Abstract
It is likely that the soil environment serves as a stable reservoir of infectious CWD and scrapie prions, as well as a potential reservoir of BSE. Prion adsorption to soil could play an important role in prion mobility, proteolysis, and infectivity. We modified previously published methods to quantify adsorbed prions via direct detection and studied prion adsorption to soil and soil minerals as a function of time through 60 days. Prion-infected brain homogenate was used as a complex, relevant prion source. We determined that maximum PrP adsorption requires days or weeks, depending on the soil or mineral, and is two to five orders of magnitude lower than previous studies using purified PrPSc or recPrP. Because PrP adsorption to soil is slow and reduced in tissue homogenate, the possibility of prion transport in soil environments cannot be excluded and requires further investigation. Our results indicate that binding to soil may protect prions from degradation, consistent with prions’ longevity in the environment. Adsorption of PrP to sterilized soil did not differ significantly from adsorption to unsterilized soil, which suggests that active biological processes do not significantly affect prion adsorption or degradation in the soil environment.
Introduction
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are fatal neurodegenerative diseases impacting a number of mammals, including cattle (bovine spongiform encephalopathy, BSE, or ‘mad cow’ disease), sheep and goats (scrapie), deer, elk, and moose (chronic wasting disease, CWD), and humans (Creutzfeldt-Jakob disease, CJD, and others) (1). Strong evidence indicates that the prion agent is solely comprised of PrPSc, an abnormally-folded isoform of a normal cellular protein, PrPc (1, 2)..
Environmental factors may be important in the transmission of animal prion diseases. Scrapie and CWD are horizontally transmissible and remain infectious after years in the environment (3–6). The CWD and scrapie agent is shed from living hosts and present in mortalities (7). It is likely that the environment serves as a stable reservoir of infectious CWD and scrapie prions, facilitating a sustained incidence of CWD in free-ranging cervid populations and complicating efforts to eliminate disease in captive livestock herds (7). In addition, the disposal of mortalities during BSE outbreaks, both in the past and potential future disposal events, serves as another environmental source of prions with the potential to infect humans.
CWD and scrapie are orally transmissible (8, 9), and the nasal cavity is also an effective route of infection (10, 11). Oral transmission studies demonstrate that soil bound prions remain infectious (12, 13). The potential for CWD or scrapie transmission by exposure to contaminated soil is possible since cervids and ruminants are known to ingest and inhale large amounts of soil (14, 15). Thus, soil and soil minerals may act as environmental reservoirs of prion infectivity.
Prion adsorption has generally been observed to be strong and fast for a wide range of surfaces, and both PrPc and PrPSc appear to have an affinity for quartz sands and soils and a particularly strong affinity for clay minerals (16–20). Prion sorption is strongly irreversible and resistant to desorption by detergent and chaotropic treatments (21, 22). Studies using recombinant PrP (recPrP) and purified PrPSc have observed maximal adsorption in less than 2 hours (17, 22, 23). However, almost all prion sorption studies have used recPrP or purified PrPSc as the source of infectious material (7). These systems do not take into account the competitive matrix (animal tissue, blood, saliva, or excreta) in which prions will enter the environment. Competition for sorption sites and interactions on mineral surfaces between PrP and other proteins, polysaccharides, lipids, nucleic acids, and ions could alter PrP adsorption. This heterogeneity can be more accurately modeled by using homogenized or intact infectious tissue.
Prion adsorption can be indirectly measured by using detergents and boiling to desorb prions into solution where they are detected by immunoblotting techniques (7). Detergent and boiling extraction methods typically have very low PrP recoveries (5–40%), presumably due to the strong and near-irreversible binding of PrP to soil particles (16, 24). In addition, it is likely that desorption selects for a certain PrP population, such as loosely-bound aggregates. A direct detection method has been developed by Genovesi and colleagues which bypasses the need to desorb and instead directly detects PrP bound to soil via immunological methods (25). While their method was not quantitative, we have modified it and used it in conjunction with a recently developed 96-well immunoassay (26) to quantify soil-bound prions.
Here we show proof-of concept for this method and apply it to quantify prion adsorption to soil and soil minerals as a function of time. The objective of this study was to obtain kinetic data of PrP adsorption using a complex and relevant prion model, infected brain homogenate, and compare these data to previous studies using recPrP or purified PrPSc. We used both sterile and nonsterile soil to determine the effect of active biological processes on prion adsorption. We found that maximum prion adsorption requires days or weeks, depending on the soil or mineral, and is two to five orders of magnitude lower than previously reported.
