Abstract
High-risk human papillomavirus (H-HPV) infection is strongly linked to cervical neoplasia but its role in detecting glandular lesions is unclear. In the cervix, carbonic anhydrase IX (CA-IX) is expressed in cervical neoplasia, but rarely in the benign cervix. The diagnostic utility of these biomarkers was evaluated in women with a cytologic diagnosis of atypical glandular cells (AGC). H-HPV was detected using Hybrid Capture 2 (HC2) in liquid based cytology and CA-IX immunoreactivity was studied on conventional Pap smears. Of 403 patients, 111 (28%) were positive for significant cervical lesions (SCLs) including CIN2, CIN3, adenocarcinoma in situ or invasive carcinoma. CA-IX testing alone (n=403) had a sensitivity of 75%, 95%, or 65% for SCLs, significant glandular lesions (GLs) or squamous lesions (SLs), respectively, with a specificity of 88%, and a false negative rate (FNR defined as one minus negative predictive value) of 10%. Testing for H-HPV (n=122) had a sensitivity of 97%, 100%, or 96% for SCLs, GLs or SLs, respectively, with a specificity of 87%, and a FNRof 1%. The combination of CA-IX and H-HPV testing (n=122), collectively, had the same sensitivity, specificity and FNR for SCLs, GLs or SLs as H-HPV testing alone. The conclusions of this study are that both H-HPV and CA-IX testing are useful diagnostic markers for GLs. However, H-HPV testing is a better diagnostic marker for SLs. The combination of CA-IX with H-HPV testing does not improve the diagnostic accuracy for cervical neoplasia in women with AGC diagnosis over that of H-HPV testing alone.
Keywords: CA-IX, HPV, AGC diagnosis, cervix
INTRODUCTION
In the United States (U.S.), an estimated 11,070 new cases of cervical cancers will be diagnosed and 3,870 women will die from cervical cancer in 2008.1 Although a majority of these cases are squamous lesions, the proportion of adenocarcinomas relative to squamous cell carcinomas is increasing, and current cervical cytologic screening fails to detect a significant proportion of glandular lesions.2
Unlike squamous lesions, the cytologic criteria for identifying glandular neoplasia are not well established. To better classify these abnormalities, the term atypical glandular cells of undetermined significance (AGUS) was introduced in the 1988 Bethesda System (TBS). In 1991, AGUS was qualified according to the possible anatomic site of origin, endocervical versus endometrial.3 The 2001 TBS replaced AGUS with the term atypical glandular cells (AGC) and classified glandular cell abnormalities less severe than adenocarcinoma into three categories: AGC of unclear cell origin, atypical endocervical cells (AEC), and atypical endometrial cells (AEMC).4 However, in clinical practice, sub-categorization of the AGC, particularly in the category of AGC/AEC remains a diagnostic challenge with poor interobserver agreement.5 In patients with AGC, the rates of CIN2 or CIN3 (high grade squamous intraepithelial lesion or HSIL) and adenocarcinoma in situ (AIS) range from 5–50 % and 0–15 %, respectively, with rates of invasive carcinoma of up to 10 %.6–9 The Society of Gynecologic Oncologists has urged more aggressive evaluation of patients with a diagnosis of AGC because of the relatively high (mean, 41%) proportion of women who eventually are found to harbor high grade cervical neoplastic lesions.9
The clinical management of AGC is also limited by the relative lack of accuracy of colposcopy and endocervical curettage for excluding cervical glandular neoplasia. Significant lesions, including invasive carcinoma, may exist in patients after negative colposcopy and endocervical sampling.10 Although AGC diagnosis only represents a small fraction (approximately 0.5%) of total Pap smear diagnoses, its association with significant underlying cervical lesions has posed a particular dilemma in clinical management, both from a cost-benefit standpoint and from a desire to avoid unnecessary invasive procedures.11,12 An accurate screening method or test is needed to determine which women with a cytologic diagnosis of AGC harbor a significant cervical lesion.
In the 1990s, the antigen MN was identified.13 MN is a transmembrane glycoprotein, is a member of the carbonic anhydrase gene family, and has been given the designation, carbonic anhydrase IX (CA-IX).14 CA-IX is a biomarker of several human tumors, including carcinomas of the cervix and kidney.15,16 CA-IX expression in cancerous tissues, and its absence in normal counterparts, has led to the speculation that it plays a role in carcinogenesis.17 Its expression is controlled by the transcription factor, hypoxia inducible factor-1 (HIF-1), and is up-regulated in hypoxic regions of tumor tissues. CA-IX expression has been associated with a poor prognosis in cervical and breast cancers.18,19
In a survey of benign and neoplastic cervical tissues and Pap smears, it was observed that virtually all AGC associated with AIS and adenocarcinoma expressed high levels of CA-IX antigen whereas endocervical cells derived from benign cervical tissues were negative, suggesting that CA-IX protein would be a useful biomarker for diagnosing AIS and invasive adenocarcinoma.6,15,20
Infection with human papillomavirus (HPV) is an etiologic factor for both glandular and squamous cervical cancer. HPV has been identified in 80 to 90% of adenocarcinomas and their precursor lesions; however, there are only limited data regarding the role of HPV testing in the detection of glandular neoplasia.12,21–26
Thus, the Gynecologic Oncology Group (GOG), a national multi-institutional clinical trials group supported by the U.S. National Cancer Institute, conducted a study of women with a cytologic diagnosis of AGC. Twenty five institutions in the U.S. participated (identified in the Acknowledgements). The objective of this study, GOG protocol #171, was to determine if CA-IX expression in a conventional Pap smear is a diagnostic biomarker for a significant cervical lesion in women with a cytologic diagnosis of AGC, and to explore the diagnostic value of HPV testing alone or in combination with CA-IX.
