in cells treated with MG132 supports the idea that the Hzf protein is targeted for degradation during prolonged exposure to genotoxic stress due to activation of an ubiquitination-proteasome pathway resulting in initiation of the apoptotic process.
Hzf−/− mice show increased sensitivity to γ-irradiation
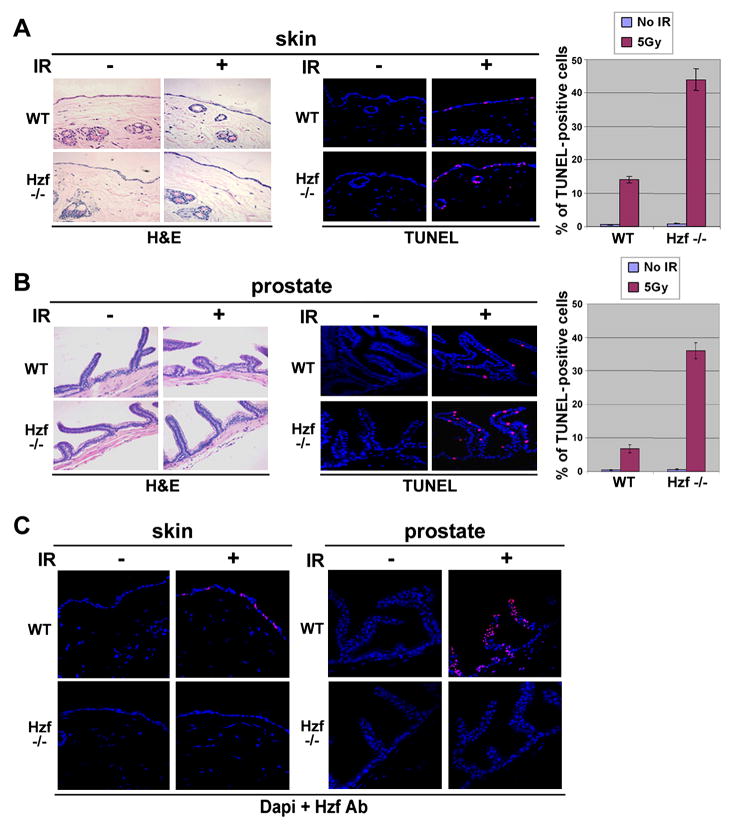
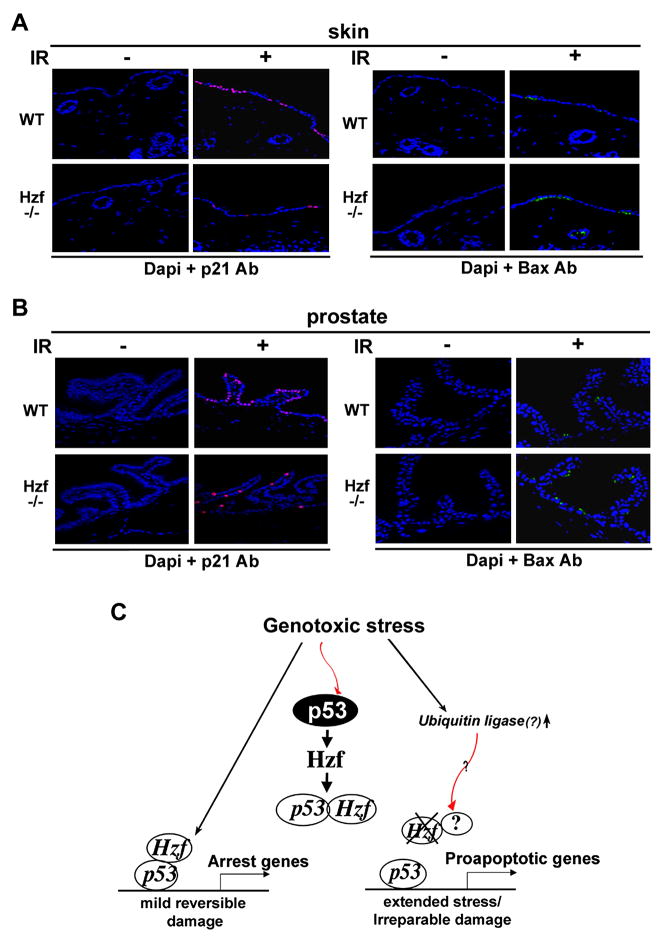
Hzf-null mice have been generated and while mice lacking Hzf seemed to be relatively normal, initial characterization suggested a role for Hzf in megakaryopoiesis and hemostasis (Kimura et al., 2002). To further study the role of Hzf in genotoxic stress response in vivo, we investigated the functional consequence of Hzf deficiency in Hzf-null mice exposed to γ-irradiation. Hzf−/− and wt-mice littermates (14 weeks old) were exposed to 5 Gy total body γ-irradiation. 6.5 hours after treatment, 3 mice from each group were evaluated by examining sensitivity to cell death using TUNEL staining and histopathological analysis of target organs including skin, spleen, small intestine, and prostate. As shown in Figure 6A and B, skin and prostate from irradiated Hzf−/− mice showed increased sensitivity to DNA damage induced by γ-irradiation as measured by TUNEL staining. However, the extent of apoptosis in wt-prostate was less than that in skin which was due to increased expression of Hzf in prostate as compared to skin (Figure 6C). TUNEL staining and immunostaining for Hzf was also carried out in IR-sensitive tissues like spleen and small intestine epithelium, where we found extensive apoptosis taking place due to meager levels of Hzf expression upon γ-irradiation (Figure S7). Consequently, in spleen no difference could be seen in the level of apoptosis between Hzf+/+ and Hzf−/− samples in response to IR but in small intestine due to marginally increased levels of Hzf expression there were slightly higher levels of apoptotis detected in Hzf−/− samples. Thus our results show that sensitivity to γ-irradiation is inversely correlated with the levels of Hzf expression in those tissues. We also performed immunostaining for p53 targets, p21 