Materials and Methods
PrP Sources
Experiments were conducted using brain homogenate from hamsters infected with the HY TME (hyper strain of transmissible mink encephalopathy (TME)) agent at terminal disease. (27). All procedures involving animals were done in accordance with the Guide for the Care and Use of Laboratory Animals (29). Brain tissue was homogenized to 10% (w/v) in Dulbecco’s phosphate-buffered saline (DPBS) without Ca++ or Mg++ (Mediatech, Herndon, VA) using a Tenbroeck tissue grinder (Kontes, Vineland, NJ).
Batch Sorption Assays
Fine white sand (particle size <0.25mm, Fisher Scientific, Pittsburgh, PA), sodium bentonite (CETCO, Arlington Heights, IL), and two whole soils, a sandy loam and a silty clay loam, were used to study prion sorption. See Tables S1 and S2 for soil characteristics. Minerals and soils were gamma (γ) irradiated with 26 kGy to sterilize soil as needed (Co-60, JS 10000 MDS Nordion, Ottawa, Canada). Brain homogenate was combined with either 10 mg fine sand, 3 mg sandy loam, 1 mg silty clay loam or 0.5 mg bentonite for a total volume of 200 μl of DPBS in 0.2ml PCR tubes (Fisher Scientific). All experiments were conducted in triplicate at pH 7 (buffered in DPBS). Soil-homogenate mixes were rotated at 24 rpm (Mini Labroller, Labnet, Edison, NJ) at 22°C. Samples were removed at specified time points and allowed to settle or centrifuged at 100 g for 5 sec. The supernatant was removed and the pellets were washed 2–3 times with DPBS. The original supernatant, first wash, and final pellet were collected and stored at −80°C until analysis. Subsequent washes do not contain measurable PrP (data not shown).
Direct Detection Assay
Aqueous controls and samples were examined using a 96-well immunoblot assay without modification (26). Soil pellets were examined in parallel using the direct detection method developed by Genovesi et al. (26) with modifications. First, pellets were saturated (30 min, 22°C) with bovine serum albumin (BSA, 200 μl, 5% in DPBS, Sigma Aldrich, St. Louis, MO). Soil pellets were then incubated (10 min, 22°C) with 3M guanidinium thiocyanate (100 μl, Sigma Aldrich), and then washed 2 times with 200 μl DPBS. Soil pellets were then incubated (30 min, 37°C) with blotto (200 μl, 5% in tris buffer solution (TBS), Biorad, Hercules, CA,) and primary antibody (150 μl TBS with 0.1% BSA and 5% blotto, 1 hr, 37°C). Samples were immunoblotted with anti-PrP mAb 3F4 (1:10,000 dilution, Chemicon, Temecula, CA). Pellets were washed 2 times with DPBS, incubated (100 μl TBS with 0.1% BSA and 5% blotto, 30 min, 37°C) with secondary antibody (horseradish-peroxidase conjugated anti-mouse IgG, 1:2000, Pierce, Rockford, IL), and washed 2 times with DPBS. Low speed centrifugation (100 g for 5 sec) was used to remove all reagents, antibody solutions, and DPBS washes from soil pellets. Pellets were loaded into the 96 well-plate (0.45 μm PVDF membrane, Pall Corp., Ann Arbor, MI) and developed with the aqueous controls and samples. Pellets were diluted if necessary to have the resulting signal within the linear range of the brain homogenate dilution standard. Well-plate membranes (with pellets added) were developed with Pierce Supersignal West Femto maximum-sensitivity substrate, according to the manufacturer’s instructions (Pierce, Rockford, IL), and imaged on a Kodak 2000R imaging station (Kodak, Rochester, NY).
Quantification of PrP
Images were analyzed using Kodak 1D 4.0 software (Kodak, Rochester, NY), which output the net intensity of each blot. For each plate, linear regressions were generated from triplicate control dilutions. Because the absolute amount of PrP in any given tissue homogenate is indeterminate, sample quantities are reported in μg brain tissue equivalents (BE), where 1 μg BE equals 1 μg starting hamster brain tissue. See Figure S1 for a typical linear regression and the Supplemental Methods Discussion for further details on PrP quantification.
Results and Discussion
Prion adsorption quantified by direct detection
We have combined the antibody-based direct detection method developed by Genovesi et al. (25) with the 96-well immunoassay method developed by Kramer and Bartz (26) to quantify PrP adsorbed to soil and minerals. The original Genovesi et al. (25) method was qualitative; therefore, a control dilution curve of brain homogenate was generated by the 96-well method (Figures 1A and S1) and used to quantify the adsorbed solid PrP concentration. We do not distinguish between PrPc and PrPSc, as we found that the PK digestion step used by Genovesi et al. does not yield consistent, quantifiable results (data not shown). An important negative control, soil not exposed to brain homogenate, confirms that the antibodies used do not bind to soil (Figure 1B–E and 25). A mock pellet, brain homogenate without soil, was negative, as was a soil pellet not exposed to primary antibody (Figure 1A). It must be considered when interpreting results that PrP conformational changes or changes in PrP-soil orientation could change the availability and/or affinity of the antibody epitope. However, the general accuracy of antibody detection for quantification of protein adsorption has been confirmed by other methods such as radiolabeling (29). See the Supplemental Methods Discussion for further details and discussion of method limitations.
