Abstract
The β1 integrins play an important role in regulating cell proliferation and survival. Using siRNA or an inhibitory antibody to β1, we show here that, in vivo, β1 integrins are essential for prostate cancer growth. Among the five known β1 integrin cytoplasmic variants, two are known to differentially affect prostate cell functions. The β1A variant promotes normal and cancer cell proliferation, whereas the β1C variant, which is downregulated in prostate cancer, inhibits tumor growth and appears to have a dominant effect on β1A. To investigate the mechanism by which β1C inhibits the tumorigenic potential of β1A, we analyzed changes in gene expression in cells transfected with either β1C or β1A. The results show that β1C expression increases the levels of an extracellular matrix protein, thrombospondin1 (TSP1), known to be an angiogenesis inhibitor. TSP1 protein levels are increased upon β1C expression in prostate cancer cells as well as in β1-null GD25 cells. We show that TSP1 does not affect proliferation, apoptosis or anchorage-independent growth of prostate cancer cells. In contrast, the newly synthesized TSP1, secreted by prostate cancer cells expressing β1C, prevents proliferation of endothelial cells. In conclusion, our novel findings suggest that expression of the β1C integrin variant in prostate glands prevents tumor progression by upregulation of TSP1 levels and inhibition of angiogenesis.
Keywords: β1 integrin variants, thrombospondin, prostate cancer, laminin, extracellular matrix
Introduction
Prostate cancer develops through a series of defined states: prostatic intraepithelial neoplasia (PIN), high-grade PIN lesions, invasive cancer and an androgen-independent state (1, 2). These defined states arise through multiple alterations in normal cell functions, including transcription, translation and post-translational processes (3).
Among the alterations described in prostate cancer, aberrant expression and abnormal functions of integrins and of their extracellular matrix (ECM) ligands have been suggested to play a pivotal role in prostate cancer progression (4, 5). In prostate diseases, cells express an abnormal integrin repertoire and are surrounded by a markedly different ECM as compared to normal prostate cells (6, 7). These changes have profound consequences, given the ability of each integrin to regulate specific cell functions. At this time, 24 integrin heterodimers, 18 α and 8 β subunits have been described (8). Five β1 variant subunits: β1A, β1B, β1C, β1C-2 and β1D, generated by alternative splicing, have been described. Among these, two variants: β1C and β1A, have been shown to be expressed in prostatic epithelium. β1C is expressed in normal prostatic epithelial cells, but is markedly downregulated in adenocarcinoma (9, 10); it inhibits cell proliferation and tumor growth, whereas the β1A variant promotes normal and cancer cell proliferation (11).
Another crucial factor promoting tumor growth is its abnormal angiogenic response (12). Tumor angiogenesis plays a critical role in the progression and growth of cancer cells by providing nutrients and other growth factors; thus, blocking angiogenesis in prostate as well as in other tissues is a therapeutic strategy (13-15). Angiogenesis is inhibited by ECM proteins like thrombospondin1 (TSP1) (16, 17). TSP1 is secreted by a wide variety of epithelial and mesenchymal cells and has been shown to bind α3β1, α4β1, α5β1, α6β1, αIIbβ3 and αVβ3 integrins (16, 17). The anti-angiogenic effect of TSP1 is partially mediated by its inhibitory effects on endothelial cell proliferation and also neovascularization in vivo (16). Besides inhibiting proliferation, TSP1 has also been shown to induce apoptosis of primary human brain microvascular endothelial cells (18). A recent study defines a novel role of TSP1 in preventing anoikis via activation of Akt (19).
In human prostate cancer, TSP1 expression is progressively decreased when normal epithelium is compared to benign prostate hyperplasia (BPH) and cancer specimens and when low-grade cancer is compared to high-grade cancer (20). In addition, prostate cancer specimens show an inverse relationship between TSP1 immunoreactivity and Gleason score (20). Decreased expression of TSP1 is especially evident in the stroma surrounding cancer areas, where decreased expression is correlated with progressive disease (20). Using 73 prostate tissue specimens (32 patients with BPH, 7 with PIN and 34 with cancer), Vallbo et al show that TSP1 is expressed in BPH, downregulated in PIN and absent in prostate cancer tissue (21). In human prostate cancer, the tumor growth inhibitory role of TSP1 begins to be investigated. TSP1 inhibits growth of LNCaP xenograft and microvessel density (22). Although in vitro TSP1 does not exert any significant growth inhibitory activity on DU145 cells, in vivo DU145 xenografts injected with TSP1-expressing plasmid show an extensive area of necrosis as compared to control tumors (23). Despite compelling evidence demonstrating widespread downregulation of TSP1 during prostate malignant progression, the factors which regulate TSP1 levels are not established nor a role for integrins in TSP1 downregulation has ever been reported.
In the present study, we investigated the mechanism by which β1C suppresses the activity of β1A in prostatic epithelium and describe a novel mechanism of prostate tumor progression mediated by TSP.
Materials and Methods
Reagents and antibodies
Reagents used for this study include: lipofectamine 2000 (Invitrogen, Carlsbad, CA); cycloheximide (CHX, Sigma); Matrigel (BD Bioscience) and Tumor Necrosis Factor-α (TNF-α, R & D, Minneapolis, MN). TSP1 was purified from human platelets as described before (24). Fibronectin (FN) was purified from human plasma. The following mouse monoclonal antibodies (mAbs) were used: to human β1 integrin TS2/16 (ATCC, Manassas, VA) used for Fluorescence-activated cell sorting (FACS); P4C10 (Chemicon) and AIIB2 (Aragen Bioscience) used for inhibition assays; clone-18 (BD Bioscience) used for immunoblotting; to chicken β1, W1B10 (Sigma) used for FACS; to hemagglutinin 12CA5 (ATCC); to TSP1: 133, previously described (25) and clone A4.1 (Thermo Scientific) (25); and to laminin α5 chain, 4C7 kindly provided by Dr. Eva Engvall (26). The following rabbit polyclonal Abs were used: to c-Jun, H-79; and to ERK1, C-16 (Santa Cruz). Normal purified rabbit IgG (rIgG), mouse IgG (mIgG), mouse IgM (mIgM, Sigma) or rat IgG (rtIgG, Pierce) were used as controls.
Cell lines and transfectants
Mouse cell line GD25, which lacks expression of the β1 family of integrin heterodimers due to disruption of the β1 integrin subunit gene, were transfected with either human β1A or β1C under the control of a doxycycline (dox)-regulated promoter and previously described (27). These transfectants were cultured as described (27).
PC3 parental or PC3 transfectants expressing chimeric β1A (clones A1 and A2), β1C (clones C1 and C2) integrin (chicken extracellular and human intracellular) or pTet (clone-6 and pool) were generated using the tetracycline (tet)-regulated expression system and cultured as described before (28).
PC3 and DU145 cells were stably transfected with plasmids containing either pEGFP or pEGFP-β1-shRNA (29) using lipofectamine 2000 (30). G418-resistant clones were pooled to generate a population.