and Bax, in the same sections of skin and prostate from wt- and Hzf−−-mice. In response to γ-irradiation, the level of Bax expression was increased in Hzf−/− skin compared to that of wt-mice while p21 levels were reduced in γ-irradiated Hzf−/− skin samples (Figure 7A). Similar results were obtained in prostate tissue from irradiated Hzf−/− mice (Figure 7B). In concurrence with TUNEL staining, Bax staining in Hzf−/− prostate was also less pronounced as compared to skin while the pattern of p21 staining was reversed. These results support the role of Hzf functioning downstream of p53 in the p53-dependent DNA damage response acting as a critical switch that prevents apoptosis by repressing transactivation of pro-apoptotic p53 targets both in vitro and in vivo. Thus, the results from the mouse model suggest that under in vivo conditions, Hzf modulates the p53 mediated stress response but there is an element of tissue specificity in this regulation due to varying degree of Hzf expression in different tissues upon γ-irradiation.
Figure 6. Increased apoptosis after radiation-induced DNA damage in skin and prostate of Hzf-deficient mice versus wild-type mice.
(A) Representative TUNEL staining of skin from Hzf−/− mice and wild-type littermates, analyzed 6.5 hours after the mice received 5 Gy total-body irradiation. Representative H&E sections are shown (left panel). Percentages of TUNEL positive cells were calculated from three independent experiments (right panel).
(B) Representative TUNEL staining of prostate tissue from the same IR treated Hzf−/−mice and wild-type littermates as in (A). Representative H&E sections are shown (left panel). Percentage of TUNEL positive cells were calculated from three independent experiments (right panel).
(C) Immunostaining for Hzf in skin and prostate from the same IR treated Hzf−/− mice and wild-type littermates as in Figure 6A.
Figure 7. Loss of Hzf enhances the expression of Bax, but the levels of p21 expression are reduced in skin and prostate.
(A) Immunostaining for p21 and Bax proteins in skin from the same IR treated Hzf−/− mice and wild-type littermates as in Figure 6A.