Materials and methods
GOG protocol #171 was initiated in 1998 when the criteria of AGC diagnosis for patient enrollment was based on the 1991 TBS classification and the conventional study Pap smears were used. HPV testing in a liquid based cytology specimen was added as a study amendment in 2003 and the protocol was closed for accrual in 2005.
Patients
Women over the age of 18 years, with a referring diagnosis of AGC who were expected, on a clinical basis, to undergo complete histologic evaluation of the cervical transformation zone were enrolled. Patients with a history of endometrial hyperplasia and/or carcinoma of the uterine corpus, cervix and vagina; prior or concurrent chemotherapy and/or radiation to the uterine corpus, cervix and vagina; or HIV infection were excluded. Informed consent consistent with federal, state, and local requirements was obtained prior to enrollment. Prior to activation, the protocol was approved by the National Cancer Institute, Division of Cancer Prevention, and the GOG Human Research Committee, and annually by the Institutional Review Board (IRB) at each of the participating institutions.
Primary end point and clinical management
The primary end point of the study was complete histologic evaluation of the cervical transformation zone within six months of the initial cytologic diagnosis. H&E stained slides of the most abnormal lesions from each diagnostic procedure were reviewed centrally by teams of two pathologists from the GOG Pathology Committee, who reached a consensus diagnosis. Disparities were arbitrated by a third GOG pathologist. Evaluation of the entire transformation zone was required to make a negative diagnosis but not to make a positive diagnosis. A positive diagnosis, coded as a significant cervical lesion (SCL), reflects the presence of CIN2, CIN3, AIS or invasive carcinoma. A negative diagnosis represents the absence of SCLs and includes CIN1 and atypia. Atypia is defined as glandular and squamous lesions in which cellular atypia falls short of AIS and CIN1. The significant lesions were restricted to the cervix and there was no case of vaginal dysplasia/neoplasia without the coexisting cervical lesions identified in the study. Cases with neoplasia detected in tissues outside of the cervix, where the cervix was histologically confirmed as benign, were classified as negative.
Patients received colposcopic examination, cervical biopsy, endocervical curettage and/or an endometrial biopsy as clinically indicated, as well as either LEEP cone biopsy of the cervix with an endocervical curettage, a cold knife cone biopsy of the cervix with/without an endocervical curettage or a hysterectomy within six months of the initial cytologic diagnosis of AGC. An endometrial biopsy or curettage was obtained in all perimenopausal and postmenopausal women, as well as in all patients with a negative cone biopsy of the cervix. Patients with a negative diagnosis after the cervical cone biopsy but not undergoing a hysterectomy were to be followed by the relevant referring gynecologist or family physician during regular visits with routine Pap smear screening every six months for two years.
Pap smear and liquid-based cytology (LBC) specimens
A spray-fixed conventional study Pap smear was prepared before collecting a LBC (ThinPrep, Cytyc/Hologic, Marlborough, MA) specimen. Both specimens were collected using a spatula and cytobrush and were collected before surgical procedures were performed. Each Pap smear collected from all patients enrolled in the study was tested for CA-IX protein expression. All cases of immunostain were manually performed in Dr. Stanbridge’s Laboratory at the University of California, Irvine. The presence of high-risk HPV (H-HPV) DNA in a LBC specimen, collected from 2003, was detected using the Digene Hybrid Capture II (HC2) system (Digene Corp., Gaithersburg, MD) at the University of Oklahoma Health Sciences Center.
Detection of CA-IX in a conventional study Pap smear
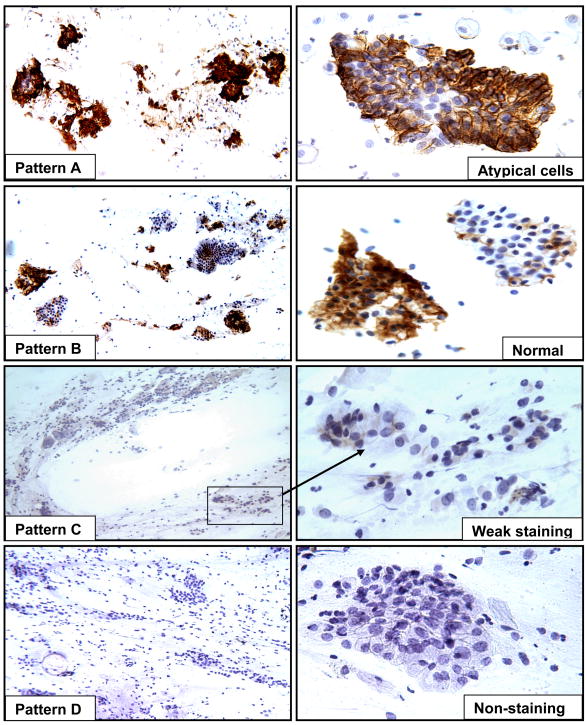
CA-IX testing was performed in conventional study Pap smears using the anti-CA-IX mouse monoclonal antibody, M75, as described previously.6,15,20 Specific immunohistochemical staining was defined by the presence of a brown reaction product on the plasma membrane under 40X magnification. Faint staining of the cytoplasm was considered negative. Cytologic criteria for atypical cells, delineated in the Bethesda System classification, were used in the diagnostic classification.27 Immunostaining was scored as positive (pattern A and B) and negative (pattern C and D) based on the staining intensity (strong versus weak/negative) and immunoreactive patterns (diffuse versus focal). Strong positivity was defined as when the dark brown immunoreactivity was easily identified at a low power magnification (4X or 10 X). The diffuse staining pattern was defined as when more than 50% of the atypical cells or normal endocervical cells in the smear showed immunoreactivity to CA-IX. The patterns A, B, C, and D were defined as: A) when individual atypical cells and/or cell clusters showed specific immunoreactivity, that was either diffuse or focal; B) when normal looking endocervical cells exhibited focal or diffuse strong specific positive staining; C) when the normal endocervical cells showed focal but weak staining and; D) when there was nonspecific faint cytoplasmic positivity or lack of staining observed at 40 × magnification (Fig. 1). A set of teaching smears with samples of known negative and positive CA-IX immunoreactivity was provided by one of authors (SYL). After the training session, the CA-IX immunostained smears were evaluated and the interpretation in each case was recorded independently by three cyto/gynecologic pathologists (SYL, WHR and TAB) without the knowledge of histologic diagnosis. Any case with a different interpretation of immunoreactive patterns (A, B, C and D) was considered to be a discrepant case. All discrepant cases were reviewed simultaneously by three study pathologists, using a multi-headed microscope. A consensus was obtained when at least two of the three study pathologists reached agreement. The results of the consensus were recorded as the final score for each patient in the study.