Human umbilical vein endothelial cells (HUVECs, Clonetics, San Diego, CA) and LNCaP cells were cultured as described (31, 32).
FACS analysis
Cells were detached and FACS analysis was used to detect surface expression of exogenous (chimera) or endogenous (human) β1 in PC3 transfectants using W1B10, TS2/16, 12CA5 or mIgG (28). Surface expression of β1A or β1C in GD25 transfectants was analyzed by FACS using TS2/16, or, as negative control, 12CA5 (30).
Prostate xenografts
PC3 transfectants (PC3-β1-shRNA or PC3-vector) were detached, washed, and resuspended in RPMI or RPMI containing Matrigel (50%). Cells (1×106) were inoculated subcutaneously (s.c.) into the right flank of six- to eight-week-old male athymic Balb/c mice (NCI Frederick).
PC3 stable cell transfectants expressing chimeric β1A integrin (chicken extracellular and human intracellular) were transiently transfected with either human β1 siRNA [R2; (29)] or control siRNA and cultured in the presence or in the absence of tet for 48 h. Cells were detached and inoculated s.c. into the right flank of athymic Balb/c mice as described above. Mice were given water supplemented with either 5% sucrose to induce β1A expression, or 5% sucrose plus 100 μg/ml tet.
PC3 cells were detached, washed, and resuspended in RPMI. Cells (1×106) were inoculated s.c. into the right flank of seven-week-old male athymic nu/nu mice (Charles River). AIIB2 or nonspecific rtIgG was injected intraperitoneally (5 mg/kg) biweekly once the tumor reached 100 mm3 (33). Subcutaneous tumors from mice described above were isolated, fixed and embedded in paraffin. Apoptosis was measured using Apoptag cell death detection kit (Chemicon).
In all cases, tumor size was determined using a caliper every other day and tumor volume was calculated as described (11).
RNA isolation and analysis
Gene expression profiles of β1A- or β1C-GD25 stable cell transfectants were generated using 1.2 Atlas Mouse cDNA Expression Arrays (Clontech) according to the manufacturer's instructions (27) (30). Briefly, GD25 stable cell lines were starved for 48 h. During the last 24 h, cells were kept in the presence of 2 μg/ml dox and then detached, washed and plated for 5 h on FN (5 μg/ml) in serum-free medium (SFM). The above conditions used for studying gene expression profiles of β1A- or β1C-GD25 stable cell transfectants were selected to allow comparable cell adhesion, as previously shown (27), and to prevent artifacts due to differential cell adhesion to tissue culture plates.
Immunoblotting
Cell lysates as well as concentrated conditioned media were immunoblotted as described before (34).
Anchorage-independent growth assay
This study utilized β1 siRNA (R1, R2 and R3) or non-specific control siRNA (Dharmacon). The siRNA specific for β1A [R1 (35)] or siRNA specific for β1 [R2 (29), R3 (36)] were described before. DU145 or LNCaP cells were transfected with siRNA and processed to analyze anchorage-independent growth as described (35). PC3-β1-shRNA or PC3-vector cells were plated on soft agar as described before (35).
β1C-PC3 transfectants were cultured as described (28). Cells were plated on soft agar in the presence of either mIgM or a-TSP (TSP blocking Ab, clone A4.1). The Abs were added twice a week. The numbers of colonies larger than 100 μm were counted.
Cell proliferation assay
β1C-PC3 transfectants were detached, plated on FN and cultured in the presence or absence of tet and TSP1. After 24 h incubation, cells were processed to measure cell proliferation using Sulforhodamine B (SRB, Sigma) assay as described (11).
HUVECs (5,000 cells per well) were plated on a 96-well plate and incubated overnight at 37°C for attachment in complete media. For each treatment, cells were plated in triplicate. Cells were treated with conditioned media from β1C-PC3 or β1A-PC3 or TSP1 (10 μg/ml). Cell proliferation was measured using SRB as described above. In some experiments, TSP blocking Ab, clone A4.1 was added at a concentration of 20 μg/ml (25, 37).
Apoptosis assay
β1C-PC3 cells were induced as described above. Cells were incubated for 42 h in the presence of CHX (3 μg/ml), TNF-α (150 ng/ml) and TSP1 (2 μg/ml) (28). Cells were processed for DNA fragmentation assay using Cell Death Detection ELISA kit (Roche). DU145 cells transfected with three β1 siRNAs or control siRNA or DU145 stable transfectants expressing β1-shRNA or vector alone were detached and processed for apoptosis detection as described above.
Statistical analysis
Data on cell proliferation, apoptosis, anchorage-independent growth and for in vivo tumor growth, were expressed as means ±SEM. Statistical analysis between different groups was conducted using the Student's t-test. All p-values were based on two-tailed tests.
Results
β1 integrins regulate tumor growth
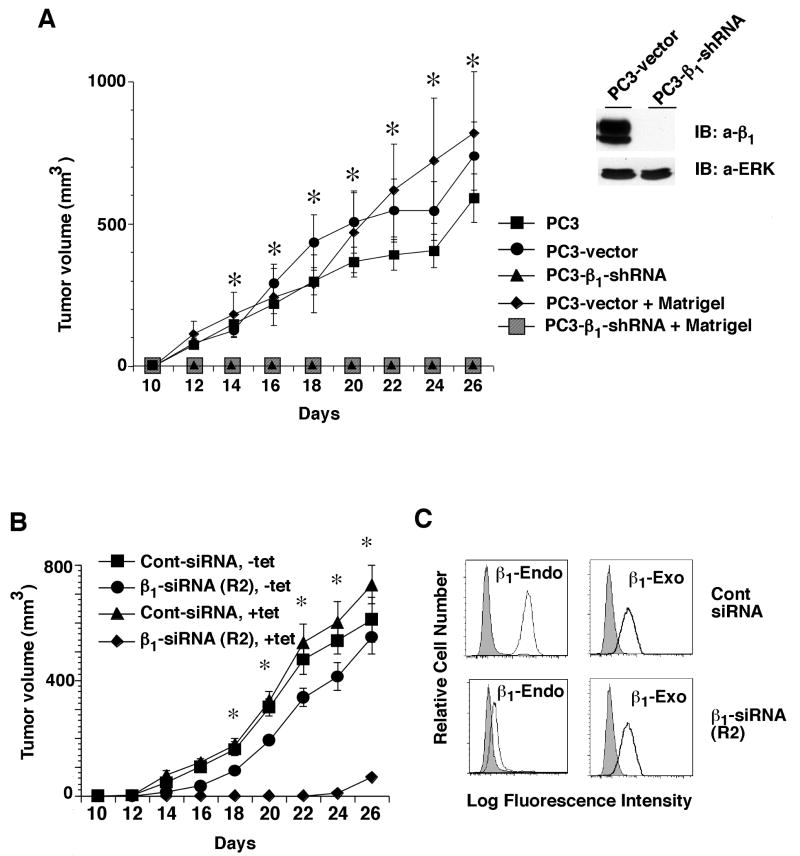
β1A and β1C integrins are differentially expressed and redistributed in prostate cancer progression in human and TRAMP (transgenic adenocarcinoma of mouse prostate) mice (35, 38, 39). To study the role of β1 integrins during prostate cancer growth in vivo, we generated PC3 stable cell transfectants expressing either a β1 specific shRNA or vector alone. We injected these PC3 transfectants (PC3-β1-shRNA or PC3-vector) in nude mice and find that downregulation of β1 integrins completely inhibits the ability of these cells to form tumors (Fig. 1A, left panel). The results show a dramatic inhibition of tumor growth in the absence of β1 integrins (11/11 mice failed to develop tumors), whereas PC3-vector transfectants form large tumors (Fig. 1A). Mice injected with PC3-β1-shRNA transfectants were followed for 60 days but they never develop tumors (data not shown). Since β1 plays a crucial role in basement membrane (BM) organization, it was investigated whether this effect on tumor growth due to β1 inhibition was a consequence of an incomplete BM (40, 41). Subcutaneous injection of PC3-β1-shRNA transfectants resuspended in Matrigel does not rescue these cells' ability to grow in nude mice, suggesting that the failure to form tumors does not result from lack of supporting BM components. We confirmed downregulation of β1 in β1-shRNA expressing cells by immunoblotting (Fig. 1A, right panel).
