Abstract
A multisite, randomized trial within the National Drug Abuse Treatment Clinical Trials Network (CTN) was conducted to test three interventions to enhance treatment initiation following detoxification: 1) a single session, therapeutic alliance intervention (TA) added to usual treatment, 2) a 2- session, counseling and education, HIV/HCV risk reduction intervention (C&E), added to usual treatment and 3) treatment as usual (TAU) only. Injection drug users (n = 632) enrolled in residential detoxification at 8 community treatment programs were randomized to 1 of the 3 study conditions. There was a significant difference between TA participants and those receiving TAU in reported outpatient treatment entry. TA participants reported entering outpatient treatment sooner and in greater numbers than TAU participants. Reported treatment entry for C&E fell between TA and TAU with no significant differences between C&E and the other conditions. There were no differences among the interventions in retention, as measured by weeks of outpatient treatment for all participants who reported treatment entry. Alliance building interventions appear to be effective in facilitating transfer from detoxification to outpatient treatment, but additional treatment engagement interventions may be necessary to improve retention.
Keywords: detoxification, treatment entry, therapeutic alliance
Substance abuse treatment reduces drug use and associated HIV/HCV risk behaviors among drug dependent individuals (Gerstein et al., 1994; Hubbard, Craddock, Flynn, Anderson & Etheridge, 1997; Hubbard et al., 1989; Sells & Simpson, 1976; Simpson, Joe & Brown, 1997; Sorenson & Copeland, 2000). Most patients leaving detoxification report plans to enter some form of treatment (Tuten, Jones, Lertch & Stitzer, 2007). Research consistently shows, however, that many do not follow through. Lundgren, Sullivan and Amodeo (2006), for example, analyzed data for injection drug users with multiple treatment admissions in Massachusetts from 1997 through 2001; the most common treatment pattern (30%) was repeated admissions to detoxification only. Kleinman, Millery, Scimeca and Polissar (2002) reported that 43% of heroin and cocaine addicted patients received no treatment in the 30 days following detoxification. Similarly, Chutuape, Jasinski, Fingerhood and Stitzer (2001) followed patients for six months following a 3-day, inpatient detoxification for opiates; most (59%) had no formal treatment during the follow-up period. Only 26% of patients in a 21-day inpatient publicly-funded detoxification center transferred to residential or outpatient treatment following discharge (McCusker, Bigelow, Luippold, Zorn & Lewis, 1995). Finally, national hospital data suggest that the percentage of patients receiving inpatient or residential treatment following detoxification dropped from 38.9% to 21.1% between 1992 and 1997 (Mark, Dilonardo, Chalk &Coffey, 2002).
Patients who enter treatment following detoxification have consistently better outcomes. Heroin users engaged in formal treatment for a minimum of seven days during the six months following detoxification reported significantly reduced drug use compared to those with no treatment (Chutuape et al., 2001). Daley, Ageriou and McCarty (1998) followed pregnant and parenting women for six months after detoxification; women who participated in treatment during that period had lower rates of re-admission to detoxification. O'Farrell, Murphy, Alter and Fals-Stewart (2007) found that engaging in aftercare treatment within 30 days after detoxification was associated with significantly fewer days of substance use relative to no treatment.
Strategies to increase engagement in treatment following detoxification are needed in order to reduce resumption of drug use and associated HIV/HCV risk behavior. Studies that evaluate such interventions have shown mixed results. Positive results have been observed with interventions conducted during residential treatment, longer term detoxification and after detoxification. Lash and colleagues reported positive outcomes with behavioral contracting, attendance prompts and social reinforcers to promote participation in aftercare following 28-day residential treatment (Lash et al 2007; Lash, Burden, Monteleone & Lehmann, 2004; Lash, Petersen, O'Connor & Lehmann, 2001; Lash & Blosser, 1999). Rawson, Mann, Tennant and Clabough (1983) compared the use of counseling to standard treatment without counseling during a 21-day, ambulatory, methadone detoxification; those who participated in counseling were more likely to enter long term treatment following detoxification. In a non-experimental design, case management services provided for up to a year following detoxification resulted in a 55% reduction in detoxification only admissions and a 70% increase in treatment participation (McClellan, Weinstein, Shen, Kendig & Levine, 2005)
Longer term detoxification, however, is not the current standard of care. The average length of hospital detoxification stays decreased from 7.7 days in 1992 to 5.5 days in 1997 (Mark et al., 2002). Shorter detoxification stays require briefer interventions to facilitate treatment entry afterward. Brief intervention studies have shown mixed results. A three-session “pre-therapy training” using a role induction, treatment preparation intervention with patients in inpatient alcohol detoxification increased the number of patients who left with a treatment referral and made an initial treatment contact (Craigie & Ross, 1980). A three-session, strength focused psychotherapy intervention and a three-session video plus discussion group, however, were not superior to treatment as usual for heroin users (Millery, Kleinman, Polissar, Millman & Scimeca. 2002). The use of a staff escort plus an incentive of $13.00 significantly increased completion of an aftercare clinic intake following detoxification relative to standard referral and referral plus incentive (Chutuape, Katz & Stitzer, 2001). A pilot study of a brief family intervention found a trend toward greater treatment entry within 30 days of discharge from detoxification (O'Farrell et al, 2007). Rates of entry into formal treatment were no different for alcoholics who received motivational interviewing during 5-day detoxification, although there was a positive difference in 12-step attendance (Schilling, El-Bassel, Finch, Roman & Hanson, 2002). Given mixed findings, additional studies are needed to identify effective brief treatment engagement interventions suited to short stay detoxification services.
Therapeutic Alliance
A strong therapeutic alliance can increase session attendance (Fiorentine, Nakashima & Anglin, 1999; Simpson et al., 1997), treatment retention (Connors, Carroll, DiClemente, Longabaugh, & Donovan, 1997; De Weert-Van Oene, Schippers, De Jong, & Schrijvers, 2001; Meier, Donmall, McElduff, Barrowclough, & Heller, 2006; Mohl, Martinez, Ticknor, Huang, M. & Cordell, 1991) and outcomes (Luborsky, McLellan, Woody, O'Brien, & Auerbach 1985; Connors et al., 1997) for substance abuse clients. Early development of a therapeutic alliance consistently predicts engagement and retention in substance abuse treatment (Meier, Barrowclough & Donmall, 2005). Although numerous studies have measured the naturally developing therapeutic alliance, few prior studies have evaluated interventions designed to facilitate its development.
The present study tested the effectiveness of three conditions, including a single session intervention focused on the development of a therapeutic alliance between outpatient counselors and patients in short term, residential detoxification, in effect serving as an interpersonal “bridge” between treatment settings.