Figure 1.
The four scoring patterns of CA-IX immunoreactivity in Pap smears containing AGC. Patterns A and B: positive immunostaining in the atypical cells/cell clusters (A) or in the normal looking endocervical cells (B). Patterns C and D: weak positive (C, arrow) or no immunoreactivity (D) in normal cervical cells (original magnification on left panels ×100 and right panels ×400).
Detection of high-risk HPV DNA in liquid-based cytology (LBC) specimens
Each LBC specimen was prepared in 20 ml of the PreservCyt Solution (Cytyc/Hologic, Marlborough, MA) and the presence of H-HPV DNA in at least 4 ml of the LBC specimen was evaluated. The specimens were tested for the presence or absence of H-HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 with the Digene HC2 system, according to the manufacturer’s protocol. This test does not distinguish between the different H-HPV types.
PCR-based HPV genotyping
The majority of specimens tested for the presence or absence of H-HPV with the HC2 method were also genotyped using the Roche LINEAR ARRAY genotyping test according to the manufacturer’s directions. This test uses a combination of amplification of target DNA by the polymerase chain reaction (PCR) and nucleic acid hybridization, and has been designed to detect a total of 37 anogenital HPV DNA genotypes, including the same 13 H-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) as the HC2 method. In this case the method identifies the individual HPV type(s) present in each positive sample.28,29
Statistical methods
Statistical analyses were performed using Statistical Analysis System (SAS) version 9.1 (SAS Institute Inc., Cary NC). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), interpreted as the risk of a SCL among women who test negative for HPV and/or CA-IX, and overall accuracy were evaluated using the definition of false negative rate (FNR) as 1-NPV to reflect the proportion of negative diagnoses that were incorrect for women diagnosed by CA-IX or HPV status, individually or jointly, relative to histologic diagnosis. When used in combination, the following decision rule was employed: diagnose those women with positive CA-IX or positive HPV as having a SCL.
Results
GOG protocol #171 was initiated in 1998 when the criteria of AGC diagnosis for patient enrollment was based on the 1991 TBS classification and the conventional study Pap smears were used. HPV testing in a liquid based cytology specimen was added as a study amendment in 2003 to incorporate advances in scientific understanding and the clinical management of women with cervical lesions. The protocol was closed for accrual in 2005.
Between September 28, 1998 and October 10, 2005, 592 women with a cytologic diagnosis of AGC were enrolled in the study. One-hundred eighty-nine women were excluded from the study. The reasons for exclusions were as the follows: withdrew consent (n=3); lack of histological evaluation of the transformation zone (n=110); and unsatisfactory study Pap smears (n=76). The clinical characteristics and histologic diagnosis of the 403 evaluable women are presented in Table 1. The ages ranged from 20 to 86 with a median of 43 years old. The distribution by race and ethnicity is illustrated in Table 1.
TABLE 1.
Clinical Characteristics and Histology Diagnosis
| Patient Age |
Race and Ethnicity |
||||||
|---|---|---|---|---|---|---|---|
| Age | n=403 (%)* | n=122 (%)** | n=403 (%)* | ||||
| HPV (HC2) | Race | Ethnicity | |||||
| HPV (+) | HPV (−) | White | 268 (67) | Hispanic | 99 (25) | ||
| <=30 | 64 (16) | 8 (6.5) | 8 (6.5) | African American | 48 (12) | ||
| 31–40 | 102 (25) | 23 (19) | 13 (11) | Asian | 8 (2) | Non-Hispanic | 287 (71) |
| 41–50 | 115 (29) | 9 (7) | 31 (25) | American Indian | 1 | ||
| 51–60 | 72 (18) | 7 (6) | 15 (12) | Not-Specified | 78 (19) | Not-Specified | 17 (4) |
| 61–70 | 37 (9) | 1 (1) | 6 (5) | ||||
| >=71 | 13 (3) | 0 | 1 (1) | ||||
| Histologic Diagnosis n=403 (%)* | |||||
| Insignificant Cervical Lesions | Significant Cervical Lesions | ||||
| 292 (72) | 111 (28) | ||||
| Negative/Benign | 232 | Squamous Lesions | 74 (67) | Glandular Lesions. | 37 (33) |
| Negative, NOS | 156 | CIN2 | 14 | AdenoCa. In Situ | 23 |
| Metaplasia, NOS | 19 | Invasive AdenoCa. | 14 | ||
| Squamous Metaplasia | 53 | CIN3 | 58 | Adenoca. NOS | 7 |
| Immature Squamous Metaplasia | 4 | Endometrioid | 1 | ||
| Atypia† | 23 | Squamous cell Ca. | 2 | Clear cell | 1 |
| Glandular Hyperplasia | 5 | Small cell | 1 | ||
| CIN1/Mild Dysplasia | 32 | Adenosquamous | 2 | ||
| Villoglandular | 1 | ||||
| Serous | 1 | ||||
403 cases were tested for CA-IX expression.