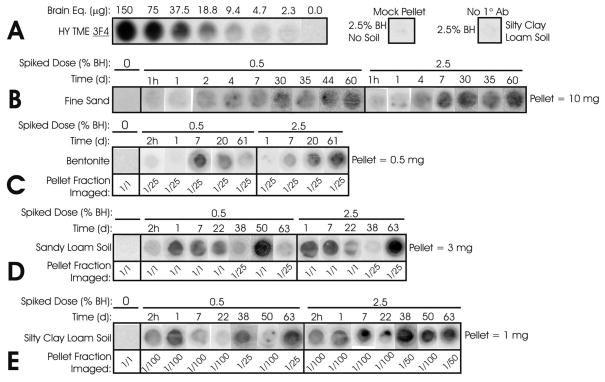
Figure 1.
Direct Detection of HY TME PrP Adsorbed to Soil and Soil Minerals. All blots detected by mAb 3F4. Results representative of at least three replicates. A: Two-fold control dilution of HY brain homogenate and negative controls. Silty clay loam soil pellet diluted 1/25. B: Fine quartz sand results. 200 μL HY brain homogenate (0.5 or 2.5% dose with 1.0% = 10 μg brain equivalents/μL) was equilibrated with 10 mg sand. C: Bentonite results. 200 μL BH was equilibrated with 0.5 mg bentonite. Pellets were diluted as noted for imaging to assure blots were in the control dilution’s linear range. D: Sandy loam soil results. 3 mg soil per sample. E: Silty clay loam results. 1 mg soil per sample.
Unsorbed, aqueous PrP concentrations can be measured using the 96-well immunoassay to obtain a complete PrP mass recovery. The direct detection (solid fraction) results are used to determine adsorbed PrP amounts as opposed to extrapolating the solid fraction from aqueous fraction, which is more variable and assumes 100% PrP conservation (e.g. no degradation). Previous reports show this is not the case for longer incubations (>6 hr) (30).
Mass recoveries for sand samples after 1 hr equilibration averaged 96% for initial doses of 0.25–5% brain homogenate (2.5–50 μg BE/μl). PrP recovery from sandy loam samples after 2 hr (0.5% dose) and 24 hr (2.5% dose) was 66% and 75%, respectively. Bentonite had more variable recoveries, ranging from 25–100% for 2–168 hr time points. Silty clay loam soil samples averaged 130% after 2 hr. Recoveries greater than 100% may be due to differences in antibody affinities for soil-bound PrP and control PrP in the 96-well PVDF membrane. With mass recoveries near 100%, this method is favorable compared to detergent and boiling extraction methods with mass recoveries of no more than 40% for sandy soils and 5% for clays (16, 24). Because desorption is not required, certain populations of PrP, such as loosely-bound aggregates, are not preferentially selected over other PrP constituencies. It is important to note that because multiple centrifugations and decantations are required, the direct detection method may select for certain soil particles. However, this selection would be constant for all samples, and mass recoveries indicate that the procedure does not remove large populations of PrP.
PrP adsorption to soil over time
We applied the direct detection method to quantify HY TME PrP adsorption over time. The amount of PrP adsorbed to fine quartz sand, bentonite clay, and two whole soils, a sandy loam and a silty clay loam, was monitored for up to 63 d using two initial brain homogenate doses, 0.5% and 2.5%, with 1000 and 5000 μg BE loaded, respectively, for initial aqueous concentrations of 5 and 25 μg BE/μl. Total PrP in the aqueous fraction (initial supernatant plus first wash) was also monitored over time for all experiments. Previous studies, which have all used recPrP or purified PrPSc, have observed maximal prion sorption in less than 2 hours (17, 22, 23). Our results using brain homogenate indicate that maximal PrP adsorption occurs over a period of days or weeks.
PrP adsorption to fine sand did not reach a maximal solid concentration (Cs) until 30 d, and remained constant thereafter through 63 d (Figures 1B and 2A). The 1 hr Cs was 3% of the maximum. Results were similar for both starting doses, with the 2.5% dose having a 30% higher Cs. The aqueous PrP decreased greatly after 7 days of incubation for the 0.5% dose to 280 μg BE (28% of initial PrP), and decreased more gradually thereafter to 60 μg BE at 60 d (Figure 3A). For the 2.5% dose, aqueous PrP decreased to 40% of initial PrP after 30 d and thereafter remained constant through 60 d (Figure 3B).
Figure 2.