Method
Study Design
This randomized, multisite clinical trial conducted within the National Institute on Drug Abuse Clinical Trials Network (CTN) tested the impact of the Therapeutic Alliance Intervention (TA) and the Counseling and Education Intervention (C&E) (Coyle, 1993) on reducing HIV/HCV risk behaviors among injection drug users in residential detoxification and on improving treatment participation after detoxification. Both interventions were added to Treatment as Usual (TAU) and compared to TAU only.
The C&E intervention targeted the study's primary aim, reducing risk behavior and, secondarily, encouraged treatment participation. The study's risk reduction outcomes are reported elsewhere (Booth et al., 2008). The TA intervention targeted treatment entry and was expected to produce the highest levels of outpatient treatment entry and retention during a six month follow up period. Specifically, the study hypothesized that participants in the TA arm of the study would: 1) enter outpatient treatment sooner, 2) enter outpatient treatment in greater numbers, and 3) have more weeks of outpatient treatment over the follow-up period.
Clinical Sites
The study was conducted at eight residential detoxification centers participating in the CTN. Efforts were made to include a variety of community treatment sites to enhance generalizability of study results. Sites were situated across the U.S. in both urban and rural catchment areas. The size of the facilities ranged from 16 to 100 beds with usual length of stay ranging from 1.5 to 10 days. Table 1 summarizes site characteristics. Recruitment of participants occurred over an enrollment period of 16 months from November 2004 through February 2006.
Table 1.
Detoxification treatment site characteristics
| Usual Care |
|||||||
|---|---|---|---|---|---|---|---|
| Site | Number Randomized |
City Population (approximate) |
Number of Beds |
Usual Length of Stay (days) |
HIV Education/ Screening |
HIV Testing |
Treatment Referral |
| A | 101 | 92,000 | 17 | 4-6 | Yes | Yes | Yes |
| B | 101 | 26,000 | 40 | 6 | No | No | Yes |
| C | 70 | 571,800 | 100 | 1.5 | Yes | No | Yes |
| D | 39 | 86,500 | 23 | 2-4 | Yes | Yes | Yes |
| E | 132 | 575,900 | 36 | 3-6 | Yes | No | Yes |
| F | 62 | 72,700 | 29 | 7-10 | Yes | No | Yes |
| G | 39 | 82,100 | 16 | 3-6 | Yes | No | Yes |
| H | 88 | 153,700 | 17 | 4-6 | Yes | No | Yes |
Participants
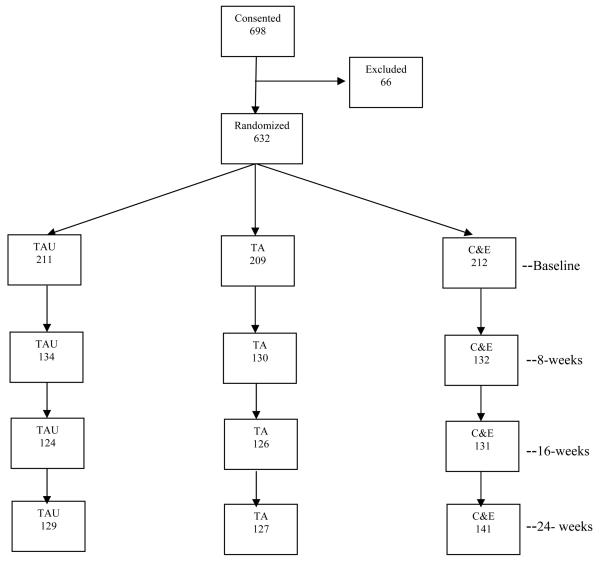
Study participants were injection drug users recruited during detoxification treatment, who were 18 years of age or older, eligible for outpatient services, had a recent history of injection drug use, and had not previously consented to participate in the study. Patients (n = 698) were evaluated for eligibility following completion of informed consent (see Figure 1) and 632 were randomized: TA = 209, C&E = 212, and TAU = 211. Most of the individuals not randomized did not meet study eligibility requirements (n = 59); the others either declined participation or left the program prior to randomization. The most common reasons for study exclusion were patients' reporting no drug injection in the prior 30 days (n = 13) and patients' requesting residential treatment (n = 15).
Figure 1.
Consort diagram of consent, randomization, baseline assessment and follow-up rates.
TAU= treatment as usual; TA = therapeutic alliance; C&E = counseling and education
Follow up assessments were conducted at 2, 8, 16 and 24 weeks. Data collection windows initially extended from one week prior to one week following the scheduled follow-up date. Windows were expanded, however, and completed follow ups were credited to the closest assessment period. Follow up rates were 70% at 2 weeks, 63% at 8 weeks, 60 % at 16 weeks and 63% at 24 weeks. The rates varied slightly by study condition. A consort diagram (Figure 1) includes the number of participants completing follow up assessments for each study condition.
Procedures
Staff members in the detoxification units presented brief descriptions of the study and study information sheets to potential participants. Patients who expressed interest generally met study eligibility and were cleared by detoxification staff as medically stable, were introduced to study personnel, who explained the study in more detail. Potential study participants read or had the consent read to them and were encouraged to ask questions. A study specific true/false quiz was administered to participants. Missed items were reviewed until understood and a 100% score achieved. After passing the quiz, participants signed the consent and received a copy. No study procedures were performed prior to the completion of informed consent. Eligible participants were randomized to one of the three study conditions following completion of the baseline assessment.
Study procedures were reviewed and approved by a Data Safety and Monitoring Board at the National Institute on Drug Abuse. Institutional review boards at the University of Colorado Health Sciences Center and participating treatment centers also reviewed and approved the research protocol.
Randomization
Following completion of baseline assessment, participants were randomly assigned to one of the three intervention conditions. A centrally administered, blocked randomization scheme was used to assign participants to one of the three conditions within each site. Blocked randomization guaranteed minimal imbalance in the number of participants assigned to each condition over time.
Data Collection Instruments
The full set of instruments used in the study included the CTN Common Assessment Battery (Addiction Severity Index- Lite, Composite International Diagnostic Interview Version 2.1-Substance Use Diagnosis), Urine Drug Screen test strips, Self-Efficacy Questionnaire for safer drug and sex behaviors, Services Received Questionnaire and a Locator Form. The measures used in this analysis were extracted from four instruments:
1) Timeline Follow-back Assessment of Treatment Behavior (TLFB) modeled after Sobell and Sobell's (1996) Timeline Follow-back Calendar. This self-report instrument measured days of any substance abuse treatment attendance, including outpatient, inpatient, residential, methadone maintenance/other opiate replacement, and 12-step meetings, since the last assessment. The TLFB assessment method has been found to be a reliable and valid measure of alcohol consumption (Sobell & Sobell, 1995; Sobell, Sobell, Leo & Cancilla, 1988), as well as other behavior including cocaine and heroin use (Ehrman & Robbins, 1994), adolescent smoking (Lewis-Esquerre et al, 2005,) panic attacks (Nelson & Clum, 2002) housing history (Tsemberis, McHugo, Williams, Hanrahan & Stefancic, 2007) and risky sexual behavior (Carey et al., 2001; Weinhart et al., 1998).