Among 403 cases, 122 were tested for high-risk HPV. NOS: not otherwise specified;
including glandular and squamous lesions in which cellular atypia falls short of AIS and CIN1.
CIN2: moderate dysplasia; CIN3: severe dysplasia/in situ squamous cell carcinoma.
Table 1 also provides the distribution of benign and neoplastic cervical lesions observed in this cohort. Of 403 patients enrolled in the study, 111 (28%) women had a SCL, 74 (18%) had significant squamous lesions (SLs), and 37 (9%) had significant glandular lesions (GLs). Among those with a SCL (n=111), 67% were SLs, including CIN2 (n=14), CIN3 (n=58) and squamous cell carcinoma (n=2), and 33% were GLs, including AIS (n=23) and invasive adenocarcinoma (n=14). The incidence of invasive carcinoma in the study was 4%. There were five women whose malignancy was found outside of the cervix. These malignant tumors involved the endometrium (n=3), ovary (n=1) and fallopian tube (n=1). All of these women with extra-cervical malignancy were diagnosed during the evaluation of AGC, either by endometrial biopsy or abnormal physical and radiographic abnormalities. All five women received a total hysterectomy and bilateral salpingo-oophorectomy. The cervix of each of these women was histologically confirmed to lack evidence of a significant lesion. The study was designed to focus only on significant lesions of the cervix, thus, these cases were coded as negative for the purposes of the CA-IX and HPV analyses
Figure 1 illustrates the four CA-IX immunohistochemical staining patterns. Diffuse or focal CA-IX immunoreactivity in the atypical cells (pattern A) and focal strong or diffuse immunoreactivity in normal looking endocervical cells (pattern B) were classified as positive. CA-IX immunoreactivity that was focal and weak in normal endocervical cells (pattern C) or negative (pattern D) was classified as negative. Pattern A was easily discriminated from pattern C and D. The rate of agreement among any two of the three reviewers reached 97% on pattern A and 100% on pattern D before the arbitrating consensus review. Five cases were upgraded from pattern C to pattern A, and five cases from pattern C to pattern B, but only two cases were downgraded from pattern B to pattern C after the consensus review.
CA-IX testing
The intent of the initial study was to determine the accuracy of CA-IX expression exclusively as an indicator of the presence of a SCL. Thus, the data shown in the upper panel (#1) of Table 2 depict the results of CA-IX testing for all of the specimens (n=403). Positive staining for CA-IX protein expression was observed in 118 (29%) conventional study Pap smear specimens. Among these positive cases, 83 (70%) had a SCL, including 48 of 74 (65%) SLs, and 35 of 37 (95%) GLs. Among 35 insignificant cervical lesions with positive CA-IX immunoreactivity, two were CIN1, one was atypia and one was glandular hyperplasia. Thus, positive CA-IX immunoreactivity in a conventional Pap smear had an overall sensitivity and specificity of 75% and 88%, respectively with a FNR of 10%, for detecting a SCL. The sensitivity for CA-IX detection in GLs was 95% and in SLs was 65%. (Table 3).
TABLE 2.
Biomarker Test (CA-IX, HPV, and CA-IX + HPV) Result by Histologic Diagnosis
| Histologic Diagnosis |
|||||
|---|---|---|---|---|---|
| Biomarker Test | Insignificant Cervical Lesions | Squamous Lesions (SLs) | Glandular Lesions (GLs) | All SCLs (SLs + GLs) | |
| Total Number (#1) | 403 (%) | 292 (73) | 74 (18) | 37 (9) | 111 (27) |
| CA-IX | |||||
| Negative | 285 (71) | 257 (88) | 26 (35) | 2 (5) | 28 (25) |
| Positive | 118 (29) | 35 (12)* | 48 (65) | 35 (95) | 83 (75) |
| Total Number (#2) | 122 (%) | 84 (69) | 28 (23) | 10 (8) | 38 (31) |
| CA-IX | |||||
| Negative | 91 (75) | 78 (93) | 12 (43) | 1 (10) | 13 (34) |
| Positive | 31 (25) | 6 (7) | 16 (57) | 9 (90) | 25 (66) |
| HPV (HC2) | |||||
| Negative | 74 (61) | 73 (87) | 1 (4) | 0 | 1 (3) |
| Positive | 48 (39) | 11 (13)** | 27 (96) | 10 (100) | 37 (97) |
| CA-IX + HPV | |||||
| Negative | 68 (56) | 67 (80) | 1 (4) | 0 | 1 (3) |
| Positive | 54 (44) | 17 (20) | 27 (96) | 10 (100) | 37 (97) |
: All cases tested for CA-IX;
: Cases in which HPV (HC2) results were available.
