Abstract
Background
Carcinomas of the major salivary glands (M-SGC) comprise a morphologically diverse group of rare tumors of largely unknown cause. To gain insight into etiology, we evaluated incidence of M-SGC utilizing the World Health Organization classification schema (WHO-2005).
Methods
We calculated age-adjusted incidence rates (IRs) and IR ratios (IRRs) for M-SGC diagnosed between 1992–2006 in the Surveillance, Epidemiology and End Results Program.
Results
Overall, 6,391 M-SGCs (IR=11.95/1,000,000 person-years) were diagnosed during 1992–2006. Nearly 85% of cases (n=5,370; IR=10.00) were encompassed within WHO-2005 and among these, males had higher IRs than females (IRR=1.51, 95%CI=1.43–1.60). Squamous cell (IR=3.44) and mucoepidermoid (IR=3.23) carcinomas occurred most frequently among males, whereas, mucoepidermoid (IR=2.67), acinic cell (IR=1.57), and adenoid cystic (IR=1.40) carcinomas were most common among females. Mucoepidermoid, acinic cell, and adenoid cystic carcinomas predominated in females through approximately age 50 years; thereafter IRs of acinic cell and adenoid cystic carcinomas were nearly equal among females and males, whereas IRs of mucoepidermoid carcinoma among males exceeded IRs among females (IRR=1.57; 95%CI=1.38–1.78). Except for mucoepidermoid and adenoid cystic carcinomas which occurred equally among all races, other subtypes had significantly lower incidence among Blacks and Asian/Pacific Islanders than among Whites. Adenoid cystic carcinoma occurred equally in the submandibular and parotid glands, and other M-SGCs evaluated had 77–98% lower IRs in the submandibular gland. Overall M-SGC IRs remained stable during 1992–2006.
Conclusion
Distinct incidence patterns according to histologic subtype suggest that M-SGCs are a diverse group of neoplasms characterized by etiologic and/or biologic heterogeneity with varying susceptibility by gender and race.
Keywords: major salivary gland cancer, epidemiology, incidence, SEER, WHO
Introduction
Cancers of the major salivary glands (M-SGC) are rare malignancies comprising 11% of all oropharyngeal neoplasms in the United States (1). However, in contrast to most head and neck cancers which are predominantly squamous cell carcinomas (1, 2), M-SGC encompass at least 20 distinct histologic subtypes (3). The first classification scheme of salivary gland tumors was proposed by Foote and Frazell (4) with subsequent refinements leading to the most recent World Health Organization classification published in 2005 (WHO-2005) (3). The rarity of M-SGC coupled with its complex and changing classification schema over time has made the diagnosis of M-SGC challenging. Further complicating the classification of salivary gland tumors is the occurrence of salivary gland cancers in the major and minor salivary glands, existence of similarly numerous benign entities, and histologic diversity within the same specimen (5).
Tobacco and alcohol use are major risk factors for cancers of the oral cavity and pharynx, but little is known about the etiology of M-SGC (6). Some studies have suggested a link between M-SGC and occupational exposures, ultraviolet light, viruses, tobacco, and alcohol; however ionizing radiation is the only well-established risk factor (6, 7).
Descriptive studies of cancer incidence can provide insight into etiology. Whereas distinct age-specific patterns may reflect differences in disease biology and/or host susceptibility, variations in temporal trends can reflect changes in exposures, methods of detection/diagnosis, and/or changing classification schemes. Precise histologic diagnoses are important not only for determining prognosis and treatment, but also for facilitating identification of risk factors in epidemiologic studies.
Epidemiologic data on M-SGC has been largely based on clinical series (8–12), with some population-based studies describing M-SGC incidence (13–19) but none considering incidence rates (IRs) according to histology. Therefore, with the paucity of population-based studies of M-SGC, we utilized the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program to provide new information on M-SGC incidence in the United States according to histologic subtype. Focusing on malignant entities localized in the major salivary glands, we limited our study to more contemporaneously diagnosed cases that would facilitate the use the WHO-2005 classification schema (3).
Materials and Methods
We evaluated the incidence of M-SGC in 13 population-based cancer registry areas of the SEER Program (SEER-13) during 1992–2006 using the Limited-Use Database, November 2008 submission (20). SEER-13 covers approximately 14% of the United states population and includes the states of Connecticut, Hawaii, Iowa, New Mexico and Utah, and the areas of Detroit, MI; San Francisco, Los Angeles, and San Jose-Monterey, CA; Seattle-Puget Sound, WA; Atlanta and rural GA, and also includes cases diagnosed among Alaskan Natives in Alaska. The SEER Program currently classifies information on morphology and topography according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) (21).