2) HIV Risk Behavior Survey (RBS) used to measure HIV and HCV risk behaviors in the areas of drug use and sex within the previous 30 days. This assessment used the Audio Computer Assisted Self Interview (ACASI) method (Needle et al., 1995; Weatherby et al., 1994). The self-conducted survey includes questions about injection drug use, sharing needles/syringes without disinfection, sexual activities, health, sexually transmitted diseases, and HCV/HIV. Reliability and validity assessments of the RBS support its use in research with injection drug users (Weatherby et al., 1994; Needle et al., 1995).
3) Demographics Questionnaire developed for use by the CTN to assess age, ethnicity/race, and gender. This was administered at baseline only.
4) Stage of Change Questionnaire (SOC) for quitting drug use, a modification of the Motivation Scales, including Drug Use Problems, Desire for Help, and Treatment Readiness from the data instruments developed by Simpson, Joe, Broome et al.(1997) for the Drug Abuse Treatment, Assessment, and Research Project. The SOC has excellent predictive validity relative to treatment entry (Booth, Kwiatkowski, Iguchi, Pinto & John, 1998) and its test-retest reliability is 90%. SOC stages are: maintenance, action, determination (or preparation), contemplation, pre-contemplation, and unstageable (i.e., responses were invalid).
Interventions
Three interventions were compared in this study:
Therapeutic Alliance Intervention (TA)
The intervention incorporated core elements of the therapeutic alliance (Bordin, 1979): 1) agreement about treatment tasks and roles, 2) agreement about the goals and expectations of treatment and 3) the positive bond between client and counselor. These elements were combined with role induction, a treatment preparation intervention designed to educate clients about what to expect in the treatment process. Studies suggest that role induction increases attendance in mental health (Walitzer, Dermen, & Conners, 1999) and substance abuse treatment (Craigie & Ross, 1980; Katz et al., 2007; Stark & Kane, 1985; Verinis, 1996). The TA intervention combined role induction's cognitive focus on treatment preparation with a relational focus on mutual agreement about treatment goals and the development of a positive bond between patient and counselor. Engagement in outpatient services was chosen as the goal of the TA because many community programs, including those that participated in the study, have immediate availability in outpatient treatment, in contrast to waiting lists for residential treatment.
The TA intervention was a 45-50 minute, single session conducted by an outpatient counselor affiliated with the outpatient treatment arm of same agency as the detoxification unit. The manualized intervention included discussion of: a) plans after detoxification b) life goals and possible treatment goals, c) developing confidence in goal attainment, d) prior treatment experiences and treatment expectations, e) what happens in treatment and f) common treatment challenges and how to handle them. Throughout the discussion, the counselor emphasized positive feedback, clarification to increase realistic understanding of treatment, mutual agreement about treatment tasks and goals, and working together as a team. When participants reported during the intervention that they would be leaving the area after detoxification, the TA counselor acted as a “stand in” for potential counselors at other programs, flexibly addressing goals, tasks and teamwork applicable to outpatient treatment in general. The session ended with the opportunity for the participant to make an appointment to see the outpatient counselor or to receive information about other treatment options.
Counseling and Education Intervention (C&E)
The C&E intervention was a manualized, individual HIV risk prevention model developed in NIDA's Cooperative Agreement (Coyle, 1993). It was designed to reduce risk behavior primarily through education, and, secondarily, by encouraging treatment participation. There was no alliance facilitating component in this intervention. The intervention consisted of two HIV/HCV risk reduction education and counseling sessions that structurally bracket and encourage confidential HIV and HCV screening. The decision to be tested was left up to the individual and the content of the intervention sessions flexibly accommodated those who declined to be tested, as well as those who tested either seropositive or seronegative. At the conclusion of the initial counseling session, participants were offered free HIV and HCV testing. For those who chose testing, test results were discussed at the beginning of the second session which was scheduled for two weeks later. The second session repeated education from the first session, and employed rehearsal of correct bleach and condom use. Alternatives to high-risk behaviors were stressed, including drug treatment, discontinuation of injection drug use and sex without protection, elimination of sharing drug equipment or the drug solution and reduction in the number of sex partners.
Treatment as Usual (TAU)
Treatment as usual varied by site and included each detoxification program's standard HIV/HCV risk assessment and testing practices as well as referrals for treatment after discharge. All sites reported that standard treatment included treatment referral; seven reported HIV screening and education; two reported on site testing (See Table 1).
Staff Training and Certification
Fifty staff members selected by the eight participating clinics were trained as interventionists and intervention supervisors. Interventionists were trained to deliver one of the two experimental interventions. Formal education for interventionists ranged from associates degrees to masters' level. Supervisors' education ranged from bachelors' degrees to a medical degree and years of experience in mental health or substance abuse treatment ranged from 6 months to 25 years. Interventionists and supervisors were trained at a 3-day centralized training by intervention experts. Following completion of the centralized training, certification was conducted at local sites for fidelity raters and interventionists using instruments developed for each intervention that rated intervention elements for completeness (adherence) and quality (competence) on 5-point scales.
Supervisors were certified as fidelity raters by achieving an 80% ratings' correspondence (i.e., within +1 and −1 on 80% of the 5-point rating scale items) with an intervention expert's rating of an audio taped session prepared by the protocol team. All supervisors met proficiency standards as fidelity raters after rating no more than two tapes.
Both interventionists and supervisors were certified as interventionists via achievement of a criterion score of 80% of items scored satisfactory or above on the fidelity ratings instrument while conducting an audio taped intervention with a treatment client or a role playing actor. Interventionist ratings were conducted by site supervisors who had been certified as raters. Experts conducted ratings for supervisors to become certified as interventionists. Most staff (49 of 50) were certified following intervention training.
Fidelity Monitoring
Fidelity monitoring included ratings of audio taped sessions and biweekly, group and/or individual supervision with local supervisors. Supervisors also met monthly with intervention experts via teleconference. Approximately 42% (n =159) of the taped sessions were randomly selected for rating by supervisors. The same score (i.e., 80% of items scored satisfactory or above) was used for initial and ongoing interventionist certification. Two interventionists lost certification after falling below the minimum score. Following retraining, they achieved recertification and were reinstated. One third of supervisor rated tapes were randomly selected for co-rating by an intervention expert. All supervisors maintained proficiency standards for ongoing certification as raters.