Insignificant cervical lesions including negative/benign, CIN1, atypia and glandular hyperplasia; SCLs: significant cervical lesions; SLs: CIN2, CIN3 and squamous cell carcinoma; GLs: AIS and adenocarcinoma.
including 2 CIN1, 1 atypia, 1 hyperplasia;
including 4 CIN1, 1 atypia.
TABLE 3.
Diagnostic Accuracy of CA-IX, HPV, and CA-IX + HPV
| Sensitivity |
||||||||
|---|---|---|---|---|---|---|---|---|
| Numbers tested |
Biomarkers | Significant Cervical Lesions |
Squamous Lesions |
Glandular Lesions |
Specificity | Negative Predictive Value (NPV) |
Positive Predictive Value |
False Negative Rate (1-NPV)* |
| 403 | CA-IX | 0.75 | 0.65 | 0.95 | 0.88 | 0.90 | 0.70 | 0.10 |
| 122 | ||||||||
| CA-IX | 0.66 | 0.57 | 0.90 | 0.93 | 0.86 | 0.81 | 0.14 | |
| HPV (HC2) | 0.97 | 0.96 | 1.00 | 0.87 | 0.99 | 0.77 | 0.01 | |
| HPV (HC2) + CA-IX | 0.97 | 0.96 | 1.00 | 0.80 | 0.99 | 0.69 | 0.01 | |
FNR: defined as 1-NPV to reflect the proportion of negative diagnosis that were incorrect
A direct comparison between CA-IX expression and HPV detection was performed on those specimens where a LBC specimen was available for HPV analysis (n=122). For these cases, 31 (25%) were positive for CA-IX expression. Among these CA-IX positive cases, 25 (80%) had a SCL, including 16 of 28 (57%) SLs, and 9 of 10 (90%) GLs. Thus, the overall sensitivity of SCLs, SLs or GLs was 66%, 57% or 90% respectively; with a specificity of 93% and a FNR of 14%. The comparative analysis of CA-IX and HPV is given below and in Tables 2 and 3.
HPV detection
The HC2 method of HPV testing was performed on 122 cases. Patient ages ranged from 20 to 71, with a median age of 36 for the HPV positive group and 45 for the HPV negative group (Table 1). H-HPV DNA was detected in 48 (39%) of LBC specimens (Table 2, #2). Among these positive cases, 37 (77%) had a SCL, including 27 of 28 (96%) SLs and 10 of 10 (100%) GLs. This provided an overall sensitivity of 97%, 96% and 100% for SCLs, SLs and GLs, respectively; with a specificity of 87% and a FNRof 1% (Table 3). There were 11 cases in the insignificant lesion category and among these, four were diagnosed as CIN1 and one was atypia.
HPV genotyping
112 of 122 cases tested for the presence of HPV by the HC2 method were also processed for HPV genotyping, using the PCR-based Roche LINEAR ARRAY (RLA) kit. Sixty-five cases (58%) were positive for H-HPV. Among these positive cases, 36 (54%) had SCLs, including 26/27 (96%) SLs, and 10 of 10 (100%) GLs. Thus, the PCR-based HPV genotyping method for detecting SCLs gives an overall sensitivity and specificity of 97% and 61%, respectively, and a FNR of 2%. In terms of the rates of positive HPV detection, the PCR-based RLA and HC2 methods did show some differences, with the RLA method detecting more positives (39% versus 13%) in the insignificant lesion category and less (97% versus 100%) in the SCL category, respectively. The comparative analysis of HC2 testing and PCR-based RLA genotyping is given in Table 4.
TABLE 4.
The Comparison of Hybrid capture 2 (HC2) and PCR-based Roche Linear Array (RLA) HPV detection
| Histologic Diagnosis |
Sensitivity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV Test | Insignificant Cervical Lesions |
Squamous Lesions (SLs) |
Glandular Lesions (GLs) |
All SCLs (SLs + GLs) |
Significant Cervical Lesions |
Squamous Lesions (SLs) |
Glandular Lesions (GLs) |
Specificity | Positive Predictive Value |
False Negative Rate |
|
| HC2 | |||||||||||
| Total Number | 122 (%) | 84 (69) | 28 (23) | 10 (8) | 38 (31) | 0.97 | 0.96 | 1.00 | 0.87 | 0.81 | 0.01 |
| Negative | 74 (61) | 73 (87) | 1 (4) | 0 | 1 (3) | ||||||
| Positive | 48 (39) | 11 (13) | 27 (96) | 10 (100) | 37 (97) | ||||||
| PCR-RLA | |||||||||||
| Total Number | 112 (%) | 75 (67) | 27 (24) | 10 (9) | 37 (33) | 0.97 | 0.96 | 1.00 | 0.61 | 0.55 | 0.02 |
| Negative | 47 (42) | 46 (61) | 1 (4) | 0 | 1 (3) | ||||||
| Positive | 65 (58) | 29 (39) | 26 (96) | 10 (100) | 36 (97) | ||||||
Insignificant cervical lesions including negative/benign, CIN 1, atypia and glandular hyperplasia; SCLs: significant cervical lesions;
SLs: CIN2, CIN3 and squamous cell carcinoma; GLs: AIS and adenocarcinoma.
A compilation of the genotyping data revealed that approximately 49% of both benign lesions and SCLs contained multiple HPV types (Table 5). There was no apparent difference with respect to whether the lesion was positive or negative by the HC2 method of detection (data not shown). The distribution of the specific H-HPV types in the cervical lesions is shown in Table 6.