Figure. 1.
β1 integrin expression is essential for prostate tumor growth. A (left panel), PC3, PC3-β1-shRNA or PC3-vector cells were injected s.c. in nude mice. Two groups of mice were also injected with PC3-β1-shRNA or PC3-vector cells in the presence of Matrigel. The graph shows kinetics of tumor growth. Tumor growth was measured up to 26 days and is expressed as tumor volume in mm3. The difference in tumor volume between cells transfected with vector or β1 shRNA from 14 to 26 days after injection are statistically significant (*p≤0.0001). A (right panel), PC3-β1-shRNA or PC3-vector cells were lysed and immunoblotted using Ab to β1 or ERK1. These experiments were repeated twice with similar results. B-C, PC3 transfectants expressing chimeric exogenous (exo) β1A (chicken extracellular and human intracellular) were transfected with β1 siRNA (siRNA sequence R2), to downregulate endogenous (endo) β1, or with control siRNA (100 nM). Cells were cultured in the presence or in the absence of tet and injected s.c. in nude mice. Cells express one of the following four combinations of β1: both endo and exo (Cont-siRNA, -tet); endo only (Cont-siRNA, +tet); exo only (β1-siRNA, -tet); none of the two (β1-siRNA, +tet). The graphs show kinetics of tumor growth, measured as described above. *p≤0.0001 from 14 to 26 days after injection (B). Data are the mean ± SEM obtained using 11 animals (A) or 5 animals (B). Cells transfected with β1A siRNA (siRNA sequence R2) or control siRNA were cultured in the absence of tet. Cells were processed for FACS analysis to determine the expression of endo (human) and exo (chimera) β1 using Ab to human β1 (TS2/16, thin black line), to chicken β1 (W1B10, thick black line) or as a negative control, 12CA5 (filled grey) (C).
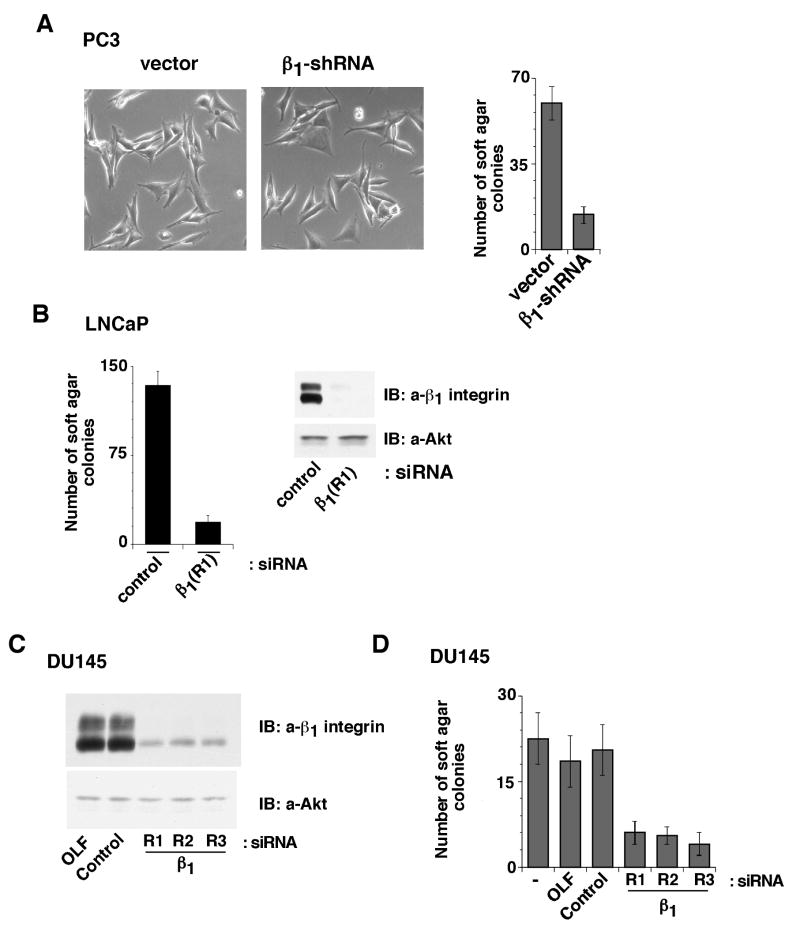
We further investigated whether expression of β1 integrins in PC3-β1-shRNA cells could rescue tumor growth. For these experiments, we transfected siRNA targeting human β1 in cells expressing the chicken/human chimera β1A integrin. We find that expression of β1 rescues the effect observed in PC3-β1-shRNA cells on tumor growth (Fig. 1B). As shown in Fig. 1C, β1 siRNA specifically downregulates the endogenous human β1 without affecting the exogenous chimeric β1A integrin. These results suggest that the β1A integrin plays a crucial role in prostate cancer tumorigenesis. β1 downregulation does not affect proliferation, adhesion (data not shown) or morphology of PC3 cells in tissue culture plates, but significantly inhibits anchorage-independent growth of these cells (Fig. 2A). We also analyzed the effect of β1 downregulation on anchorage-independent growth in androgen-dependent LNCaP cells. Using siRNA to β1, we achieved complete downregulation of β1 which causes a significant inhibition of anchorage-independent growth of LNCaP cells (Fig. 2B). A similar effect was observed in another androgen-independent cell line, DU145. We further confirmed the effect of β1 downregulation by using three different siRNA to β1 in DU145 cells (Fig. 2C-D). These results show that β1 downregulation impairs androgen-dependent and -independent prostate cancer cells' ability to grow in an anchorage-independent manner.