Data analysis
The dependent variables were self-reported treatment entry (i.e., the first treatment visit of a particular treatment type reported by participants) and dates of treatment services from the Timeline Follow-back Assessment of Treatment Behavior. Reported treatment dates were analyzed using two different methods. First, the time to entering a particular treatment was examined using the product-limit survival analysis method to take into account participants' different lengths of follow up observation due to loss to follow up. This descriptive method was supplemented by the proportional hazards regression model for multivariate analyses. Several variables were evaluated for inclusion as covariates to adjust for group differences that may not have been balanced by randomization: age, race, gender, drug type (any heroin use past 30 days, yes/no; any cocaine or amphetamine use past 30 days, yes/no), severity of baseline injection (days of reported injection past 30 days), and stage of change (preparation, contemplation/precontemplation, or un-staged) at baseline. Study site was also included in multivariate analysis to examine possible interaction with intervention condition. Variables related to outpatient treatment entry (p < .10) were included in analysis of covariance. The analysis was performed using SAS software, Version 9.1 (SAS Institute, Inc., Cary, NC, 2004). Weeks of treatment were examined using the ratio estimation method based on the number of person-months of follow-up after reported treatment entry. This analysis was conducted using the RATIO procedure in SUDAAN software, Version 9.0.1 (Research Triangle Institute, Research Triangle Park, NC, 2005).
Results
Study Participants
Table 2 shows the age, gender, ethnicity, racial composition and other participant characteristics at baseline according to intervention group. Participants averaged 36 years of age with a range from 19 to 65. Approximately 24% were female, 8% were African American, 10% were multi-racial and 9% reported Latino or Hispanic ethnicity. Overall, 82% of participants scored in the preparation stage for quitting drug use, and 14% were in pre-contemplation or contemplation stage at baseline. Over 80% reported injecting heroin within the past 30 days, nearly 60% reported stimulant (i.e., amphetamine or cocaine) injection, and 38% reported injecting “speedballs”, a combination of heroin and cocaine.
Table 2.
Participant characteristics at baseline by intervention group
| Characteristics | TAU a (n=211) |
TA b (n=209) |
C&E c (n=212) |
Total (n=632) |
|---|---|---|---|---|
| Age: | ||||
| Mean age (years) | 35.6 | 36.3 | 35.7 | 35.9 |
| (Range) | (19 - 62) | (19 - 61) | (19 - 65) | (19 - 65) |
| Gender: | ||||
| Female (%) | 26.5 | 23.4 | 23.1 | 24.4 |
| Race: | ||||
| White/Caucasian (%) | 73.9 | 69.4 | 77.4 | 73.4 |
| African American (%) | 9.0 | 10.1 | 5.2 | 8.1 |
| Multi-Racial (%) | 9.0 | 11.5 | 8.5 | 9.7 |
| Others (%) | 8.1 | 9.0 | 8.9 | 8.8 |
| Ethnicity: | ||||
| Hispanic/Latino (%) | 10.9 | 9.1 | 7.6 | 9.2 |
| Stage of Change: | ||||
| Preparation (%) | 84.4 | 79.0 | 81.1 | 81.5 |
| Pre-contemplation/Contemplation (%) | 10.0 | 18.7 | 13.2 | 13.9 |
| Unstageable (%) | 5.6 | 2.3 | 5.3 | 4.6 |
| Drug Use Past 30 Days: | ||||
| Heroin Use (%) | 81.3 | 83.9 | 77.1 | 80.7 |
| Stimulant Use (%) | 61.4 | 56.7 | 61.1 | 59.7 |
| Other Opiates (%) | 54.5 | 52.3 | 52.7 | 53.2 |
| Speedball Use (%) | 39.6 | 33.8 | 40.4 | 37.9 |
Treatment as Usual
Therapeutic Alliance
Counseling and Education
Treatment Entry
Table 3 summarizes the cumulative frequencies and probabilities of reported, first time treatment entry across months during the follow up period. The probability of initiating a treatment was estimated at each treatment entry point taking into account dropouts that occurred prior to that point. Because of dropout, cumulative frequency cannot be directly compared across experimental groups. Probability of treatment entry at each time point allows such a comparison. Four types of treatment are reported: 1) outpatient, 2) methadone maintenance/other opiate replacement treatment, 3) residential/inpatient, and 4) 12-step meetings. Participants reporting entry into more than one treatment type are counted for each type they reported. The total number of participants reporting treatment entry (i.e., at least one treatment date) during the six month follow up period is listed for each treatment type under “Month 6” in Table 3.
Table 3.
Cumulative frequency and probability* of entering treatment by month's end for treatment type and intervention group
| Treatment | Group |
Month 1 No. (prob.) |
Month 2 No. (prob.) |
Month 4 No. (prob.) |
Month 6 No. (prob.) |
|---|---|---|---|---|---|
| Outpatient Treatment: | |||||
| TAU | 25 (0.138) | 37 (0.214) | 46 (0.279) | 50 (0.311) | |
| TA | 37 (0.212) | 49 (0.290) | 60 (0.375) | 66 (0.455) | |
| C&E | 38 (0.206) | 46 (0.254) | 61 (0.351) | 63 (0.373) | |
| Methadone Maintenance: | |||||
| TAU | 9 (0.065) | 12 (0.088) | 20 (0.148) | 22 (0.163) | |
| TA | 7 (0.055) | 14 (0.110) | 25 (0.181) | 25 (0.198) | |
| C&E | 8 (0.056) | 15 (0.106) | 24 (0.169) | 29 (0.205) | |
| Residential / Inpatient: | |||||
| TAU | 28 (0.199) | 37 (0.264) | 46 (0.330) | 47 (0.337) | |
| TA | 29 (0.203) | 33 (0.233) | 42 (0.300) | 46 (0.330) | |
| C&E | 32 (0.204) | 37 (0.238) | 45 (0.291) | 47 (0.305) | |
| 12-Step Meetings: | |||||
| TAU | 66 (0.419) | 82 (0.535) | 97 (0.593) | 97 (0.645) | |
| TA | 73 (0.473) | 88 (0.580) | 102 (0.680) | 107 (0.742) | |
| C&E | 67 (0.394) | 81 (0.483) | 92 (0.554) | 101 (0.633) | |
Estimated by the product-limit survival analysis method
Outpatient Treatment Entry
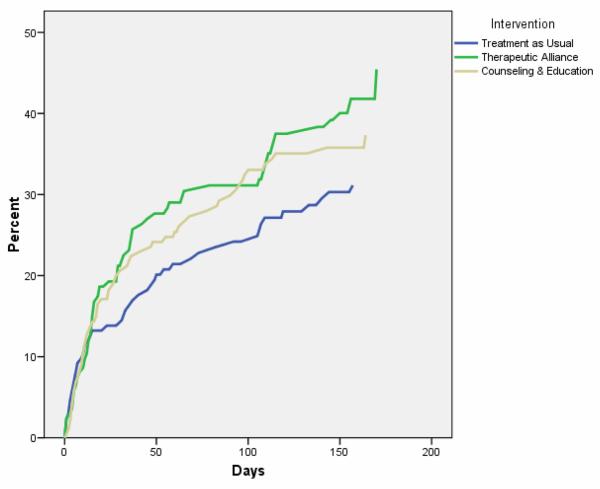
Differences among the three treatment conditions in cumulative probability of entering outpatient treatment are shown graphically in Figure 2. The treatment entry curves for TA and C&E are consistently above TAU throughout the follow-up period, suggesting that the participants in the two intervention groups were more likely to report at least one outpatient treatment visit and to enter care sooner than those in TAU.