Quantified Adsorbed PrP Over Time. A: Fine quartz sand results. B: Bentonite results. C: Sandy loam soil results. Note the 1512 hr 2.5% data point is 3660 μg BE/mg soil (±155 μg BE SE). D: Silty clay loam soil results. Error bars show ± the standard error.
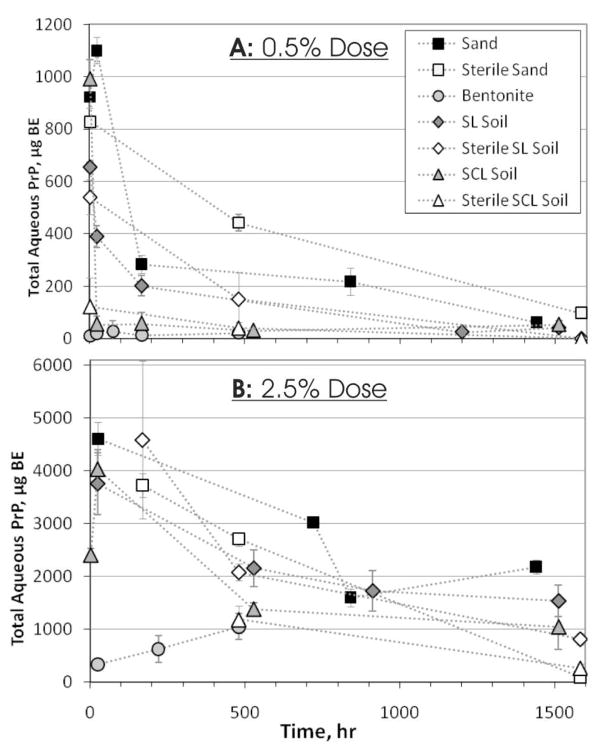
Figure 3.
Total Aqueous PrP Quantified Over Time. A: Initial BH dose of 0.5% (an initial aqueous fraction of 1000 μg BE). B: Initial 2.5% BH dose (an initial aqueous fraction of 5000 μg BE). Data points are connected by straight lines for clarity. Error bars show ± the standard error.
Decreases of PrP in the aqueous fraction over time could be due to PrP degradation, sorption to soil, sorption to the tube, or inactivation of the 3F4 epitope. Inactivation of the antibody epitope would imply a conformational change in PrP, which is unlikely considering the conformational stability of PrPSc. Adsorption of PrP to the polypropylene PCR tube is possible; however, in a previous experiment utilizing polypropylene tubes and CWD-infected brain homogenate, no decrease in PrP signal was observed through 30 d (30), suggesting that PrP adsorption to the tube is minimal. PrP adsorption to sand was inconsequential compared to the remaining aqueous fraction (a 1:200 ratio). Thus, the decreases in aqueous PrP indicate degradation of PrP. The relatively constant PrP in the aqueous fraction between 30 and 60 d could be due to near-complete adsorption of the proteases responsible for PrP degradation. Constant aqueous and solid phase PrP from 30 d to 60 d suggests the system reached equilibrium after 30 d.
PrP adsorption to bentonite reached a maximum Cs after 1 week at the 0.5% dose and decreased slightly thereafter through 61 d (Figures 1C and 2B). The 2.5% dose Cs values were similar or lower than the 0.5% dose except at 61 d. Aqueous PrP was consistently low from 2 hr through 20 d for both doses. At the 0.5% dose, aqueous PrP was never more than 0.3% of initial PrP, which indicates that all PrP adsorbed to the bentonite and remained bound (Figure 3A). Aqueous PrP at the 2.5% dose actually increased from 24 hr to 20 d, from 330 to 1050 μg BE (20% of initial PrP) (Figure 3B). This finding suggests that some PrP may have desorbed, although solid fraction PrP did not decrease (Figure 2B).
Adsorption of PrP to a sandy loam soil reached an initial maximum at 1 week and remained relatively constant through 22 d (Figures 1D and 2C). The initial Cs,max for the 0.5% dose was 50% higher than the Cs,max for the 2.5% dose. Cs values greatly increased after 22 d for both 0.5 % and 2.5% doses with 40- and 520-fold increases at 63 d, respectively, over the initial Cs,max. This large increase could be due to additional PrP binding or changes in antibody affinity. Aqueous PrP decreased rapidly for the 0.5% dose, similar to sand, from 200 μg BE at 7 d to 20 μg BE at 50 d (Figure 3A). Aqueous PrP at the 2.5% dose also decreased similarly to sand (Figure 3B). Based on these results, the large increase in the solid fraction PrP signal after 22 d cannot be fully explained by additional binding of aqueous PrP. Thus, the increased signal could be due to conformational changes in PrP, PrP aggregation, and/or changes and degradation of other bound constituents which increase the antibody (mAb 3F4) epitope accessibility and affinity.