Figure 2.
Downregulation of β1 integrins inhibits growth of androgen-dependent and -independent cells. A, PC3-β1-shRNA or PC3-vector cells were plated on tissue culture plates. Cells on tissue culture plates were observed under phase contrast microscope (left panels); alternatively, cells were processed to measure anchorage-independent growth (right panel). B, LNCaP cells were transiently transfected with either β1A siRNA (siRNA sequence R1) or control siRNA. Cells were processed to measure anchorage-independent growth (left panel); alternatively, the cells were lysed and immunoblotted with an Ab to β1 or as a loading control, Akt (right panel). C-D, DU145 cells were transiently transfected with three β1 siRNAs (R1, R2 and R3) or control siRNA or oligofectamine (OLF) alone. Cells were lysed and immunoblotted with an Ab to β1 integrins or as a loading control, Akt (C); alternatively, the cells are processed to measure anchorage-independent growth (D). Triplicate observations in three separate experiments were performed.
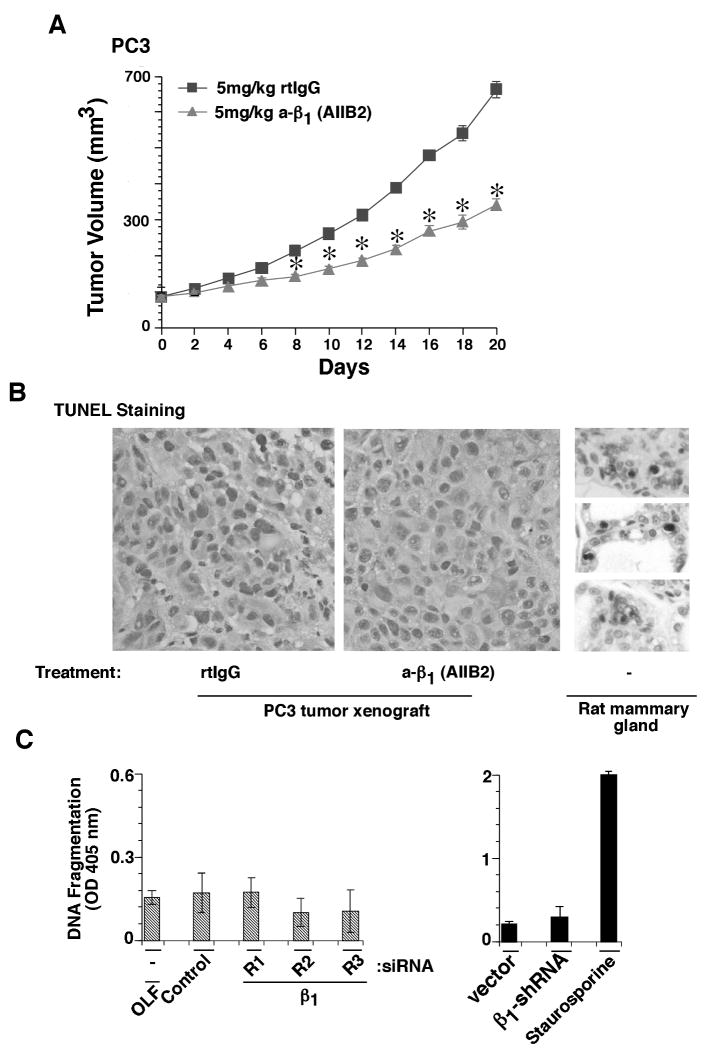
To study the effect of inhibiting β1 on growth of preformed prostate tumors, we implanted PC3 cells in nude mice. An inhibitory Ab to β1 (AIIB2) or an irrelevant control Ab (rtIgG) was injected intraperitoneally when the tumor volume reached 100 mm3. Compared with tumors propagated in animals that received rtIgG, there is a significant decrease in the volume of AIIB2 treated tumors (Fig. 3A). Furthermore, we analyzed whether inhibition or downregulation of β1 integrins induces apoptosis, but we do not detect any apoptotic cells in prostate tumors from mice treated with AIIB2 or rtIgG (Fig. 3B). A section from rat mammary gland, which undergoes apoptosis after weaning, shows apoptotic nuclei and was used as a positive control (Fig. 3B). Similarly, no apoptosis is detected in DU145 (Fig. 3C) and PC3 (data not shown) cells upon β1 downregulation. These results show that β1 integrin inhibition prevents prostate tumor growth without inducing apoptosis.
Figure 3.
β1 integrin inhibition does not induce apoptosis in vivo. A, PC3 xenograft (100 mm3) bearing nude mice were injected with either AIIB2 or rat IgG (rtIgG) biweekly. Tumor growth was measured up to 20 days and is expressed as tumor volume in mm3. Data are the mean ± SEM of 6 animals per group. The differences in tumor volume in the range of 10 to 20 days after injection are statistically significant between the mice injected with AIIB2 versus mice injected with rtIgG (*p≤0.001). The graph shows kinetics of tumor growth. B, Subcutaneous tumors from mice described in panel (A) were dissected and fixed in 10% neutral buffered formalin. The fixed tissues were then embedded in paraffin. A section from female rat mammary gland, 3-5 days after rat pups weaning, was used as a positive control. Apoptosis was measured using Apoptag cell death detection kit from Chemicon. C, DU145 cells transiently transfected with three β1 siRNAs (R1, R2 and R3) or control siRNA or oligofectamine (OLF) alone (left panel) or DU145 stable transfectants expressing β1-shRNA or vector alone (right panel) were detached and processed for DNA fragmentation assay. DU145 cells treated with staurosporine (1 μM) were used as a positive control for apoptosis.