Figure 2.
Cumulative probability of entering outpatient treatment by intervention group estimated by the product-limit survival analysis method.
Five potential covariates influenced outpatient treatment entry patterns: gender, stage of change at baseline, heroin use in the 30 days prior to baseline, stimulant use in the 30 days prior to baseline, and clinical sites. See Table 4 for covariates. Women reported entering outpatient treatment sooner than men. Participants scoring in the preparation stage of change at baseline were nearly twice as likely to enter outpatient treatment within 6 months compared to contemplators and pre-contemplators (41.7% vs. 22.1%). Heroin users and stimulant nonusers were more likely to enter outpatient treatment than their counterparts. Finally, the clinical sites variable exerted widely varying effects on outpatient treatment entry.
Table 4.
Cumulative probability* of entering outpatient treatment by month's end for selected covariates
| Covariates | Month 1 | Month 2 | Month 4 | Month 6 | Log-rank test |
|---|---|---|---|---|---|
| Gender: | |||||
| Male (n=478) | 0.182 | 0.245 | 0.324 | 0.350 | χ2 = 2.85 |
| Female (n=154) | 0.198 | 0.281 | 0.371 | 0.466 | p = 0.09 |
| Stage of Change: | |||||
| Preparation (n=515) | 0.201 | 0.273 | 0.368 | 0.417 |
χ2 = 10.79 |
| Pre-contemplation/Contemplation (n=88) | 0.147 | 0.204 | 0.204 | 0.221 | p = 0.005 |
| Un-staged (n=29) | 0.040 | 0.040 | 0.147 | 0.147 | |
| Heroin Use: | |||||
| Users (n=503) | 0.197 | 0.280 | 0.363 | 0.411 | χ2 = 7.26 |
| Non-Users (n=120) | 0.132 | 0.132 | 0.215 | 0.242 | p = 0.007 |
| Stimulant Use: | |||||
| Users (n=372) | 0.188 | 0.245 | 0.297 | 0.329 | χ2 = 2.81 |
| Non-users (n=251) | 0.185 | 0.260 | 0.388 | 0.447 | p = 0.09 |
| Clinical Sitea | |||||
| A | 0.383 | 0.475 | 0.624 | 0.717 | χ2=84.65 |
| B | 0.210 | 0.291 | 0.429 | 0.429 | p < 0.001 |
| C | 0.064 | 0.064 | 0.064 | 0.064 | |
| D | 0.112 | 0.112 | 0.189 | 0.189 | |
| E | 0.081 | 0.245 | 0.312 | 0.410 | |
| F | 0.344 | 0.365 | 0.460 | 0.488 | |
| G | 0.238 | 0.305 | 0.378 | 0.451 | |
| H | 0.097 | 0.097 | 0.130 | 0.150 |
See Table 1 for clinical site sample sizes.
Estimated by the product-limit survival analysis method
Multivariate analysis was conducted using the proportional hazards model as summarized in Table 5. Two way comparisons of outpatient treatment entry were performed between the intervention groups and TAU. Model 1 shows unadjusted group differences. There was a trend for TA participants to report treatment entry sooner and in greater numbers than those in TAU (X2 = 3.44, p< .06).
Table 5.
Comparisons of intervention group differences in entering outpatient treatment adjusted for selected covariates
| Model 1a | Model 2b | Model 3c | |||||
|---|---|---|---|---|---|---|---|
| Comparison | Covariate | Chi-Sq | p-value | Chi-Sq | p-value | Chi-Sq | p-value |
| TAU vs. TA: | |||||||
| Intervention Group | 3.44 | 0.064 | 4.96 | 0.026 | 5.21 | 0.023 | |
| Gender | -- | -- | 3.32 | 0.069 | 2.90 | 0.089 | |
| Stage of Change | -- | -- | 8.97 | 0.003 | 7.03 | 0.008 | |
| Heroin Use | -- | -- | -- | -- | 0.14 | 0.713 | |
| Stimulant Use | -- | -- | -- | -- | 0.20 | 0.657 | |
| Clinical Site | -- | -- | -- | -- | 17.29 | <0.001 | |
| TAU vs. C&E: | |||||||
| Intervention Group | 1.04 | 0.309 | 1.23 | 0.268 | 1.84 | 0.175 | |
| Gender | -- | -- | 1.12 | 0.291 | 0.60 | 0.437 | |
| Stage of Change | -- | -- | 4.21 | 0.040 | 2.33 | 0.127 | |
| Heroin Use | -- | -- | -- | -- | 0.30 | 0.585 | |
| Stimulant Use | -- | -- | -- | -- | 0.13 | 0.714 | |
| Clinical Site | -- | -- | -- | -- | 11.94 | 0.001 | |
| C&E vs. TA: | |||||||
| Intervention Group | 0.75 | 0.387 | 1.01 | 0.315 | 0.55 | 0.457 | |
| Gender | -- | -- | 0.74 | 0.388 | 1.62 | 0.203 | |
| Stage of Change | -- | -- | 7.14 | 0.008 | 4.62 | 0.032 | |
| Heroin Use | -- | -- | -- | -- | 1.13 | 0.288 | |
| Stimulant Use | -- | -- | -- | -- | 0.89 | 0.345 | |
| Clinical Site | -- | -- | -- | -- | 15.94 | <0.001 | |
Unadjusted
Adjusted for gender and stage of change using proportional hazards model
Adjusted for gender, stage of change, heroin use, stimulant use, and clinical site using proportional hazards model
Models 2 and 3 adjust group differences for covariates. Model 2 adjusted for gender and stage of change at baseline and indicates a significant difference between TA and TAU (X2 = 4.96, p<.026). Model 3 included five covariates: gender, stage of change, heroin use, stimulant use and sites. The TA vs. TAU difference remained significant. Differences between TA and C&E were not significant. It is important to note that the intervention effect was not changed by the introduction of the sites variable, suggesting that randomization controlled site effects. However, the site variable altered the influence of the substance use variables (heroin and stimulant) so that they were no longer significant. These variables may have been confounded with the sites variable.