We considered all M-SGCs (ICD-O-3 topography codes C07.9, C08.0–08.9) with malignant behavior diagnosed during 1992–2006. All lymphohematopoietic malignancies were excluded from the analysis (n=1,255) as were cases that were not microscopically confirmed (n=74). ICD-O-3 histology codes that were specified in the WHO-2005 classification were included in a category entitled “total, WHO” and those histology codes not specified in the WHO-2005 classification were collectively included in a group entitled “total, non-WHO.” Specific histology categories were based on the WHO-2005 classification (3), with ICD-O-3 codes as described in Table 1. Except for polymorphous low-grade adenocarcinoma (PLGA) (M-8525) and myoepithelial carcinoma (M-8982) which were introduced with ICD-O-3 in 2000, all other histology codes were included in ICD-O-2 (22).
Table 1.
Age-adjusted incidence rates and incidence rate ratios of carcinomas of the major salivary glands diagnosed in SEER-13 according to histology, overall and by gender, 1992–2006†
| ICD-O-3 | Mean/median age (years) |
All | Males | Females | Male:female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| codes | Males | Females | No. | IR | No. | IR | No. | IR | IRR | (95% CI) | |
| All cases | * | 63/65 | 59/61 | 6,391 | 11.95 | 3,601 | 15.67 | 2,790 | 9.51 | 1.65 | (1.57–1.73) |
| Total, WHO | * | 62/64 | 58/59 | 5,370 | 10.00 | 2,932 | 12.62 | 2,438 | 8.33 | 1.51 | (1.43–1.60) |
| Mucoepidermoid carcinoma | 8430 | 58/61 | 53/54 | 1,554 | 2.85 | 775 | 3.23 | 779 | 2.67 | 1.21 | (1.09–1.34) |
| Squamous cell carcinoma | 8070 | 72/73.5 | 74/77 | 942 | 1.83 | 720 | 3.44 | 222 | 0.73 | 4.70 | (4.03–5.49) |
| Adenoid cystic carcinoma | 8200 | 56/55 | 56/57 | 709 | 1.30 | 303 | 1.21 | 406 | 1.40 | 0.86 | (0.74–1.00) |
| Adenocarcinomas | |||||||||||
| Acinic cell carcinoma | 8550 | 51/50 | 49/48 | 770 | 1.38 | 313 | 1.20 | 457 | 1.57 | 0.77 | (0.66–0.89) |
| Adenocarcinoma-NOS | 8140 | 65/66 | 65/68 | 641 | 1.22 | 396 | 1.71 | 245 | 0.83 | 2.06 | (1.75–2.42) |
| Salivary duct carcinoma | 8500 | 65/67 | 67/66 | 92 | 0.18 | 67 | 0.29 | 25 | 0.09 | 3.38 | (2.10–5.59) |
| Basal cell adenocarcinoma | 8147 | 62/61.5 | 66/70 | 80 | 0.15 | 36 | 0.15 | 44 | 0.15 | 0.98 | (0.61–1.56) |
| Oncocytic carcinoma | 8290 | 71/70 | 70/75.5 | 43 | 0.08 | 25 | 0.12 | 18 | 0.06 | 1.94 | (1.01–3.77) |
| Clear cell adenocarcinoma, NOS | 8310 | 65/66.5 | 70/71 | 30 | 0.06 | 22 | 0.09 | 8 | ~ | ~ | |
| Cystadenocarcinoma | 8440 | 65/60 | 61/64 | 23 | 0.04 | 17 | 0.07 | 6 | ~ | ~ | |
| Mucinous adenocarcinoma | 8480 | 61/63 | 50/54 | 16 | 0.03 | 9 | ~ | 7 | ~ | ~ | |
| Polymorphous low-grade adenocarcinoma | 8525 | 65/64 | 60/62.5 | 15 | ~ | 11 | ~ | 4 | ~ | ~ | |
| Sebaceous carcinoma/lymphadenocarcinoma | 8410 | 51/56 | ~~ | 3 | ~ | 3 | ~ | 0 | ~ | ~ | |
| Mixed tumors | |||||||||||
| Carcinoma ex pleomorphic adenoma | 8941 | 59/59 | 64/65 | 174 | 0.33 | 91 | 0.37 | 83 | 0.28 | 1.31 | (0.96–1.78) |
| Carcinosarcoma | 8980 | 60/60 | 62/61 | 15 | ~ | 8 | ~ | 7 | ~ | ~ | |
| Other rare carcinomas | |||||||||||
| Epithelial-myoepithelial carcinoma | 8562 | 65/66 | 68/70 | 96 | 0.18 | 39 | 0.17 | 57 | 0.19 | 0.87 | (0.56–1.33) |
| Lymphoepithelial carcinoma | 8082 | 56/58 | 60/61 | 55 | 0.10 | 25 | 0.10 | 30 | 0.10 | 1.00 | (0.56–1.76) |
| Small cell carcinoma | 8041 | 72/74 | 76/81 | 45 | 0.09 | 32 | 0.15 | 13 | ~ | ~ | |
| Large cell carcinoma | 8012 | 72/73.5 | 72/73.5 | 38 | 0.07 | 28 | 0.13 | 10 | ~ | ~ | |
| Myoepithelial carcinoma | 8982 | 61/64 | 63/63 | 29 | 0.06 | 12 | ~ | 17 | 0.06 | 0.87 | (0.37–1.93) |
| Total, non-WHO | * | 67/70 | 67/69 | 1,021 | 1.95 | 669 | 3.05 | 352 | 1.19 | 2.58 | (2.26–2.94) |
Abbreviations: ICD-O-3, International Classification of Diseases for Oncology, third edition; No., number of cases; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; NOS, not otherwise specified; ~, IR and IRRs not calculated for fewer than 16 cases; ~~ no cases.