β1C integrin regulates expression of TSP1
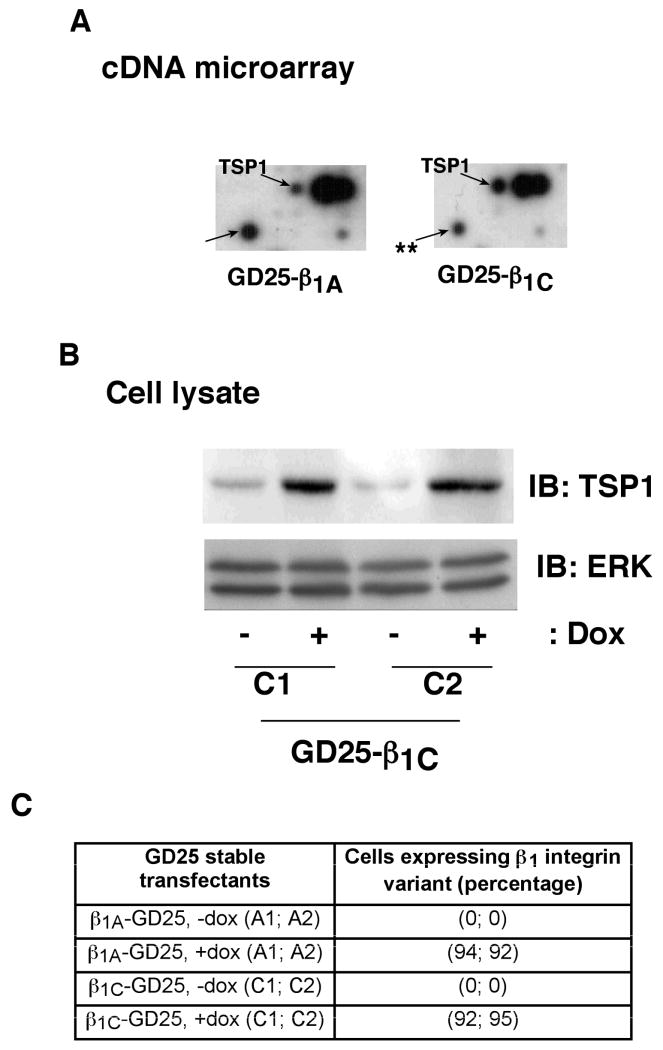
To study the mechanism by which β1C blocks prostate tumor growth, we analyzed the gene expression profile of GD25 cells expressing either β1C or β1A integrin variants. These stable transfectants express β1C or β1A integrin variants under the control of dox (30). Among the genes, which are differentially regulated, TSP1 levels are significantly increased upon β1C expression as compared to β1A (Fig. 4A). In contrast, other genes are downregulated (one representative spot is shown as **). To confirm these results, we performed immunoblotting using an Ab to TSP1. β1C causes a significant increase in TSP1 protein expression (Fig. 4B), whereas β1A integrin expression does not change TSP1 levels (data not shown). The GD25 stable transfectants used for the above-described experiments show comparable surface expression of β1A or β1C variants (Fig. 4C). These results show that TSP1 is specifically upregulated in cells expressing the β1C variant.
Figure 4.
β1C integrin expression increases TSP1 mRNA and protein levels in β1–null GD25 cells. A, β1C- and β1A-GD25 transfectants were induced to express β1C or β1A and processed for cDNA array expression analysis. The arrows indicate the spots corresponding to TSP1 cDNA or an another gene, which is downregulated (shown as **) in β1C-expressing cells. Similar results were obtained in two separate experiments. B, β1C-GD25 transfectants (clones C1 and C2) were cultured as described above. Cells were lysed and immunoblotted using Ab to TSP1 or ERK1. C, β1C-GD25 (clones C1 and C2) or β1A-GD25 (clones A1 and A2) transfectants induced to express β1C or β1A, were detached and analyzed by FACS using either TS2/16 or 12CA5. The table summarizes the results of the FACS analysis.
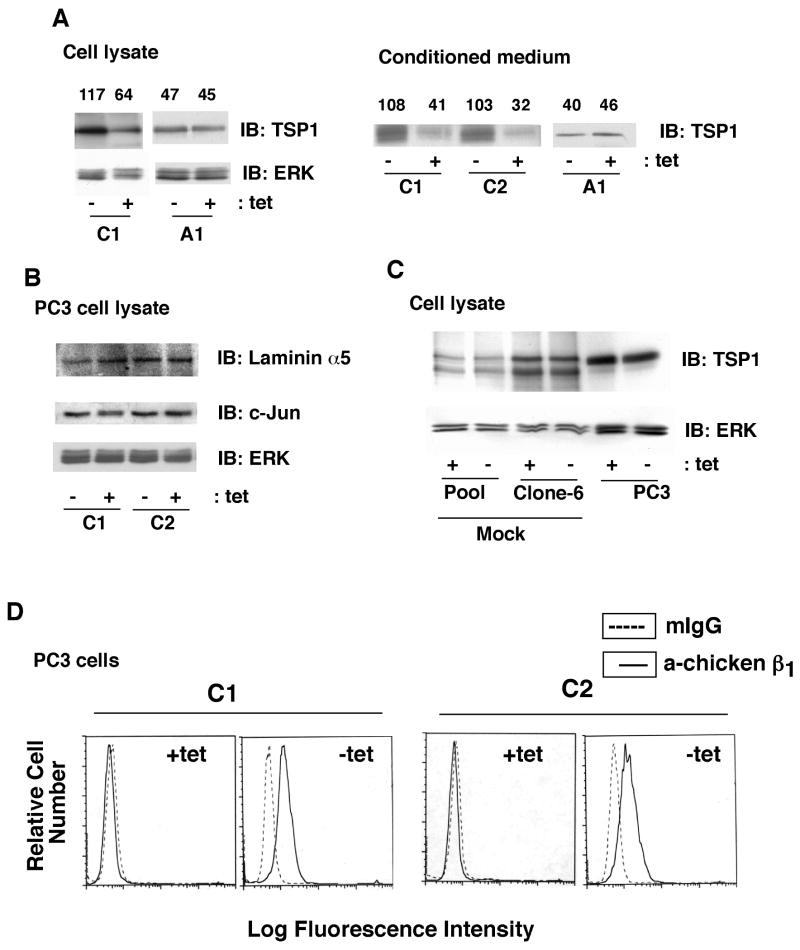
The β1C integrin variant has previously been shown to inhibit prostate tumor growth (11). We investigated the effect of the β1C integrin variant on TSP1 expression in prostate cancer cells. We used PC3 stable transfectants expressing either chimeric β1C or β1A integrin (chicken extracellular and human intracellular) (28). These cells were induced to express β1 variants by removing tet, and expression of TSP1 in cell lysates and in conditioned media was analyzed. We find increased TSP1 expression upon β1C transfectants' attachment to FN (Fig. 5A, left panel and data not shown). Conditioned media from cells expressing β1C also show upregulation of TSP1 (Fig. 5A, right panel). However, TSP1 expression in cell lysates as well as in conditioned media is not affected upon β1A transfectants' attachment to FN (Fig. 5A). In contrast, the levels of laminin α5, another ECM protein shown to inhibit migration, invasion and angiogenesis (42), are not affected upon β1C expression (Fig. 5B). We also analyzed the expression of c-Jun, which has been shown to inhibit TSP1 levels (43, 44). The results show that β1C expression does not affect c-Jun protein levels (Fig. 5B). Moreover, tet removal does not affect TSP1 levels in cells transfected with vector alone (ptet, pool and clone-6) or in parental PC3 cells (Fig. 5C). We confirmed surface expression of the transfected chimeric β1C (clones C1 and C2) upon tet withdrawal using FACS analysis (Fig. 5D). These results indicate that β1C induces expression and secretion of TSP1 in prostate cancer cells.
Figure 5.