(B) Immunostaining for p21 and Bax proteins in prostate tissue from the same IR treated Hzf−/− mice and wild-type littermates as in Figure 6B.
(C) Model for p53-dependent cell fate control through the p53→Hzf→p53/Hzf autoregulatory feedback loop. p53 activation by DNA damage induces Hzf, and it binds to p53. This directs p53 preferentially to cell cycle arrest gene promoters resulting in growth arrest. Upon extended or irreparable stress, Hzf ubiquitination/degradation prevents this from occurring, thus allowing p53 to activate pro-apoptotic targets, and resulting in cell to trigger apoptosis.
Discussion
It is widely accepted that the p53 tumor suppressor restricts the proliferation of cells exposed to genotoxic stress by induction of growth arrest or by triggering cell death (Vousden and Lu, 2002). This process depends mainly on the expression of genes that regulate cell-cycle arrest or apoptosis. A critical unresolved issue about the DNA damage response is how the resulting up-regulation of the p53 tumor suppressor can lead either to cell cycle arrest and DNA repair, or to apoptosis. Here we present evidence that Hzf plays a critical role in p53-mediated transcription and functions as a key player in controlling a cellular regulatory switch that dictates the cellular decision toward cell cycle arrest in response to stress. An autoregulatory feedback loop involving p53 and its target proteins such as Mdm2, Pirh2, and Cop1 is well established (Dornan et al., 2004; Leng et al., 2003; Wu et al., 1993). These genes are known to have ubiquitin ligase activity, and can target p53 for ubiquitination and degradation to maintain low basal levels of the p53 protein. Unlike these p53 target proteins, Hzf encodes a zinc-finger domain protein, and its binding to p53 does not seem to regulate p53 stability in response to stress. Instead, induction of Hzf and its binding to p53 selectively influences the specificity of the p53-mediated transcription, resulting in preferential transactivation of its cell cycle arrest mediating target genes such as p21 and 14-3-3σ.
It has been previously shown that Hzf is a direct p53 transcriptional target in NIH3T3 cells (Sugimoto et al., 2006). Promoter analyses revealed that p53 could bind directly to the p53 responsive element in the first intron of the Hzf gene. Moreover, Hzf was found to have a role in cell cycle checkpoint control, specifically at the G2/M checkpoint. Strikingly, knockdown of Hzf expression promoted p21 ubiquitination and degradation, the mechanism of which was not elucidated. We independently identified Hzf as a p53 target gene in human cells, and our promoter analysis strongly suggest that unlike mouse Hzf, the p53-responsive element in human Hzf is at the −1044 bp of the promoter region (Figure S1). Our results show further that Hzf binds to p53, which results in preferential transactivation for pro-arrest p53 target genes. Thus, upon genotoxic stress, abrogation of Hzf expression results in preferential transactivation of pro-apoptotic p53 target genes such as Bax, Perp, Puma and Noxa over its pro-arrest targets such as p21 and 14-3-3σ. Hence, our results argue that down regulation of p21 and other pro-arrest p53 target genes such as 14-3-3σ in the absence of Hzf is transcriptional in nature.
Our results also indicate that the level of Hzf protein is inversely correlated with the extent of genotoxic stress. Prolonged p53 activation/expression or extended exposure to stress induced Hzf protein degradation through activation of an ubiquitination-proteasome pathway whose consequences were induction of pro-apoptotic p53 targets such as Bax, Puma, Noxa and Perp and apoptosis. Thus, we propose a model (Figure 7C) wherein p53 activates Hzf, which then binds to p53 in an autoregulatory feedback loop. Hzf-bound p53 is specifically recruited to cell cycle arrest gene promoters resulting in growth arrest. Prolonged p53 expression is associated with Hzf degradation, which then allows p53 activation of pro-apoptotic targets, resulting in apoptosis. However, if the cells lack the mechanism for Hzf degradation, prolonged p53 expression leads to senescence and shRNA mediated inhibition of Hzf expression makes the cells highly apoptotic (Figure S8). Therefore, our results provide a novel mechanism for modulation of p53-dependent DNA damage response. It will be of interest to determine the expression pattern of p53-regulated genes by the microarray analysis in the absence or presence of Hzf as well as the specific interacting partner(s) that mediates Hzf ubiquitination/degradation. Recently, it was shown that Tip60-dependent acetylation of p53 at lysine120 modulates the decision between cell-cycle arrest and apoptosis, leading to enhanced p53-dependent apoptotic response (Sykes et al., 2006; Tang et al., 2006). Thus it will be interesting to know whether the acetylation of K120 within the DNA-binding domain of p53 affects Hzf-mediated preferential transactivation of pro-arrest p53 target genes. While the role of Mdm2 in p53 regulation is well-established, we found that there is no role of Mdm2 in Hzf degradation (Figure S9).