PrP adsorption to a silty clay loam soil was faster, reaching an initial maximal Cs at 24 hr (Figures 1E and 2D). Subsequent adsorption at the 0.5% dose remained constant through 63 d. However, at the 2.5% dose, Cs increased approximately 3-fold between 22 d and 63 d. This was similar to the sandy loam results, but only occurred at the 2.5% dose. Aqueous PrP decreased very rapidly at the 0.5% dose, with 990 μg BE at 2 hr but ≤ 60 μg BE from 1–63 d (Figure 3A). Consistently low aqueous PrP mirrors the solid results and indicates that equilibrium was reached, similar to sand. At the 2.5% dose, results were similar to sand and sandy loam soil, decreasing to 30% of initial PrP at 22 d and remaining approximately constant thereafter through 63 d (Figure 3B). Thus, as with the sandy loam results, the increase in Cs at later time points at the 2.5% dose cannot be explained by additional binding of PrP and may be due to changes in PrP or other bound constituents increasing antibody affinity. The 0.5% dose did not exhibit this behavior. It is possible that at the lower dose, the majority of PrP adsorbed more avidly, resisting conformational or aggregation changes.
PrP adsorption to sterilized soil over time
To determine the effect of soil microbial populations on adsorption and degradation, kinetic experiments were repeated through 20 d with soil sterilized by gamma irradiation. Gamma irradiation (at the 25kGy dose used) effectively kills all microorganisms and does not significantly affect physiochemical soil properties or extracellular enzymes (32, 33). Adsorption of PrP to sterile sand yielded a maximum capacity approximately equal to unsterile results, but sorption appeared faster, with the 2 hr Cs equal to 67% of Cs,max (Figure 2A). PrP adsorption to sterilized sandy loam was similar to unsterile results through 20 d (Figure 2C). Maximal sorption occurred at 1 week and remained high through 20 d. Sterile silty clay loam adsorbed PrP at somewhat lower levels than unsterile samples (Figure 2D), and maximum sorption was approximately 50% lower than unsterile soil. Sterile kinetics were similar to unsterile samples, with Cs, max reached at 24 hr. The 2 hr Cs was 45% of Cs, max.
Aqueous concentrations of PrP during the sterile experiments decreased over time similar to the nonsterile experiments for both soils and sand (Figure 3). Therefore, PrP was not significantly degraded by active bacterial populations present in the soil. Degradation of recPrP by soluble soil enzymes has been demonstrated (34), and extracellular proteases in the soil are not inactivated by gamma irradiation, so native soil proteases could be responsible for some of the PrP degradation. However, organic matter was negligible for the sand samples, which still had aqueous PrP degradation. Thus, it is more likely that the observed PrP degradation is due to proteases present in the brain homogenate, which we previously demonstrated can significantly degrade HY TME at 22°C and pH 7 (30). These proteases are as yet unidentified.
Approximate Soil PrP adsorption capacities
Maximum observed adsorption to the silty clay loam and sandy loam soils was approximately 8000 μg BE/mg and 3700 μg BE/mg, respectively, and maximum observed adsorption to pure bentonite and sand was 3200 μg BE/mg and 6 μg BE/mg, respectively (Figure 2 and Table 1). These results confirm previous results which indicate that PrP has a high affinity for clay minerals per gram compared with sand (18, 20). However, our measured maximum Cs values are much lower than previously reported PrP sorption capacities. Using a recent study which measured 19 μg PrPSc/brain μg in a 263K-infected hamster (35) to convert μg BE to μg PrPSc, we measured capacities for quartz sand, bentonite clay, and whole soils that were 2–5 orders of magnitude lower (Table 1). It is important to note that we tested only two initial brain homogenate doses and that measured prion adsorption capacities could differ with other experimental designs such as flow-through or column studies. However, the experimental conditions for our study (batch assay, incubation with slow rotation at room temperature and neutral pH) were similar to those employed in previous studies (17–19, 23). At our maximum spiked-brain to soil mass ratios (between 0.5:1 for sand and 10:1 for bentonite), measured PrP absorption was 150–500,000 times lower than results reported previously for recPrP and purified PrPSc. Buffer chemistry may also affect PrP adsorption. Although Polano et al. observed increasing PrP adsorption to montmorillonite as DPBS concentration decreases (19), preliminary results using brain homogenate indicate that PrP adsorption to sand, bentonite, and silty clay loam soil may decrease somewhat as DPBS concentration decreases, however additional research is needed to fully eluicidate the impact of solution chemistry on PrP adsorption from brain homogenate.
Table 1.