β1C integrin expression increases TSP1 protein levels in PC3 cells. A-B, β1C-PC3 (clones C1 and C2) or β1A-PC3 (clone A1) cells cultured in presence or absence of tet were detached, plated on FN and attached cells were cultured in growth medium in the presence or absence of tet. Cells were lysed or conditioned media were collected and concentrated. Cell lysates as well as conditioned media were immunoblotted using Ab to TSP1, ERK1 (A), laminin α5, c-Jun or ERK1 (B). Densitometric values were shown for each lane. C, PC3 cells stably transfected with pTet either pooled (pool) or used as a separate clone (clone 6) were cultured in the presence or absence of tet, lysed and immunoblotted using Ab to TSP1 or ERK1. D, β1C-PC3 (clones C1 and C2) cells were cultured in the presence or absence of tet. Cells were detached and analyzed by FACS using either W1B10 or mIgG.
TSP1 does not affect proliferation or apoptosis of β1C expressing PC3 cells
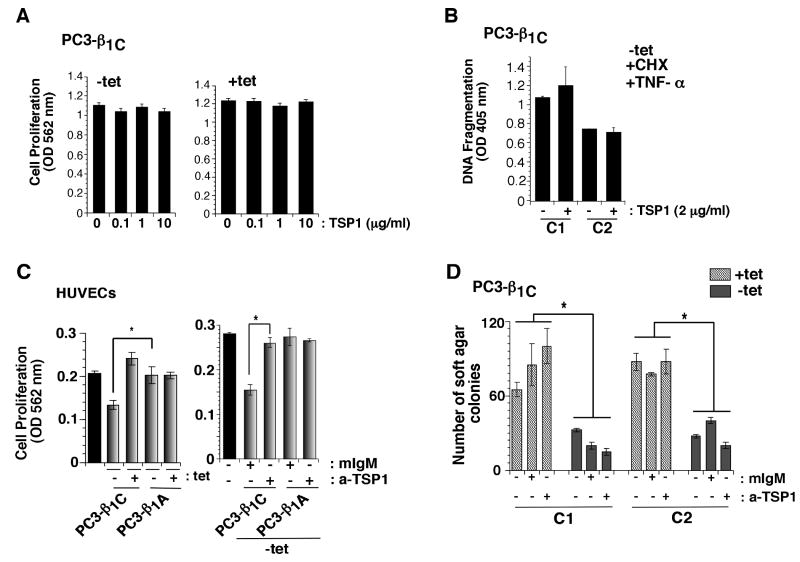
To investigate whether secreted TSP1 blocks PC3 cell growth in an autocrine manner, β1C-PC3 transfectants were cultured in the presence or absence of tet and cell proliferation was studied at different doses of TSP1. As shown in Fig. 6A, TSP1 does not affect proliferation of PC3 cells in the presence (-tet) or in the absence (+tet) of β1C integrin. In addition, TSP1 does not affect apoptosis of β1C-PC3 cells induced by CHX and TNF-α treatment (Fig. 6B). We confirmed the homogeneity of two different preparations of TSP1 used in this study by SDS-PAGE (Supplementary Fig. S1A). We show that PC3 cells adhere to TSP1 and this attachment is inhibited by pretreatment with an inhibitory Ab to β1 integrins (Fig. S1B-C). These results suggest that increased levels of TSP1 in response to β1C expression do not affect prostate cancer cell proliferation or apoptosis.
Figure 6.
TSP1 inhibits proliferation of HUVECs. A-B, β1C-PC3 cells were cultured either in the absence (-tet, left panel) or presence (+tet, right panel) of tet. Cells were incubated in the presence or absence of TSP1 (0, 0.1, 1 or 10 μg/ml). After 24 h of incubation, cells were processed to measure cell proliferation using Sulforhodamine B (SRB) assay (A). Cells were detached, plated on FN and attached cells were incubated in the presence of CHX, TNF-α or TSP1 (2 μg/ml). Cells were detached and processed for DNA fragmentation assay (B). C, HUVECs were plated on 96-well plate and incubated overnight for attachment. Cells were washed with PBS and incubated in serum free medium for 6 h. HUVECs were treated with conditioned media from β1C-PC3 (clone C1, -tet or +tet) or β1A-PC3 (clone A2, -tet or +tet) transfectants for 24 h. Cell proliferation was measured using SRB. TSP1 secreted by β1C-PC3 cells inhibits proliferation of HUVECs (*p<0.05) (left panel). C, right panel, cells were plated and treated with conditioned media from β1C-PC3 (-tet) or β1A-PC3 (-tet) as described above in the presence of either mIgM or TSP Ab (A4.1, 20 μg/ml) and proliferation was measured using SRB after 48 h. The TSP1 inhibitory Ab prevents the effect of β1C-PC3 conditioned medium on proliferation of HUVECs (*p<0.05). Grey bars, conditioned medium; black bars, HUVEC culture medium. D, β1C-PC3 transfectants (clones C1 and C2) were cultured in the presence or absence of tet. Cells were processed to measure anchorage-independent growth in the presence of either mIgM or a-TSP (TSP blocking Ab, clone A4.1). The expression of β1C suppresses anchorage-independent growth of PC3 cells (*p<0.05).
TSP1 secreted by β1C expressing-PC3 cells inhibits proliferation of HUVECs
Since TSP1 is known to inhibit angiogenesis and proliferation of endothelial cells (37), we investigated the effect of conditioned media from PC3 cells expressing β1C or β1A integrin on HUVECs' proliferation. As shown in Fig. 6C, we find significant inhibition (range 36-45%) of HUVECs proliferation by conditioned media of β1C-PC3 transfectants compared to β1A-PC3 transfectants. An Ab specific for TSP1, clone A4.1, has been shown to inhibit the anti-angiogenic activity of TSP1 (25, 37); we observe that TSP1 blocks HUVECs proliferation and this inhibitory effect is rescued by clone A4.1 (data not shown) (25, 37). Our data show that addition of A4.1 Ab prevents the effect of β1C-PC3 conditioned media on proliferation of HUVECs (Fig. 6C). To investigate the effect of TSP1 inhibition on β1C's ability to suppress tumor growth, we performed an anchorage-independent growth assay using PC3 cells expressing β1C. Our results show that expression of β1C suppresses anchorage-independent growth of PC3 cells. However, inhibition of secreted TSP1 could not rescue PC3 cells from β1C's blocking effect (Fig. 6D). We find similar results when anchorage-independent growth of β1C-PC3 was analyzed in the presence of β1C-PC3 conditioned medium (data not shown) previously tested for its ability to prevent endothelial cell proliferation via TSP1 (Fig. 6C). Our data show that TSP1 inhibition does not rescue anchorage-independent growth of β1C-expressing PC3 cells, thus supporting our hypothesis that TSP1 secreted by PC3-β1C cells acts by inhibiting proliferation of endothelial cells, rather than affecting prostate cancer cells.
Discussion
The present study describes a novel mechanism mediated by the β1 integrins that may contribute to prostate cancer progression. The results describe for the first time the ability of integrins to regulate the levels of TSP1, an angiogenesis inhibitor. These findings also characterize the β1C integrin variant, a tumor growth inhibitor, as a positive modulator of TSP1 expression.