Another question remains as to whether cells that have deficiency of p53 pro-arrest or pro-apoptotic target(s) can affect Hzf-mediated cell fate decision upon genotoxic stress. In HCT116 and HCT116 p21−/− cells genotoxic stress results in cell cycle arrest in both cells lines with p21−/− cells showing a higher extent of G2/M arrest. If under such conditions Hzf is also knocked down, the cells show much increased apoptosis levels with marginal differences between them (Figure S10). Thus, Hzf is likely to play a key role in sustaining p53 mediated cell cycle arrest through other pro-arrest p53 target genes such as 14-3-3σ in addition to p21. It is well established that HCT116 Bax−/− cells are resistant to apoptosis upon DNA damage (Zhang et al., 2000). When Hzf was depleted in parental HCT116 and HCT116 Bax−/− cells and then exposed to DNA damage, there was a significant induction of apoptosis in both the cell types, but the extent of apoptosis was higher in the wild type cells (data not shown). Thus, Hzf can act through modulation of p53-mediated transactivation of multiple pro-arrest and pro-apoptotic genes.
Targeted Hzf disruption in the mouse germ line has no reported effects on cell cycle dynamics, but general growth retardation and internal hemorrhage in brain of Hzf −/− mice were observed (Kimura et al., 2002). While mice lacking Hzf seemed to be otherwise normal, the initial characterization of Hzf-null mice suggested a role for Hzf in megakaryopoiesis and hemostasis. Based on our in vitro characterization of the role of Hzf in p53-dependent stress response, we carried out an analysis of Hzf function in response to irradiation under in vivo conditions using Hzf knock out mice. We observed that exposure to ionizing radiation results in increased cell death in Hzf−/− mice as compared to their wild type littermates, especially in the epidermis and prostate epithelium. However, comparison of the extent of apoptosis between the different tissues of γ-irradiated Hzf−/− mice revealed significant differences indicative of some tissue specificity with respect to Hzf function due to varying degree of Hzf expression in different tissues upon γ-irradiation. While our preliminary investigations reveal some evidence for epithelial hyperplasia in organs like lung, mammary gland, etc. of Hzf−/−mice, further studies will be needed to elucidate the role of Hzf in tumor susceptibility of mice and its implications for human cancer. Nevertheless, our present findings establish that Hzf functions downstream of p53 in DNA damage response and acts as a critical switch that favors cell cycle arrest over apoptosis by promoting preferential transactivation of pro-arrest targets of p53 both in vitro and in vivo. Thus, Hzf is both a p53 target and an important modulator of p53 functions, adding a new layer of complexity to the mechanisms by which p53 determines cell fate decisions in response to cellular stresses.
Experimental Procedures
Cell lines and culture conditions
U2OS, LNCaP, Saos2, HCT116, HCT116 p53−/− (Bunz et al., 1998) HCT116 p21−/−(Waldman et al., 1996) and HCT116 bax−/− (Zhang et al., 2000) were cultured in DMEM containing fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 100 U/ml penicillin and 100μg/ml streptomycin at 37°C. Hzf+/+ and Hzf−/− early passage MEFs (Kimura et al., 2002) were also maintained in DMEM containing 10% FBS. For drug treatment, cells were grown to ~50% confluency prior to exposure to the DNA-damaging agents for the indicated time. Adenoviruses expressing Hzf or GFP were generated, amplified and titrated as previously reported (Ongusaha et al., 2003). Cells were grown to ~50–70% confluency and infected with recombinant adenovirus at multiplicity of infection (MOI) of 10–20 for the indicated time. GFP expressing adenovirus (Ad-GFP) was used as control.