TABLE 5.
Detection of High Risk HPV types by PCR-Based Roche Linear Array Testing
| Histologic Diagnosis | Total Number of HPV Positive Cases n (%) | Multiple HPV Types (with or without low risk) | Single HPV type |
|---|---|---|---|
| Total Numbers (negative + positive lesions) | 65 (%) | 32 (49) | 33 (51) |
| Negative/Benign | 29 (45) | 13 (45) | 16 (55) |
| CIN2, CIN3 (Moderate/Severe Dysplasia/in-situ squamous carcinoma) | 25 (38) | 12 (48) | 13 (52) |
| Squamous Cell Carcinoma | 1 (1) | 0 | 1 (100) |
| Adenocarcinoma In-Situ | 5 (8) | 4 (80) | 1 (20) |
| Invasive Adenocarcinoma | 5 (8) | 3 (60) | 2 (40) |
Table 6.
High-Risk HPV Genotypes using the Roche Linear Array Kit
| Histologic Diagnosis/HPV type | 16 | 18 | 31 | 33 | 35 | 39 | 45 | 51 | 52 | 53 | 56 | 58 | 59 | 66 | 68 | 82 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative/Benign | 4 | 2 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | |||||||
| Glandular Hyperplasia | ||||||||||||||||
| Atypia | 1 | 1 | ||||||||||||||
| CIN1 (Mild dysplasia) | 1 | 1 | 1 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | |||||
| CIN2 (Moderate dysplasia) | 1 | 1 | 1 | 2 | 1 | 1 | ||||||||||
| CIN3 (Severe Dysplasia/in situ squamous Ca. | 8 | 1 | 4 | 2 | 1 | 3 | 1 | 1 | 1 | 4 | 2 | |||||
| Squamous cell Ca. | 1 | |||||||||||||||
| Adenocarcinoma In Situ | 1 | 3 | 1 | |||||||||||||
| Adenocarcinoma, NOS | 2 | 1 | 1 | 1 | 1 | |||||||||||
| Adenosquamous cell Ca. | 1 | |||||||||||||||
| Villoglandular AdenoCa. | 1 | 1 | ||||||||||||||
| Total Number | 17 | 11 | 10 | 3 | 5 | 5 | 5 | 3 | 6 | 2 | 8 | 4 | 2 | 1 | 0 | |
The combined accuracy of CA-IX and HPV testing
The data were also evaluated based on the combined CA-IX and HPV testing (HC2 method) performed in 122 cases. For the combination of CA-IX with HPV, a case was negative if there was a negative result for both CA-IX and HPV. If either or both were positive, the case was then called positive. The combined CA-IX and HPV testing had an overall sensitivity and specificity of 97% and 80% and a FNR of 1%. Details are given in Table 3.
Discussion
GOG protocol #171 is the first cooperative group-wide prospective cohort study of women with AGC diagnoses. The participants were enrolled from 25 medical institutions. In agreement with previous studies, a wide spectrum of benign and clinical significant lesions was identified in patients with a diagnosis of AGC enrolled in the study.6–9 The rate of significant uterine lesions in published studies of AGC has ranged from 17 to 80% (mean, 41%), with ranges of 0–34% (mean, 11%) with GLs, 5–43 % (mean, 17%) with SLs, and 0–23% (mean, 9%) with invasive carcinomas. Most of this latter category were of endocervical or endometrial origin.9 In the study described here, we found 28% of women had a SCL (CIN2, CIN3, AIS or invasive carcinoma), 18% had a SL and 9% had a GL. The overall rate of invasive carcinoma was 4%. Among the SCLs, 33% were GLs (AIS/adenocarcinomas) and 14% were invasive cervical carcinomas. These results are similar to published findings.6–9
With the exception of two adenocarcinomas, all AIS and conventional endocervical adenocarcinomas showed the strong and diffuse CA-IX immunoreactive pattern A, which is easily discriminated from the weak or negative immunostaining patterns under 4× or 10× magnification. The observations of diffuse positivity in cases of AIS and adenocarcinoma are identical to those previously reported, thus the current study confirms the diagnostic utility of CA-IX for glandular neoplasia of the cervix.15 Although CA-IX testing missed two adenocarcinomas, no abnormal cells were seen in the Pap smear (sampling error) in one case, and the other was a clear cell adenocarcinoma of the cervix, which does not express CA-IX.15 There were five cases in which malignancy was identified outside of the cervix, including endometrium (3), fallopian tube (1) and ovary (1) but in all of these cases the cervix was histologically confirmed to be benign. In each instance there were few exfoliated atypical cells in the smears, and all three carcinomas of the endometrium were diagnosed by endometrial biopsy. In the 2001 TBS these atypical cells would be classified as atypical endometrial cells. Furthermore, the goal of the cervical Pap screening program does not include identification of malignancies originating outside of the uterus.
H-HPV DNA has been strongly associated with SCLs; however, only limited, albeit promising, published data on HPV testing with AGC exist. In this study we detected H-HPV types in 39% of the 122 women enrolled, including 96% of those with CIN2, CIN3 or squamous cell carcinoma, and 100% with AIS and adenocarcinoma. Of the 122 cases tested by HC2, 112 were genotyped using the PCR-based RLA genotyping kit. The sensitivity of detection of H-HPV in SCLs is similar between the two methods and this also has been seen in other large population studies.28,30,31 However, significantly more cases with benign lesions were positive by the RLA method. Another interesting feature of the H-HPV analysis is that, in the positive cases, multiple HPV types were detected in both benign and SCLs at approximately equal frequencies to those that identified individual genotypes.