In the first part of the study, we show that β1 downregulation inhibits prostate cancer growth in vivo. Although the effect of inhibiting β1 on other cancer types has been described by others, the finding that β1 downregulation inhibits prostate cancer growth in vivo had never been reported. This finding had to be investigated given that our previous study, performed in vitro, had shown that β1 downregulation in aggressive PC3 prostate cancer cells did not prevent cell proliferation in response to serum (35). In addition, previous in vivo studies had shown that β1 expression either prevents (45) or promotes (46) tumor growth. We, then, extended our study to several prostate cancer cell lines and confirm that β1 downregulation impairs androgen-dependent and -independent prostate cancer cells' ability to grow in an anchorage-independent manner. Although we cannot exclude that these β1 receptors become inactive in poorly differentiated tumors (35), these findings highlight a crucial role for β1 in androgen-dependent and -independent prostate cancer growth.
In the second part of the study, we focused our attention on two of the five known β1 integrin cytoplasmic variants, β1C and β1A. These variants are known to differentially affect prostate cell functions. The β1C variant, which is downregulated in prostate cancer and is co-expressed with the β1A integrin variant (9), inhibits cell proliferation and tumor growth and appears to have a dominant effect on the β1A variant which instead promotes normal and cancer cell proliferation. To investigate the mechanism by which β1C inhibits the tumorigenic potential of β1A, we analyzed changes in gene expression in cells transfected with either β1C or β1A. We show here that TSP1 is a downstream effector of β1C, and acts in a paracrine manner by inhibiting endothelial cell proliferation rather than affecting cancer cell proliferation or apoptosis. The differential response of prostate cancer and endothelial cells may be ascribed to the different repertoire of integrins in the two different cell types. Among the receptors for TSP1, both PC3 and HUVECs express predominantly β1 and β3 integrins (16, 17); however, these integrins may heterodimerize with different α-subunits in these cell types and thus provide different ligand specificity.
Our observation that changes in RNA and protein levels of TSP1 in prostate cancer cells occur in response to β1C expression is novel and is also highly specific. We show that the levels of laminin α5, another ECM protein, known to inhibit migration, invasion and angiogenesis (42) are not affected. The described gene regulation of an ECM protein in response to β1C expression seems, therefore, to be specific for TSP1. Other integrins share this ability to regulate gene expression; among others, αVβ3 expression has been documented to increase cdc2 levels (32). Similarly, several other molecules [cyclin A, cyclin D1, cyclin E-cdk2 kinase activity, gelatinase, metalloproteinases, immediate–early response genes] have been shown to be upregulated in response to integrin expression, although these receptors' engagement is also required to generate a significant response (32, 47).
Among the possible TSP1 regulators activated by β1C, the following potential downstream effectors of integrins are likely to contribute to this pathway. We have shown earlier that β1C inhibits activation of ERK (35); thus, ERK inhibition may be responsible for TSP1 regulation, although a role for ERK in this pathway remains to be investigated. Additional pathways may be involved. First, β1C may affect TSP1 expression via downregulation of VEGF, a factor known to stimulate angiogenesis, but also to mediate apoptosis of endothelial cells in the presence of TGF-β1 (48). This is conceivable since an inverse correlation between expression of TSP1 and VEGF in prostate cancer specimens has been shown (49) and remains to be investigated. Second, the tumor suppressor protein p53 is known to be regulated by integrins (50). In cultured fibroblasts, loss of the wild-type allele of p53 results in reduced expression of TSP1, while re-expression of p53 in these cells restores TSP1 mRNA levels (51). Correlation between reduced TSP1 expression with p53 alterations was also observed in patients with invasive transition carcinoma of the bladder (52). Since PC3 cells used in this study are p53-null, we can rule out the possible involvement of p53 in the regulation of TSP1 levels. Third, several oncogenes like ras and src, known to be activated by integrins, cause reduction in TSP1 mRNA levels and may act as downstream effectors of integrins (16, 53). Finally, c-jun inhibits TSP1 mRNA levels, but is unlikely to be responsible for TSP1 downregulation (43, 44), since our data show that c-Jun expression levels remain unchanged in the presence of β1C integrin. However, further investigation is required to determine the role of the basal level of c-Jun in TSP1 regulation.
Taken together, our results suggest that selective loss of specific integrin variants, particularly of the β1C integrin, may facilitate neoplastic transformation not only by inducing a burst in cell proliferation (54), but also by promoting changes in the microenvironment that would favor angiogenesis.
Supplementary Material
Acknowledgments
This study was supported by grants from NIH (CA109874 and CA89720); DOD (PCRP DAMD PC040221) to LRL, from American Cancer Society (Institutional Research Grant-93-033) to HLG, from NIH (HL79644 and DK78038) to JEM. We would like to thank Heather Ehlers for help during the preparation of this article. We thank Dr. E. Sahai for β1-shRNA plasmid. Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
Grant support: National Institute of Health CA109874, CA89720 (LRL), HL79644 and DK78038 (JEM); DOD PC040221 (LRL); American Cancer Society Institutional Research Grant IRG-93-033 (HLG).