PrP Adsorption Is Lower than Previously Reported
| Soil/Mineral | Sorption Capacity (μg PrP/mg) | Equilibration Time | Prion Material Used | Buffer Used | Reference |
|---|---|---|---|---|---|
| Loam soil (3% OC¶) | 30.5 | 24h | ovine recPrP | DI Water | 23 |
| Loamy sand soil (2% OC) | 15.5 | 24h | ovine recPrP | DI Water | 23 |
| Sandy Loam (1% OC) | 0.07 | 1h-60d | HY (hamster) BH | 1X DPBS | This paper |
| Silty Clay Loam (1% OC) | 0.2 | 1h-60d | HY (hamster) BH | 1X DPBS | This paper |
| Fine Quartz Sand | >50† | 4h | purified HY (hamster) PrPSc | 10mM MOPS | 20 |
| Fine Quartz Sand | 0.0001 | 1h-60d | HY (hamster) BH | 1X DPBS | This paper |
| Montmorillonite- Ca2+ | >400† | 10 min | murine recPrP | DI Water | 19 |
| Montmorillonite- Na+ | 1000†§ | 2h | ovine recPrP | 20mM Na-Acetate | 20 |
| Montmorillonite- Na+ | 87–174 | 2h | purified HY (hamster) PrPSc | 10mM MOPS | 17 |
| Montmorillonite- Na+ | 600† | 10 min | murine recPrP | 1X PBS | 19 |
| Bentonite- Na+ | 0.04 | 1h-20d | HY (hamster) BH | 1X DPBS | This paper |
Organic carbon content
Estimated from graph. All others reported.
at pH 5. All other at pH 7 or unspecified.
Sorption capacity per square meter surface area varied: 130 BE/m2 for bentonite, 40 BE/m2 for pure sand, 400 BE/m2 for silty clay loam soil, and 2200 BE/m2 for sandy loam soil. The large differences in the PrP surface affinity of sandy loam soil and silty clay loam soil compared with pure quartz sand and bentonite are not likely due to mineralogy since sand and smectite are majority constituents in the whole soils. Previous studies found that recPrP sorption to organic matter is high, approximately equal to that calculated for clay mineral surfaces (19, 23, 35), and pure solid humic acid was found to adsorb about 10 times as much recPrP as montmorillonite (19). However, organic carbon content was not high in either soil used in this study (1%). It is possible that the physical size of soil particles and aggregates has a greater influence on PrP binding than surface chemistry. An intermediately-sized particle (silt or very fine sand) might interact more favorably with PrPSc aggregates, which may be larger than 200 nm (36).
Implications for prion environmental transmissibility
Prion adsorption to soil could play an important role in prion transport or immobilization, provide protection from or enhance in situ proteolysis, and induce conformational changes that enhance or decrease infectivity, all of which could affect the transmissibility of CWD, scrapie, and BSE in the environment.
In this study, PrP adsorption increased or remained constant through 60 d for all sorbents tested, while unbound PrP degraded over time. These results indicate that soil-bound PrP fraction does not degrade as readily as unbound PrP. PrPSc adsorption and subsequent protection from enzymatic degradation could explain the longevity of prions in the environment. Alternately, degradation-resistant PRPSC may preferentially bind. To date, only one study has directly evaluated the mobility of prions in soil, and it found only slight migration of recPrP over 6 months (24). However, we found PrP adsorption was slow and reduced when studied as a component in a tissue homogenate. Thus, the possibility of prion transport in soil environments due to incomplete binding cannot be excluded and requires further investigation. A recent report suggested that soil-bound prions are more infectious that unbound prions; however, this study used a 2 hr equilibration time and purified PrPSc (12). It is possible that equilibration of PRPSC to soil for longer times in brain homogenate may yield different results. Additional bioassay studies are required to clarify this point.
Protein adsorption in a heterogeneous environment such as brain homogenate and soil is complex (7). Adsorbed proteins may reorient, relax in conformation, or be displaced by other proteins (37). Degradation of bound or unbound constituents by extracellular enzymes, as well as binding and degradation of the enzymes themselves may also occur. It is likely that PrP adsorption is slower and less avid than previously reported due to the competitive sorption effects of the brain homogenate matrix, which was not used in previous studies. PrP will compete for sorption sites with thousands of other constituents, likely slowing PrP transport to the soil surface and inhibiting initial binding. Over time, more surface area may become available due to degradation, reorientation, or conformational changes of bound molecules, allowing aqueous PrP to adsorb. In addition, PrP adsorption may be more favorable after an initial (organic) surface coating. Changes in aqueous PrP conformation or aggregation might also favor additional adsorption. Although no studies to-date have used a complex tissue homogenate to investigate protein adsorption, studies of blood plasma have found significant effects on adsorption capacity due to competition between plasma proteins, which also affects conformational changes upon adsorption (38).