Incidence rates are age-adjusted to the 2000 U.S. standard population and expressed per 1,000,000 person-years. IRRs are based on unrounded rates.
"All cases" includes ICD-O-3 histology codes in "total, WHO" and "total, non-WHO" categories. "Total, WHO" includes all codes in the specified subcategories, and "total, non-WHO" includes all ICD-O-3 codes not specified within “total, WHO” category.
We estimated age-adjusted IRs, IR ratios (IRRs), and annual percent change (APC) in incidence using SEER*Stat 6.5.1. All IRs were expressed per 1,000,000 person-years (PY) and age adjusted to the 2000 U.S. standard population. IRs were calculated according to histology, race (White, Black, Asian/Pacific Islander (API), other and unknown), gender (male, female), age at diagnosis (<50, ≥50 years or <65, 65+ years), and site (parotid gland (C07.9), submandibular gland (C08.0), sublingual gland (C08.1), overlapping sites and site not specified (C08.8–C08.9)). Age-adjusted temporal trends (1992–1996, 1997–2001, 2002–2006) and age-specific IRs (<15, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, ≥75 years) were plotted on a log-linear scale, where a slope change of 10 degrees approximates a rate change of 1% per year, as previously described (23). Incidence rates were not calculated for fewer than 16 cases (1).
Results
Overall, 6,391 M-SGCs (IR=11.95/1,000,000 PY) were diagnosed in SEER-13 during 1992 to 2006 (Table 1). Nearly 85% of cases were encompassed within the WHO-2005 classification (n=5,370; IR=10.00), with males having 51% higher IR than females (IRR=1.51, 95%CI 1.43–1.60). For males and females, mean and median ages at diagnosis were younger for cases included in the total WHO category than for those in the non-WHO category. There was marked variation in age at diagnosis ranging from mean/median ages of 51/50 years and 49/48 years for acinic cell carcinoma among males and females, respectively, to mean/median ages of 72 years and older for squamous cell, small cell, and large cell carcinomas among males and females.
When considering the WHO M-SGC subtypes, the highest IRs were observed for squamous cell (IR=3.44), mucoepidermoid (IR=3.23), and adenocarcinoma, not otherwise specified (adenocarcinoma-NOS) (IR=1.71) among males. Among females, the highest IRs were noted for mucoepidermoid (IR=2.67), acinic cell (IR=1.57), and adenoid cystic (IR=1.40) carcinomas. Squamous cell, adenocarcinoma-NOS, and salivary duct carcinomas were associated with greater than 2-fold higher incidence among males than among females, whereas acinic cell and adenoid cystic carcinoma had substantially lower IR rates among males than among females.
Except for adenoid cystic carcinoma which occurred equally in the submandibular and parotid glands, M-SGCs of the submandibular gland had 77–98% lower IRs than those diagnosed in the parotid gland (Table 2). Overall IRs of total WHO and non-WHO M-SGCs were significantly lower among Blacks and APIs than among Whites. Mucoepidermoid carcinoma occurred equally among Whites, Blacks, and APIs (Black:White IRR=1.00 and API:White IRR=1.00) and similar IRs were also noted for adenoid cystic carcinoma (Black:White IRR=0.94 and API:White IRR=1.11). All histologic subtypes included in Table 2 were more frequent among those ≥50 years of age compared to those <50 years; however, a broad range of IRRs were observed, from approximately 3- to 5-fold higher IRs for mucoepidermoid, adenoid cystic, and acinic cell carcinomas to greater than 50-fold risks for squamous cell carcinoma. Among individuals diagnosed prior to age 50 years, IRs of mucoepidermoid (IRR=0.72), adenoid cystic (IRR=0.76), and acinic cell (IRR=0.62) carcinomas were significantly lower in males compared to females; in contrast, a 2-fold higher incidence of squamous cell carcinoma (IRR=2.22) was observed among males than females. Adenoid cystic and acinic cell carcinoma IRs were generally similar among males and females diagnosed at ages ≥50 years, whereas significantly higher IRs of mucoepidermoid and squamous cell carcinomas and adenocarcinoma-NOS occurred among older males compared to older females.