Entry into Other Treatment Types
Group differences in entry into other treatments (methadone maintenance/other opiate replacement, residential/inpatient, and 12-step meetings) were not as prominent. Only one significant relationship was observed; TA participants compared to C&E participants were more likely to report attending at least one 12-step meeting (X2 = 4.554, p<.05).
Treatment Retention
Treatment retention was examined for participants who reported entering care, taking into account person-months of follow up after treatment entry. See Table 6 for the number of weeks during which at least one treatment visit was reported per person-month of follow up after entering treatment. TAU participants who reported beginning outpatient treatment had 3.2 weeks of outpatient treatment per person-month of follow up, TA participants reported 2.64 weeks and C&E participants reported 2.98 weeks. There is no evidence that intervention group affected retention in care.
Table 6.
Number of weeks of treatment per person-month of follow-up by intervention group (95% confidence intervals in parentheses)
| Treatment | TAU | TA | C&E | Total |
|---|---|---|---|---|
| Outpatient Treatment | 3.20 (2.76, 3.65) |
2.64 (2.31, 2.98) |
2.98 (2.52, 3.43) |
2.92 (2.69, 3.16) |
| Methadone Maintenance | 4.22 (4.14, 4.30) |
3.85 (3.51, 4.19) |
4.05 (3.78, 4.32) |
4.02 (3.86, 4.19) |
| Residential/Inpatient | 3.44 (3.09, 3.78) |
3.68 (3.43, 3.92) |
3.32 (2.95, 3.69) |
3.47 (3.28, 3.67) |
| 12-Step Meetings | 3.01 (2.72, 3.31) |
3.00 (2.72, 3.29) |
3.21 (2.91, 3.51) |
3.07 (2.90, 3.24) |
Discussion
Results supported the hypothesis that a therapeutic alliance intervention conducted by outpatient counselors would increase outpatient treatment entry. Participants who received the TA intervention reported entering treatment sooner and in greater numbers than individuals who received treatment as usual. The success of the TA intervention in facilitating treatment entry is consistent with a body of research indicating that positive therapeutic alliance increases treatment engagement (Meier et al., 2005). Although its “active ingredients” cannot be determined by the study's design, some components of the TA intervention are similar to intervention components in two other studies with positive results in treatment facilitation after detoxification. The first (Craigie & Ross, 1980 ) used a “pre-therapy training” role induction in inpatient alcohol detoxification to promote initial post detoxification treatment contact. The TA intervention included education about what to expect in treatment, a role induction component which is also a core component of the alliance. The TA intervention also involved direct contact with an outpatient counselor who conducted the intervention. Chuatape et al., (2001) used direct contact with a staff escort to assist nurses and patients prepare for discharge and accompany patients on a bus to aftercare treatment. Perhaps the personal contact bridging the two treatment settings was impactful in both studies.
The expectation that the TA intervention would also lead to better treatment retention was not supported. There were no differences among groups in weeks of outpatient treatment for those who had begun treatment. The impact of the intervention was limited to facilitating first outpatient treatment contact. In retrospect, this is not surprising. Given the myriad of factors that have been found to contribute to treatment dropout (Stark, 1992) and extensive research on interventions designed to increase retention during various stages of treatment (Onken, Blaine & Boren, 1997), one session may have been an insufficient dose of “alliance facilitating behaviors” (Luborsky, Barber, Siqueland, McLellan, & Woody, 1997, p.238). As the study was designed, outpatient counselors who conducted the interventions planned to be available to continue working with patients who entered treatment at their clinics, but the study protocol covered the TA intervention during detoxification only. Patients who reported treatment entry may have seen different counselors once they entered treatment and may have entered different outpatient programs altogether. Thus, a positive relationship with a specific counselor may have been limited to the single TA session.
There are several limitations in study design. Foremost is reliance on self-report using a calendar recall method for treatment attendance. Records of actual treatment attendance would strengthen confidence in findings. Another limitation is the incomplete follow-up data from approximately one third of the participants. Our findings are limited to the cohort of participants who may have been functioning better than their counterparts who missed some or all follow-up assessments. Finally, the study did not collect a measure of therapeutic alliance following the interventions, thus cannot inform identification of the mechanism of action of the TA intervention with alliance measures.
Strengths of the study include its rigorous application of fidelity monitoring which assured adherence to the intervention that was intended for evaluation. Another strength is the applicability of findings to “real world” community treatment settings. The multisite study was conducted in a wide range of programs with a diverse clientele, from rural to urban, small to large, using regular staff members as interventionists. The outcome of improving outpatient treatment entry occurred across these heterogeneous sites. Although sites exerted a strong effect and had a wide range of outcomes in reported treatment entry, there was no site by treatment interaction, indicating overall effectiveness of the TA intervention relative to TAU at many different locations. Practicality is another benefit. This brief intervention should be relatively easy to incorporate into community treatment settings, particularly programs whose detoxification units and outpatient programs are in close proximity.
Future directions include replication of current findings in a study with treatment records documenting attendance. If findings are replicated, actual mechanisms of action for the TA intervention should be investigated, including the relationship between the intervention and the therapists who deliver it. In addition, TA treatment engagement interventions may be evaluated for applicability beyond detoxification settings. Staff feedback interviews conducted at sites indicated support for the TA intervention and interest in employing it during outpatient treatment entry and beyond. Other possibilities for using TA style interventions include recruiting addicts into treatment, and for patients transitioning from one form of treatment to another. At the clinical level, training counselors in specific alliance developing skills, such as those used in the TA intervention, has been strongly advocated (Luborsky et al, 1997; Newman, 1997). The findings of the current study support that position.
Acknowledgments
The study was conducted within the National Drug Abuse Treatment Clinical Trials Network and supported through cooperative agreements with the National Institute on Drug Abuse Northern New England Node (U10 DA15831), Great Lakes Node (U10 DA13710), Rocky Mountain Node (U10 DA13716), Oregon/Hawaii Node (U10 DA13036), and Pacific Northwest Node (U10 DA13714). Data analysis for this publication received assistance from the Biostatistics Shared Resource of Oregon Health and Science University and from the Oregon Clinical and Translational Research Institute (OCTRI; grant number UL1 RR024140 01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research).
We are grateful to the eight participating detoxification centers, their directors and staff: Stanley Street Treatment and Resources (SSTAR), Fall River, MA; Stanley Street Treatment and Resources, North Kingstown, RI; Jim Gilmore Jr. Community Healing Center, Kalamazoo, MI; Denver Health and Hospital Authority, Denver, CO; Island Grove Regional Treatment, Greeley, CO; Willamette Family Treatment Services, Eugene, OR, and Recovery Centers of King County in Seattle and Kent, WA.. We also thank Node Coordinators from each participating node, as well as Marilyn Macdonald and Suzell Klein who served as National Research Coordinators for this study.