Our data suggest that, in the U.S., H-HPV testing is a useful biomarker for identifying SCLs in women with a cytologic diagnosis of AGC, and has a sensitivity of 97% and specificity of 87%, with a false negative rate of 1%. CA-IX expression is less sensitive than HPV in detecting SLs (65% versus 96%, respectively). The sensitivity of HPV for detection of GLs appears to be also better than CA-IX (100% [10/10] vs 95% [35/37]). However, the confidence intervals overlapped and the difference did not achieve statistical significance. This trial was not designed to detect such a difference.
A replacement protocol (GOG-237) has been activated, using LBC specimens, to evaluate the diagnostic accuracy of H-HPV DNA in combination with CA-IX, p16, mini-chromosome maintenance proteins (MCMs), and/or Ki67, for cervical dysplasia/neoplasia in women with a cytologic diagnosis of AGC. In this future study all experimental procedures, including HPV testing protocols, will be identical. The major goal of this study will be to determine whether the specificity and PPV of HPV testing can be improved by incorporating, any of these additional biomarkers into the diagnostic test, without sacrificing the excellent sensitivity observed in the current study for H-HPV alone.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the GOG Tissue Bank (CA 11479), and GOG Statistical and Data Center (CA 37517). As well as the funding from the Department of Obstetrics and Gynecology at University of Oklahoma Health Sciences Center (JLW), Department of Microbiology & Molecular Genetics at the University of California at Irvine School of Medicine (EJS) and a supplemental award from the Division of Center Prevention at the National Cancer Institute (GOG 38886). The Cytyc Company provided the free samples of PreservCyt medium in vials and none of the authors have conflicts to disclose. The following member institutions participated in this study: Abington Memorial Hospital, Walter Reed Army Medical Center, University of Mississippi Medical Center, University of California at Los Angeles, University of Pennsylvania Cancer Center, University of Cincinnati, University of Texas Southwestern Medical Center at Dallas, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, The Cleveland Clinic Foundation, SUNY at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, Fox Chase Cancer Center, Women’s Cancer Center – University of Nevada, University of Oklahoma, Tacoma General Hospital, Tampa Bay Cancer Consortium, Gynecologic Oncology Network/Brody School of Medicine, Ellis Fischel Cancer Center, Fletcher Allen Health Care, University of Wisconsin Hospital, University of Texas-Galveston.
The authors thank Kim Blaser and Anne Reardon for their assistance in preparing this manuscript for publication, Jan Barnes and Amy Speaker for coordinating the clinical and pathology data for this protocol, and to David Brown and Terry Dunn at the University of Oklahoma Health Sciences Center for performing the HC2 and PCR-based RLA in the U.S. cohort. A special acknowledgement is also extended to the clinic staff at the various GOG institutions for their efforts in providing conventional Pap smears, the liquid-based cytology specimens and the stained and unstained tissue sections for this study. Finally, we thank the GOG Publications Subcommittee for their critical review of the manuscript and for their helpful recommendations.
Abbreviations
- GOG
Gynecologic Oncology Group
- AGC
atypical glandular cells
- LBC
liquid-based cytology
- SCL
significant cervical lesion
- SLs
significant squamous lesions
- GLs
significant glandular lesions
- U.S
United States
- AGUS
atypical glandular cells of undetermined significance
- CA-IX
carbonic anhydrase IX
- TBS
The Bethesda System
- AEC
atypical endocervical cells
- AEMC
atypical endometrial cells
- AIS
adenocarcinoma in-situ
- HSIL
high grade squamous intraepithelial lesion
- H-HPV
high-risk human papillomavirus
- IRB
Institutional Review Board
- SAS
Statistical Analysis System
- HC2
Hybrid Capture II
- RLA
Roche Linear Array
- PPV
positive predictive value
- NPV
negative predictive value
- FNR
false negative rate defined as 1-NPV to reflect the proportion of negative diagnoses that were incorrect
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. Ca Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States-a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous The Bethesda System for reporting cervical/vaginal cytologic diagnosis: revised after the second National Cancer Institute Workshop, April 29–30, 1991. Acta Cytol. 1993;37:115–124. [PubMed] [Google Scholar]
- 4.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Jr, Young N Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda system- Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 5.Lee KR, Darragh TM, Joste NE, Krane JF, Sherman ME, Hurley LB, Allred EM, Manos MM. Atypical glandular cells of undetermined significance (AGUS): Interobserver reproducibility in cervical smears and corresponding thin-layer preparations. Am J Clin Pathol. 2002;117:96–102. doi: 10.1309/HL0B-C7Y6-AC77-ND2U. [DOI] [PubMed] [Google Scholar]
- 6.Liao SY, Stanbridge EJ. Expression of MN/CA9 protein in Papanicolaou smears containing atypical glandular cells of undetermined significance is a diagnostic biomarker of cervical dysplasia and neoplasia. Cancer. 2000;88:1108–21. doi: 10.1002/(sici)1097-0142(20000301)88:5<1108::aid-cncr23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AW, Salmieri SS, Wirth SL, Biscotti CV, Tuason LJ, Travarca MJ. Results of the clinical evaluation of atypical glandular cells of undetermined significance (AGCUS) detected on cervical cytology screening. Gynecol Oncol. 1996;3:14–8. doi: 10.1006/gyno.1996.0270. [DOI] [PubMed] [Google Scholar]