References
- 1.Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99:373–81. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- 2.De Marzo AM, DeWeese TL, Platz EA, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–77. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 3.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 4.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–31. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 5.Cooper CR, Chay CH, Pienta KJ. The role of αvβ3 in prostate cancer progression. Neoplasia (New York, NY) 2002;4:191–4. doi: 10.1038/sj.neo.7900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;3:657–64. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudsen BS, Miranti CK. The impact of cell adhesion changes on proliferation and survival during prostate cancer development and progression. J Cell Biochem. 2006;99:345–61. doi: 10.1002/jcb.20934. [DOI] [PubMed] [Google Scholar]
- 8.Alam N, Goel HL, Zarif MJ, et al. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–53. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 9.Fornaro M, Tallini G, Zheng DQ, Flanagan WM, Manzotti M, Languino LR. p27(kip1) acts as a downstream effector of and is coexpressed with the β1C integrin in prostatic adenocarcinoma. J Clin Invest. 1999;103:321–9. doi: 10.1172/JCI4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlino E, Lovecchio M, Vacca RA, et al. Regulation of mRNA and protein levels of β1 integrin variants in human prostate carcinoma. Am J Pathol. 2000;157:1727–34. doi: 10.1016/s0002-9440(10)64809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel HL, Fornaro M, Moro L, et al. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J Cell Biol. 2004;166:407–18. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto S, Ryan AJ, Kyprianou N. Targeting vasculature in urologic tumors: Mechanistic and therapeutic significance. J Cell Biochem. 2008;103:691–708. doi: 10.1002/jcb.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rege TA, Fears CY, Gladson CL. Endogenous inhibitors of angiogenesis in malignant gliomas: nature's antiangiogenic therapy. Neuro Oncol. 2005;7:106–21. doi: 10.1215/S115285170400119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson B, Theodorescu D. Angiogenesis and prostate cancer tumor growth. J Cell Biochem. 2004;91:125–50. doi: 10.1002/jcb.10772. [DOI] [PubMed] [Google Scholar]
- 15.Arap W, Haedicke W, Bernasconi M, et al. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci USA. 2002;99:1527–31. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Fraipont F, Nicholson AC, Feige JJ, Van Meir EG. Thrombospondins and tumor angiogenesis. Trends Mol Med. 2001;7:401–7. doi: 10.1016/s1471-4914(01)02102-5. [DOI] [PubMed] [Google Scholar]
- 17.Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Rege TA, Stewart J, Jr, Dranka B, Benveniste EN, Silverstein RL, Gladson CL. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Physiol. 2009;218:94–103. doi: 10.1002/jcp.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallero MA, Elzie CA, Chen J, Mosher DF, Murphy-Ullrich JE. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. Faseb J. 2008;22:3968–79. doi: 10.1096/fj.07-104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP. Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate. 2001;49:293–305. doi: 10.1002/pros.10025. [DOI] [PubMed] [Google Scholar]
- 21.Vallbo C, Wang W, Damber JE. The expression of thrombospondin-1 in benign prostatic hyperplasia and prostatic intraepithelial neoplasia is decreased in prostate cancer. BJU Int. 2004;93:1339–43. doi: 10.1111/j.1464-410x.2004.04818.x. [DOI] [PubMed] [Google Scholar]
- 22.Colombel M, Filleur S, Fournier P, et al. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res. 2005;65:300–8. [PubMed] [Google Scholar]
- 23.Jin RJ, Kwak C, Lee SG, et al. The application of an anti-angiogenic gene (thrombospondin-1) in the treatment of human prostate cancer xenografts. Cancer Gene Ther. 2000;7:1537–42. doi: 10.1038/sj.cgt.7700266. [DOI] [PubMed] [Google Scholar]
- 24.Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Hook M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–9. [PubMed] [Google Scholar]
- 25.Annis DS, Murphy-Ullrich JE, Mosher DF. Function-blocking antithrombospondin-1 monoclonal antibodies. J Thromb Haemost. 2006;4:459–68. doi: 10.1111/j.1538-7836.2006.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986;103:2457–65. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moro L, Fornaro M, Steger CA, Languino LR. Regulation of MCP-3 and BRCA2 mRNA expression levels by β1 integrins. Exp Mol Pathol. 2001;70:239–47. doi: 10.1006/exmp.2001.2359. [DOI] [PubMed] [Google Scholar]
- 28.Fornaro M, Plescia J, Chheang S, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor α-induced apoptosis via the AKT/Survivin pathway. J Biol Chem. 2003;278:50402–11. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 29.Brockbank EC, Bridges J, Marshall CJ, Sahai E. Integrin β1 is required for the invasive behaviour but not proliferation of squamous cell carcinoma cells in vivo. Br J Cancer. 2005;92:102–12. doi: 10.1038/sj.bjc.6602255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel HL, Moro L, King M, et al. β1 integrins modulate cell adhesion by regulating insulin-like growth factor-II levels in the microenvironment. Cancer Res. 2006;66:331–42. doi: 10.1158/0008-5472.CAN-05-2588. [DOI] [PubMed] [Google Scholar]
- 31.Altieri DC, Duperray A, Plescia J, Thornton GB, Languino LR. Structural recognition of a novel fibrinogen gamma chain sequence (117-133) by ICAM-1 mediates leukocyte-endothelium interaction. J Biol Chem. 1995;270:696–9. doi: 10.1074/jbc.270.2.696. [DOI] [PubMed] [Google Scholar]
- 32.Manes T, Zheng DQ, Tognin S, Woodard AS, Marchisio PC, Languino LR. αvβ3 integrin expression up-regulates cdc2, which modulates cell migration. J Cell Biol. 2003;161:817–26. doi: 10.1083/jcb.200212172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. β1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornaro M, Steger CA, Bennett AM, Wu JJ, Languino LR. Differential role of β1C and β1A integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/Mitogen-activated protein kinase pathways. Mol Biol Cell. 2000;11:2235–49. doi: 10.1091/mbc.11.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel HL, Breen M, Zhang J, et al. β1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 36.Cordes N, Hansmeier B, Beinke C, Meineke V, van Beuningen D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br J Cancer. 2003;89:2122–32. doi: 10.1038/sj.bjc.6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knox JD, Cress AE, Clark V, et al. Differential expression of extracellular matrix molecules and the α6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–74. [PMC free article] [PubMed] [Google Scholar]
- 39.Fornaro M, Tallini G, Bofetiado CJ, Bosari S, Languino LR. Down-regulation of β1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am J Pathol. 1996;149:765–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki T, Forsberg E, Bloch W, Addicks K, Fassler R, Timpl R. Deficiency of β1 integrins in teratoma interferes with basement membrane assembly and laminin-1 expression. Exp Cell Res. 1998;238 doi: 10.1006/excr.1997.3837. [DOI] [PubMed] [Google Scholar]
- 41.Bloch W, Forsberg E, Lentini S, et al. β1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol. 1997;139:265–78. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–6. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- 43.Dejong V, Degeorges A, Filleur S, et al. The Wilms' tumor gene product represses the transcription of thrombospondin 1 in response to overexpression of c-Jun. Oncogene. 1999;18:3143–51. doi: 10.1038/sj.onc.1202654. [DOI] [PubMed] [Google Scholar]
- 44.Mettouchi A, Cabon F, Montreau N, et al. SPARC and thrombospondin genes are repressed by the c-jun oncogene in rat embryo fibroblasts. The EMBO journal. 1994;13:5668–78. doi: 10.1002/j.1460-2075.1994.tb06905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giancotti FG, Ruoslahti E. Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of chinese hamster ovary cells. Cell. 1990;60:849–59. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 46.White DE, Kurpios NA, Zuo D, et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–60. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 48.Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF- β1 to induce endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2006;103:17260–5. doi: 10.1073/pnas.0605556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwak C, Jin RJ, Lee C, Park MS, Lee SE. Thrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int. 2002;89:303–9. doi: 10.1046/j.1464-4096.2001.01417.x. [DOI] [PubMed] [Google Scholar]
- 50.Lewis JM, Truong TN, Schwartz MA. Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc Natl Acad Sci USA. 2002;99:3627–32. doi: 10.1073/pnas.062698499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–4. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 52.Grossfeld GD, Ginsberg DA, Stein JP, et al. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst. 1997;89:219–27. doi: 10.1093/jnci/89.3.219. [DOI] [PubMed] [Google Scholar]
- 53.Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells. 2004;17:188–202. [PubMed] [Google Scholar]
- 54.Fornaro M, Manzotti M, Tallini G, et al. β1C integrin in epithelial cells correlates with a nonproliferative phenotype: forced expression of β1C inhibits prostate epithelial cell proliferation. Am J Pathol. 1998;153:1079–87. doi: 10.1016/s0002-9440(10)65652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.