Footnotes
The Therapeutic Alliance Treatment Manual can be downloaded from the CTN Dissemination Library at http://ctndisseminationlibrary.org/display/284.htm
References
- Booth RE, Kwiatkowski C, Iguchi MY, Pinto F, John D. Facilitating treatment entry among out-of-treatment injection drug users. Public Health Reports. 1998;113(Supplement 1):116–128. [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Mikulich-Gilbertson S, Thompson L, Fuller BE, Campbell BK, Klein, Dempsey C, Zammarelli L, Summers S, Bryant K, Jenkins L, Darton, Liepman M, Calsyn D, Paull N, Robinson J, Mandler R. HIV and HCV risk reduction interventions in drug detoxification and treatment settings. 2008 Manuscript in preparation. [Google Scholar]
- Bordin E. The generalizability of the psychoanalytic concept of the working alliance. Psychotherapy: Theory, Research, and Practice. 1979;16:252–260. [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gordon CM, Weinhardt LS. Assessing sexual risk behaviour with the Timeline Followback (TLFB) approach: Continued development and psychometric evaluation with psychiatric outpatients. International Journal of STD & AIDS. 2001;12(6):365–375. doi: 10.1258/0956462011923309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. American Journal of Drug and Alcohol Abuse. 2001;27(1):19–44. doi: 10.1081/ada-100103117. [DOI] [PubMed] [Google Scholar]
- Chutuape MA, Katz EC, Stitzer ML. Methods for enhancing transition of substance dependent patients from inpatient to outpatient treatment. Drug and Alcohol Dependence. 2001;61(2):137–143. doi: 10.1016/s0376-8716(00)00133-2. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Carroll KM, DiClemente CC, Longabaugh R, Donovan DM. The therapeutic alliance and its relationship to alcoholism treatment participation and outcome. Journal of Consulting and Clinical Psychology. 1997;65(4):588–598. doi: 10.1037//0022-006x.65.4.588. [DOI] [PubMed] [Google Scholar]
- Coyle SL, National Institute on Drug Abuse. Community Research Branch & NOVA Research Company (Bethesda Md.) The NIDA HIV counseling and education intervention model intervention manual. Rockville, MD: 1993. (On NIH publication: no. 93-3580). [Google Scholar]
- Craigie F, Ross S. The use of a videotape pretherapy training program to encourage treatment-seeking among alcohol detoxification patients. Behavior Therapy. 1980;11(2):141–147. [Google Scholar]
- Daley M, Argeriou M, McCarty D. Substance abuse treatment for pregnant women: a window of opportunity? Addictive Behaviors. 1998;23(2):239–249. doi: 10.1016/s0306-4603(97)00029-4. [DOI] [PubMed] [Google Scholar]
- De Weert-Van Oene GH, Schippers GM, De Jong CA, Schrijvers GJ. Retention in substance dependence treatment: the relevance of in-treatment factors. Journal of Substance Abuse Treatment. 2001;20(4):253–261. doi: 10.1016/s0740-5472(01)00160-x. discussion 263-254. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology. 1994;62:843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Fiorentine R, Nakashima J, Anglin MD. Client engagement in drug treatment. Journal of Substance Abuse Treatment. 1999;17(3):199–206. doi: 10.1016/s0740-5472(98)00076-2. [DOI] [PubMed] [Google Scholar]
- Gerstein DR, Johnson RB, Harwood HJ, Fountain D, Suter N, Malloy K. Evaluating recovery services: The California drug and alcohol treatment assessment (CALDATA) California Department of Alcohol and Drug Programs; Sacramento, CA: 1994. [Google Scholar]
- Hubbard RL, Craddock SG, Flynn PM, Anderson J, Etheridge RM. Overview of 1-year follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Psychology of Addictive Behaviors. 1997;11(1):261–278. [Google Scholar]
- Hubbard RL, Marsden ME, Rachal JV, Harwood HJ, Cavanaugh ER, Ginzberg HM. Drug abuse treatment: A national study of effectiveness. University of North Carolina Press; Chapel Hill, NC: 1989. [Google Scholar]
- Katz EC, Brown BS, Schwartz RP, King SD, Weintraub E, Barksdale W. Impact of role induction on long-term drug treatment outcomes. Journal of Addictive Diseases. 2007;26(2):81–90. doi: 10.1300/J069v26n02_10. [DOI] [PubMed] [Google Scholar]
- Kleinman BP, Millery M, Scimeca M, Polissar N. Predicting long-term treatment utilization among addicts entering detoxification: The contribution of help-seeking models. Journal of Drug Issues. 2002;32(1):209–230. [Google Scholar]
- Lash SJ, Stephens RS, Burden JL, Grambow SC, DeMarce JM, Jones ME, Lozano BE, Jeffreys AS, Fearer SA, Horner RD. Contracting, prompting, and reinforcing substance use disorder continuing care: A randomized clinical trial. Psychology of Addictive Behaviors. 2007;21(3):387–397. doi: 10.1037/0893-164X.21.3.387. [DOI] [PubMed] [Google Scholar]
- Lash SJ, Burden JL, Monteleone BR, Lehmann LP. Social reinforcement of substance abuse treatment aftercare participation: Impact on outcome. Addictive Behaviors. 2004;29(2):337–342. doi: 10.1016/j.addbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Lash SJ, Petersen GE, O'Connor EA, Lehmann LP. Social reinforcement of substance abuse aftercare group therapy attendance. Journal of Substance Abuse Treatment. 2001;20(1):3–8. doi: 10.1016/s0740-5472(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Lash SJ, Blosser SL. Increasing adherence to substance abuse aftercare group therapy. Journal of Substance Abuse Treatment. 1999;16(1):55–60. doi: 10.1016/s0740-5472(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, Monti PM. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug and Alcohol Dependence. 2005;79(1):33–43. doi: 10.1016/j.drugalcdep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Luborsky L, Barber JP, Siqueland L, McLellan AT, Woody G. Establishing a therapeutic alliance with substance abusers. NIDA Research Monograph. 1997;165:233–244. [PubMed] [Google Scholar]
- Luborsky L, McLellan AT, Woody GE, O'Brien CP, Auerbach A. Therapist success and its determinants. Archives of General Psychiatry. 1985;42(6):602–611. doi: 10.1001/archpsyc.1985.01790290084010. [DOI] [PubMed] [Google Scholar]
- Lundgren LM, Sullivan L, Amodeo M. Racial and ethnic differences in drug treatment entry of injection drug users in Massachusetts. Journal of Substance Abuse Treatment. 2006;21(3):145–153. doi: 10.1016/s0740-5472(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Mark TL, Dilonardo JD, Chalk M, Coffey RM. Trends in inpatient detoxification services. 1992-1997. Journal of Substance Abuse Treatment. 2002;23:253–260. doi: 10.1016/s0740-5472(02)00271-4. [DOI] [PubMed] [Google Scholar]