GST-pull down assay and yeast two hybrid assay
Cells were lysed in lysis buffer (20 mM Tris-HCl at pH 7.4, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP-40, 1 mM phenyl methylsulphonyl fluoride (PMSF), 1X Protease inhibitor cocktail (Roche). 500μg cell extracts pre-treated with MNase as described (Groisman et al., 2003) was subjected to GST-pull down following the manufacturer’s protocol (Amersham). Yeast two hybrid assay was performed following the manufacturer’s protocol (Clontech). Briefly, Hzf cloned in the pGADT7 vector was transformed into yeast strain Y187, mated with yeast strain AH109 carrying the pGBKT7-p53 plasmid and then plated on SD/-Trp/-Leu and SD/-Trp/-Leu/-Ade/-His plates. Representative colonies for the positive control, negative controls and the test interaction were then streaked on to a SD/-Trp/-Leu/-Ade/-His/X-α-Gal plate.
Apoptosis and flow cytometry
Apoptosis was detected using the Cell Death Detection ELISA (Roche) and TUNEL assay (Roche). For the Cell Death Detection ELISA cells were lysed and the amount of nucleosomes in the cytoplasmic fraction of the cell lysates was measured using anti-histone antibody and anti-DNA antibody linked to peroxidase in a sandwich ELISA based protocol. For calculating relative nucleosome content the absorbance of all the samples was normalized with respect to that of untreated/untransfected cells. Error bars are means ± SD of three independent experiments with triplicate samples. For TUNEL assay the cells were fixed with paraformaldehyde and stained by TUNEL reaction using TMR red conjugated nucleotides and counterstained with DAPI. For each sample 5 random fields were counted and error bars are means ± SD of three such independent experiments. Cell cycle analysis using flow cytometry was carried out as described previously (Han et al., 2002).
shRNA
Vectors expressing shRNA against Hzf (5′-ATCCGCTTCAATTCTCAGA-3′), and the scrambled sequence (5′-GAGCCCTATTTCACAACTT-3′) were generated using the pBabe-U6-shRNA plasmid. Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Total body irradiation and immunohisochemistry
14 weeks old Hzf−/− mice and age- and sex-matched wild-type littermates were subject to 5 Gy of total body irradiation with a 137Cs gamma source at a rate of 0.6 Gy/min. All animal protocols were approved by an Institutional Animal Care and Use Committee. 6.5 hours later the animals were euthanised. Tissues were harvested, fixed in formalin, washed with PBS and finally embedded in paraffin for sectioning. Immunohistochemical analyses of the tissues were carried out by fluorescence microscopy. Briefly, 5μm paraffin sections were dewaxed and immunostained with Bax (Santa-Cruz) or p21 (Calbiochem) antibody followed by counterstaining with TO-PRO-3 (Invitrogen). TUNEL staining of dewaxed sections were carried out as described above.