Conformational changes in protein structure upon interaction with soil minerals are well documented and can range from slight unfolding to significant changes in secondary structure and can also include aggregation or disaggregation (37). Because the structure of PrP is likely the sole determinant of infectivity and strain properties (2, 36, 39–41), changes in PrP structure due to environmental mechanisms could directly impact transmissibility and pathogenesis.
We used infected brain homogenate as a model for prion interactions with soil. Other matrices in which prions might enter soil environments (e.g. feces, urine, saliva) are similarly competitive in nature, but consist of different constituents and a lower concentration of PrP. A recent paper reported differences in PrP adsorption in brain homogenate due to prion strain and host species (42). Our results indicate that PrP model selection (i.e. infected homogenate, recPrP, or purified PrPSc) can be important in studies of prion environmental fate, as it is clear that the brain homogenate matrix can greatly affect PrP interactions with soil. Changes in PrPSc aggregation and conformation over time and upon binding to soil require further study, specifically how these changes affect agent survival and infectivity. Future studies of prion environmental fate should consider adsorption kinetics as an important variable.
Supplementary Material
Acknowledgments
We thank Ronald Shikiya and Michelle Kramer for technical assistance. Thanks to James Smith for specific surface area analysis, and Michael Beard at Becton-Dickenson, Columbus, NE for gamma irradiation. This research was supported in part by the UNL Research Council and the National Center for Research Resources (P20 RR0115635-6 and C06 RR17417-01).
Footnotes
Supporting Information Available: Table S1. Soil and Soil Mineral Characteristics. Table S2. Soil Mineralogy (Weight Percent). Supplemental Methods Discussion. Figure S1. Typical Linear Regression of Control Dilution.
Literature Cited
- 1.Prusiner SB. An introduction to prion biology and diseases. In: Prusiner SB, editor. Prion Biology and Diseases. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2004. [Google Scholar]
- 2.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greig JR. Scrapie: Observation on the transmission of the disease by mediate contact. Vet J. 1940;96:203–206. [Google Scholar]
- 4.Hadlow WJ, Kennedy RC, Race RE. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 5.Miller MW, Williams ES. Horizontal prion transmission in mule deer. Nature. 2003;425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 6.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental source of prion transmission in mule deer. Emerging Infect Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders SE, Bartelt-Hunt SL, Bartz JC. Prions in the environment: Occurrence, fate, and mitigation. Prion. 2009;2:162–169. doi: 10.4161/pri.2.4.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke KI, Holyoak GR, Clark WW, Mickelson JR, Wang S, Melco RP, Besser TE, Foote WC. PrP genotypes and experimental scrapie in orally inoculated suffolk sheep in the united states. J Gen Virol. 1997;78:975–978. doi: 10.1099/0022-1317-78-4-975. [DOI] [PubMed] [Google Scholar]
- 10.Kincaid AE, Bartz JC. The nasal cavity is a route for prion infection in hamsters. J Virol. 2007;81:4482–4491. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamir AN, Kunkle RA, Richt JA, Miller JM, Greenlee JJ. Experimental transmission of us scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Vet Pathol. 2008;45:7–11. doi: 10.1354/vp.45-1-7. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. Oral transmissibility of prion disease is enhanced by binding of soil particles. PLoS Pathog. 2007;3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS ONE. 2007;3:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur WJ, Alldredge AW. Soil ingestion by mule deer in northcentral Colorado. Range Ecol Manage. 1979;32:67–71. [Google Scholar]
- 15.Beyer WN, Connor EE, Gerould S. Estimates of soil ingestion by wildlife. J Wildlife Manage. 1994;58:375–382. [Google Scholar]
- 16.Cooke CM, Rodger J, Smith A, Fernie K, Shaw G, Somerville RA. Fate of prions in soil: Detergent extraction of PrP from soils. Environ Sci Technol. 2007;41:811–817. doi: 10.1021/es0618189. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2:296–302. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Benson CH, McKenzie D, Aiken JM, Pedersen JA. Adsorption of pathogenic prion protein to quartz sand. Environ Sci Technol. 2007;41:2324–2330. doi: 10.1021/es062122i. [DOI] [PubMed] [Google Scholar]