- McClellan AT, Weinstein RL, Shen Q, Kendig C, Levine M. Improving continuity of care in a public addiction treatment system with clinical case management. American Journal on Addictions. 2005;14(5):426–440. doi: 10.1080/10550490500247099. [DOI] [PubMed] [Google Scholar]
- McCusker J, Bigelow C, Luippold R, Zorn M, Lewis BF. Outcomes of a 21-day drug detoxification program: retention, transfer to further treatment, and HIV risk reduction. American Journal of Drug and Alcohol Abuse. 1995;21(1):1–16. doi: 10.3109/00952999509095225. [DOI] [PubMed] [Google Scholar]
- Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304–316. doi: 10.1111/j.1360-0443.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- Meier PS, Donmall MC, McElduff P, Barrowclough C, Heller RF. The role of the early therapeutic alliance in predicting drug treatment dropout. Drug and Alcohol Dependence. 2006;83(1):57–64. doi: 10.1016/j.drugalcdep.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Millery M, Kleinman BP, Polissar NL, Millman RB, Scimeca M. Detoxification as a gateway to long-term treatment: assessing two interventions. Journal of Substance Abuse Treatment. 2002;23(3):183–190. doi: 10.1016/s0740-5472(02)00246-5. [DOI] [PubMed] [Google Scholar]
- Mohl PC, Martinez D, Ticknor C, Huang M, Cordell L. Early dropouts from psychotherapy. Journal of Nervous and Mental Disease. 1991;179(8):478–481. doi: 10.1097/00005053-199108000-00005. [DOI] [PubMed] [Google Scholar]
- Needle RH, Fisher DG, Weatherby N, Chitwood D, Brown B, Cesari H, Booth R, Williams ML, Watters J, Andersen M, Braunstein M. Reliability of self-reported HIV risk behaviors of drug users. Psychology of Addictive Behaviors. 1995;9(4):242–250. [Google Scholar]
- Nelson WA, Clum GA. Assessment of Panic Frequency: Reliability and validity of a time-line follow-back method. Journal of Psychopathology and Behavioral Assessment. 2002;24(1):47–54. [Google Scholar]
- Newman CF. Establishing and maintaining a therapeutic alliance with substance abuse patients: a cognitive therapy approach. NIDA Research Monograph. 1997;165:181–206. [PubMed] [Google Scholar]
- O'Farrell TJ, Murphy M, Alter J, Fals-Stewart W. Brief family treatment intervention to promote aftercare among male substance abusing patients in inpatient detoxification: A quasi-experimental pilot study. Addictive Behaviors. 2007;32(8):1681–1691. doi: 10.1016/j.addbeh.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken LS, Blaine JD, Boren JJ. Treatment for drug addiction: it won't work if they don't receive it. NIDA Research Monograph. 1997;165:1–3. [PubMed] [Google Scholar]
- Rawson RA, Mann AJ, Tennant FS, Jr., Clabough D. Efficacy of psychotherapeutic counseling during 21-day ambulatory heroin detoxification. Drug and Alcohol Dependence. 1983;12(2):197–200. doi: 10.1016/0376-8716(83)90045-5. [DOI] [PubMed] [Google Scholar]
- Research Triangle Institute . SUDAAN (Release 9.0.1) [Computer Software] Research Triangle Institute; Research Triangle Park, NC: 2005. [Google Scholar]
- SAS Institute . SAS/STAT user's guide: version 9.1. SAS Institute Inc; Cary, NC: 2004. [Google Scholar]
- Schilling RF, El-Bassel N, Finch JB, Roman RR, Hanson M. Motivational Interviewing to encourage self-help participation following alcohol detoxification. Research on Social Work Practice. 2002;12(6):711–730. [Google Scholar]
- Sells SB, Simpson D. Effectiveness of drug abuse treatment. Ballinger; Cambridge, MA: 1976. [Google Scholar]
- Simpson D, Joe G, Broome K, Hiller M, Knight K, Rowan-Szal G. Program diversity and treatment retention rates in the Drug Abuse Treatment Outcome Study (DATOS) Psychology of Addictive Behaviors. 1997;11(1):279–293. [Google Scholar]
- Simpson D, Joe G, Brown B. Treatment retention and follow-up outcomes in the Drug Treatment Outcomes Study (DATOS) Psychology of Addictive Behaviors. 1997;11:294–307. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M, editors. Assessing alcohol problems: A guide for clinician and researchers. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1995. pp. 55–73. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback user's guide: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Ontario: 1996. [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sorenson J, Copeland A. Drug abuse treatment as an HIV prevention strategy: A review. Drug and Alcohol Dependence. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- Stark M. Dropping out of substance abuse treatment: A clinically oriented review. Clinical Psychology Review. 1992;12(1):93–116. [Google Scholar]
- Stark MJ, Kane BJ. General and specific psychotherapy role induction with substance-abusing clients. International Journal of the Addictions. 1985;20(8):1135–1141. doi: 10.3109/10826088509056355. [DOI] [PubMed] [Google Scholar]
- Tsemberis S, McHugo G, Williams V, Hanrahan P, Stefancic A. Measuring Homelessness and Residential Stability: The Residential Time-Line Follow-Back Inventory. Journal of Community Psychology. 2007;35(1):29–42. [Google Scholar]
- Tuten M, Jones HE, Lertch EW, Stitzer ML. Aftercare plans of inpatients undergoing detoxification. American Journal of Drug and Alcohol Abuse. 2007;33(4):547–555. doi: 10.1080/00952990701407454. [DOI] [PubMed] [Google Scholar]
- Verinis JS. The effect of an orientation-to-treatment group on the retention of alcoholics in outpatient treatment. Substance Use and Misuse. 1996;31(10):1423–1431. doi: 10.3109/10826089609063985. [DOI] [PubMed] [Google Scholar]
- Walitzer KS, Dermen KH, Connors GJ. Strategies for preparing clients for treatment. A review. Behavior Modification. 1999;23(1):129–151. doi: 10.1177/0145445599231006. [DOI] [PubMed] [Google Scholar]
- Weatherby N, Needle R, Cesari C, Booth RE, McCoy CB, Watters JK, Williams M, Chitwood D. Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street outreach. Evaluation and Program Planning. 1994;17(4):347–355. [Google Scholar]
- Weinhart LS, Carey MP, Maisto SA, Carey KB, Cohen MM, Wickramasinghe SM. Reliability of the Timeline Follow-Back sexual behavior interview. Annals of Behavioral Medicine. 1998;20(1):25–30. doi: 10.1007/BF02893805. [DOI] [PMC free article] [PubMed] [Google Scholar]