Supplementary Material
Acknowledgments
We would like to thank the Cutaneous Biology Research Center for supporting the work, S. Boswell, A. Mandinova and L. Brown for reading the manuscript, and Y. Minamishima for initiation of the project. We also thank J. Manfredi, Mount Sinai School of Medicine for providing the mutant p53 constructs. This work was supported by NIH grants (2RO1 CA085681, 1RO1 CA097216 and 2RO1 CA078356 to SWL, and 2RO1 CA085214 and PO1 CA80058 to SAA), and Shiseido Research Core funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000;14:704–718. [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, Del Sal G, Syed N, Smith P, Gasco M, et al. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet. 2006;38:1133–1141. doi: 10.1038/ng1879. [DOI] [PubMed] [Google Scholar]
- Braithwaite AW, Del Sal G, Lu X. Some p53-binding proteins that can function as arbiters of life and death. Cell Death Differ. 2006;13:984–993. doi: 10.1038/sj.cdd.4401924. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O’Rourke K, Koeppen H, Dixit VM. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, Kisselev AF, Tanaka K, Nakatani Y. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell. 2003;113:357–367. doi: 10.1016/s0092-8674(03)00316-7. [DOI] [PubMed] [Google Scholar]
- Han JA, Kim JI, Ongusaha PP, Hwang DH, Ballou LR, Mahale A, Aaronson SA, Lee SW. P53-mediated induction of Cox-2 counteracts p53- or genotoxic stress-induced apoptosis. Embo J. 2002;21:5635–5644. doi: 10.1093/emboj/cdf591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Hidaka M, Caruana G, Stanford WL, Sam M, Correll PH, Bernstein A. Gene trapping of two novel genes, Hzf and Hhl, expressed in hematopoietic cells. Mech Dev. 2000;90:3–15. doi: 10.1016/s0925-4773(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Hupp TR, Meek DW, Midgley CA, Lane DP. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Li B, Bartel P, Fields S. Use of the two-hybrid system to identify the domain of p53 involved in oligomerization. Oncogene. 1993;8:1693–1696. [PubMed] [Google Scholar]
- Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991;252:1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Hart A, Hirashima M, Wang C, Holmyard D, Pittman J, Pang XL, Jackson CW, Bernstein A. Zinc finger protein, Hzf, is required for megakaryocyte development and hemostasis. J Exp Med. 2002;195:941–952. doi: 10.1084/jem.20011522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptenko O, Prives C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 2006;13:951–961. doi: 10.1038/sj.cdd.4401916. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–950. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. Faseb J. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ma W, Benchimol S. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat Genet. 2000;26:122–127. doi: 10.1038/79102. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, Lee SW. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. Embo J. 2003;22:1289–1301. doi: 10.1093/emboj/cdg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MS, Desterro JM, Lain S, Midgley CA, Lane DP, Hay RT. SUMO-1 modification activates the transcriptional response of p53. Embo J. 1999;18:6455–6461. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Ludwig RL, Haupt Y, Bates S, Lu X, Oren M, Vousden KH. Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. Embo J. 1996;15:827–838. [PMC free article] [PubMed] [Google Scholar]
- Samuels-Lev Y, O’Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T, Lu X. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Sharma S, Dimasi D, Higginson K, Della NG. RZF, a zinc-finger protein in the photoreceptors of human retina. Gene. 2004;342:219–229. doi: 10.1016/j.gene.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Gromley A, Sherr CJ. Hzf, a p53-responsive gene, regulates maintenance of the G2 phase checkpoint induced by DNA damage. Mol Cell Biol. 2006;26:502–512. doi: 10.1128/MCB.26.2.502-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, McMahon SB. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Thornborrow EC, Patel S, Mastropietro AE, Schwartzfarb EM, Manfredi JJ. A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax genes. Oncogene. 2002;21:990–999. doi: 10.1038/sj.onc.1205069. [DOI] [PubMed] [Google Scholar]
- Trigiante G, Lu X. ASPP and cancer. Nat Rev Cancer. 2006;6:217–226. doi: 10.1038/nrc1818. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Prives C. Increased and altered DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent kinases. Nature. 1995;376:88–91. doi: 10.1038/376088a0. [DOI] [PubMed] [Google Scholar]
- Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S, Ronai Z, Blandino G, Schneider C, Del Sal G. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature. 2002;419:853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zhang W, Guo XY, Hu GY, Liu WB, Shay JW, Deisseroth AB. A temperature-sensitive mutant of human p53. Embo J. 1994;13:2535–2544. doi: 10.1002/j.1460-2075.1994.tb06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G, Gu L, Tang X, Lu KP, Xiao ZX. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature. 2002;419:849–853. doi: 10.1038/nature01116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.