- 19.Polano M, Anselmi C, Leita L, Negro A, Nobili MD. Organic polyanions act as complexants of prion protein in soil. Biochem Biophys Res Com. 2008;367:323–329. doi: 10.1016/j.bbrc.2007.12.143. [DOI] [PubMed] [Google Scholar]
- 20.Rigou P, Rezaei H, Grosclaude J, Staunton S, Quiquampoix H. Fate of prions in soil: Adsorption and extraction by electroelution of recombinant ovine prion protein from montmorillonite and natural soils. Environ Sci Technol. 2006;40:1497–1503. doi: 10.1021/es0516965. [DOI] [PubMed] [Google Scholar]
- 21.Leita L, Fornasier F, Nobili MD, Bertoli A, Genovesi S, Sequi P. Interactions of prion proteins with soil. Soil Biol Biochem. 2006;38:1638–1644. [Google Scholar]
- 22.Vasina EN, Dejardin P, Rezaei H, Grosclaude J, Quiquampoix H. Fate of prions in soil: Adsorption kinetics of recombinant unglycosylated ovine prion protein onto mica in laminar flow conditions and subsequent desorption. Biomacromolecules. 2005;6:3425–3432. doi: 10.1021/bm050492d. [DOI] [PubMed] [Google Scholar]
- 23.Pucci A, D’Acqui LP, Calamai L. Fate of prions in soil: Interactions of recPrP with organic matter of soil aggregates as revealed by LTA-PAS. Environ Sci Technol. 2008;42:728–733. doi: 10.1021/es071314q. [DOI] [PubMed] [Google Scholar]
- 24.Cooke CM, Shaw G. Fate of prions in soil: Longevity and migration of recPrP in soil columns. Soil Biol Biochem. 2007;39:1181–1191. [Google Scholar]
- 25.Genovesi S, Lieta L, Sequi P, Andrighetto I, Sorgato MC, Bertoli A. Direct detection of soil-bound prions. PLoS ONE. 2007;2:e1069. doi: 10.1371/journal.pone.0001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer ML, Bartz JC. Rapid, high-throughput detection of PrPSc by 96-well immunoassay. Prion. 2009;3:44–48. doi: 10.4161/pri.3.1.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartz JC, Kramer ML, Sheehan MH, Hutter JAL, Ayers JI, Bessen RA, Kincaid AE. Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol. 2007;81:689–697. doi: 10.1128/JVI.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 29.Slack SM, Posso SE, Horbett TA. Measurement of fibrinogen adsorption from blood plasma using 125I-fibrinogen and a direct ELISA technique. J Biomat Sci Poly Ed. 1991;3:49–67. doi: 10.1163/156856292x00079. [DOI] [PubMed] [Google Scholar]
- 30.Saunders SE, Bartz JC, Telling GC, Bartelt-Hunt SL. Environmentally-relevant forms of the prion protein. Environ Sci Technol. 2008;42:6573 – 6579. doi: 10.1021/es800590k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotrario JB, Stuart J, Lam T, Arands RR, O’Connor OA, Kosson DS. Effects of sterilization methods on the physical characteristics of soil: Implications for sorption isotherm analyses. Bullet Environ Contamin Toxicol. 1995;54:668–675. doi: 10.1007/BF00206097. [DOI] [PubMed] [Google Scholar]
- 32.Wolf DC, THD, Scott HD, Lavy TL. Influence of sterilization methods on selected soil microbiological physical, and chemical properties. J Environ Qual. 1989;18:39–44. [Google Scholar]
- 33.Rapp D, Potier P, Jocteur-Monrozier L, Richaume A. Prion degradation in soil: Possible role of microbial enzymes stimulated by the decomposition of buried carcasses. Environ Sci Technol. 2006;40:6324–6329. doi: 10.1021/es060943h. [DOI] [PubMed] [Google Scholar]
- 34.Onisko B, Dynin I, Requena J, Silva C, Erickson M, Carter J. Mass spectrometric detection of attomole amounts of the prion protein by NanoLC/MS/MS. J Am Soc Mass Spec. 2007;18:1070–1079. doi: 10.1016/j.jasms.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Rao MA, Russo F, Granata V, Berisio R, Zagari A, Gianfreda L. Fate of prions in soil: Interaction of a recombinant ovine prion protein with synthetic humic-like mineral complexes. Soil Biol Biochem. 2007;39:493–504. [Google Scholar]
- 36.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brash JL, Horbett TA. Proteins at interfaces: An overview. In: Brash JL, Horbett TA, editors. Proteins at Interfaces II: Fundamentals and Applications. American Chemical Society; Washington, DC: 1995. [Google Scholar]
- 38.Slack SM, Horbett TA. The Vroman effect. In: Brash JL, Horbett TA, editors. Proteins at Interfaces II: Fundamentals and Applications. American Chemical Society; Washington, DC: 1995. [Google Scholar]
- 39.Bessen RA, Marsh RF. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deleault AM, Deleault NR, Harris BT, Rees JR, Supattapone S. The effects of prion protein proteolysis and disaggregation on the strain properties of hamster scrapie. J Gen Virol. 2008;89:2646–2650. doi: 10.1099/vir.0.2008/002303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safar J, Willie H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 42.Saunders SE, Bartz JC, Bartelt-Hunt SL. Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environmental Science & Technology. 2009;43:5242–5248. doi: 10.1021/es900502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.