- 8.Veljovich DS, Stoler MH, Andersen WA, Covell JL, Rice LW. Atypical glandular cells of undetermined significance: a five-year retrospective histopathologic study. Am J Obstet Gynecol. 1998;179:382–90. doi: 10.1016/s0002-9378(98)70368-0. [DOI] [PubMed] [Google Scholar]
- 9.Cangiarella JF, Chhieng DC. Atypical glandular cells-an update. Diagn Cytopathol. 2003;29:271–9. doi: 10.1002/dc.10316. [DOI] [PubMed] [Google Scholar]
- 10.Andersen ES, Arffmann E. Adenocarcinoma in situ of the uterine cervix: A clinico-pathologic study of 36 cases. Gynecol Oncol. 1989;35:1–7. doi: 10.1016/0090-8258(89)90001-2. [DOI] [PubMed] [Google Scholar]
- 11.Hecht JL, Sheets EE, Lee KR. Atypical glandular cells of undetermined significance (AGUS) in conventional cervical/vaginal smears and thin-layer preparations. Cancer. 2002;96:1–4. [PubMed] [Google Scholar]
- 12.Ronnett BM, Manos MM, Ransley JE, Fetterman BJ, Kinney WK, Hurley LB, Ngai JS, Kurman RJ, Sherman ME. Atypical glandular cells of undetermined significance (AGUS): Cytopathologic features, histopathologic results, and human papillomavirus DNA detection. Hum Pathol. 1999;30:816–25. doi: 10.1016/s0046-8177(99)90143-0. [DOI] [PubMed] [Google Scholar]
- 13.Zavada J, Zavadova Z, Pastorekova S, Ciampor F, Pastorek J, Zelnik V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–74. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 14.Pastorek J, Pastorekova S, Callebaut I, Mornon JP, Zelnik V, Opavský R, Zat’ovicová M, Liao S, Portetelle D, Stanbridge EJ. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–88. [PubMed] [Google Scholar]
- 15.Liao SY, Brewer C, Zavada J, Pastorek J, Pastorekova S, Manetta A, Berman ML, DiSaia PJ, Stanbridge EJ. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinoma. Am J Pathol. 1994;145:598–609. [PMC free article] [PubMed] [Google Scholar]
- 16.Liao SY, Aurelio ON, Jan K, Závada J, Stanbridge EJ. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–31. [PubMed] [Google Scholar]
- 17.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miaqkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–19. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–8. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 19.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM. Carbonic anhydrase(CA-IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–9. [PubMed] [Google Scholar]
- 20.Liao SY, Stanbridge EJ. Expression of the MN antigen in cervical Papanicolaou smears is an early diagnostic biomarker of cervical dysplasia. Cancer Epidemiol Biomarkers Prev. 1996;5:549–57. [PubMed] [Google Scholar]
- 21.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. Epidemiology and natural history of human papillomavirus infections and type specific implications in cervical neoplasia. Vaccine. 2008;26S:K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol. 2000;157:1055–62. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ ASCCP-Sponsored Consensus Conference. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–9. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 24.Krane JF, Lee KR, Sun D, Yuan L, Crum CP. Atypical glandular cells of undetermined significance: Outcome predictions based on human papillomavirus testing. Am J Clin Pathol. 2004;121:87–92. doi: 10.1309/N7KC-UP0V-D59G-DJEL. [DOI] [PubMed] [Google Scholar]
- 25.Irvin W, Evans SR, Andersen W, Jazaeri A, Taylor P, Stoler M, Pastore L, Rice L. The utility of HPV triage in the management of cytological AGC. Am J Obstet Gynecol. 2005;193:559–67. doi: 10.1016/j.ajog.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Saqi A, Gupta PK, Erroll M, Babiac A, Blackmun D, Mansukhani M, Vazquez M. High-Risk Human Papillomavirus DNA testing: A marker for Atypical Glandular Cells. Diag Cytopathol. 2006;34:235–9. doi: 10.1002/dc.20369. [DOI] [PubMed] [Google Scholar]
- 27.Kurman RJ, Solomon D. The Bethesda System: Terminology for reporting cervical/vaginal cytologic diagnoses: definitions, criteria and explanatory notes for terminology and specimen adequacy. New York: Springer Verlag; 1994. [Google Scholar]
- 28.Gravitt PE, Schiffman M, Solomon D, Wheeler CM, Castle PE. A comparison of Linear Array and Hybrid Capture 2 for detection of carcinogenic human papillomaviruses and cervical pre-cancer in ASCUS-LSIL Triage Study. Cancer Epidemiol Biomarkers Prev. 2008;17:1248–54. doi: 10.1158/1055-9965.EPI-07-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castle PE, Sadorra M, Garcia F, Holladay EB, Kornegay J. Pilot study of a commercialized human papillomavirus (HPV) genotyping assay: comparison of HPV risk group to cytology and histology. J Clin Microbiol. 2006;44:3915–7. doi: 10.1128/JCM.01305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki H, Sasagawa T, Basha W, Segawa T, Inoue M. Hybrid capture-II and LCR-E7 PCR assays for HPV typing in cervical cytologic samples. Int J Cancer. 2001;94:222–7. doi: 10.1002/ijc.1455. [DOI] [PubMed] [Google Scholar]
- 31.Inoue M, Sakaguchi J, Sasagawa T, Tango M. The evaluation of human papillomavirus DNA testing in primary screening for cervical lesions in a large Japanese population. Int J Gynecol Cancer. 2006;16:1007–13. doi: 10.1111/j.1525-1438.2006.00460.x. [DOI] [PubMed] [Google Scholar]