Table 2.
Age-adjusted incidence rates and incidence rate ratios of carcinomas of the major salivary glands diagnosed in SEER-13 according to histologic subtype, 1992–2006†
| Total, WHO | Mucoepidermoid | Squamous cell | Adenoid cystic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | IR | IRR | (95% CI) | No. | IR | IRR | (95% CI) | No. | IR | IRR | (95% CI) | No. | IR | IRR | (95% CI) | |
| Site | ||||||||||||||||
| Parotid | 4,265 | 7.94 | 1.00 | (referent) | 1,326 | 2.43 | 1.00 | (referent) | 796 | 1.54 | 1.00 | (referent) | 323 | 0.59 | 1.00 | (referent) |
| Submandibular | 845 | 1.58 | 0.20 | (0.18–0.21) | 159 | 0.29 | 0.12 | (0.10–0.14) | 126 | 0.24 | 0.16 | (0.13–0.19) | 327 | 0.60 | 1.03 | (0.88–1.20) |
| Sublingual | 58 | 0.11 | 0.01 | (0.01–0.02) | 24 | 0.05 | 0.02 | (0.01–0.03) | 7 | ~ | ~ | 23 | 0.04 | 0.07 | (0.04–0.11) | |
| Other/unspecified | 202 | 0.38 | 0.05 | (0.04–0.05) | 45 | 0.08 | 0.03 | (0.02–0.05) | 13 | ~ | ~ | 36 | 0.07 | 0.11 | (0.08–0.16) | |
| Race | ||||||||||||||||
| Whites | 4,394 | 10.30 | 1.00 | (referent) | 1,210 | 2.82 | 1.00 | (referent) | 847 | 2.01 | 1.00 | (referent) | 555 | 1.29 | 1.00 | (referent) |
| Blacks | 415 | 8.13 | 0.79 | (0.71–0.88) | 146 | 2.82 | 1.00 | (0.83–1.19) | 49 | 1.10 | 0.55 | (0.40–0.73) | 62 | 1.22 | 0.94 | (0.71–1.24) |
| API | 474 | 8.20 | 0.80 | (0.72–0.88) | 169 | 2.82 | 1.00 | (0.84–1.18) | 38 | 0.74 | 0.37 | (0.26–0.51) | 84 | 1.44 | 1.11 | (0.87–1.41) |
| Other/unspecified | 87 | ~ | ~ | 29 | ~ | ~ | 8 | ~ | ~ | 8 | ~ | ~ | ||||
| Age (years) | ||||||||||||||||
| <50 | 1,513 | 3.55 | 1.00 | (referent) | 574 | 1.34 | 1.00 | (referent) | 51 | 0.12 | 1.00 | (referent) | 258 | 0.61 | 1.00 | (referent) |
| 50+ | 3,857 | 26.89 | 7.57 | (7.13–8.04) | 980 | 6.81 | 5.09 | (4.58–5.65) | 891 | 6.29 | 51.42 | (38.77–69.63) | 451 | 3.11 | 5.10 | (4.37–5.97) |
| Age and gender | ||||||||||||||||
| <50 years | ||||||||||||||||
| Females | 848 | 3.99 | 1.00 | (referent) | 333 | 1.56 | 1.00 | (referent) | 16 | 0.08 | 1.00 | (referent) | 147 | 0.69 | 0.69 | (referent) |
| Males | 665 | 3.13 | 0.78 | (0.71–0.87) | 241 | 1.12 | 0.72 | (0.61–0.85) | 35 | 0.17 | 2.22 | (1.20–4.30) | 111 | 0.53 | 0.53 | (0.59–0.98) |
| 50+ years | ||||||||||||||||
| Females | 1,590 | 19.67 | 1.00 | (referent) | 446 | 5.58 | 1.00 | (referent) | 206 | 2.45 | 1.00 | (referent) | 259 | 3.25 | 1.00 | (referent) |
| Males | 2,267 | 37.46 | 1.90 | (1.78–2.03) | 534 | 8.74 | 1.57 | (1.38–1.78) | 685 | 11.99 | 4.90 | (4.18–5.76) | 192 | 2.98 | 0.92 | (0.76–1.11) |
| Acinic cell | Adenocarcinoma-NOS | carcinoma ex pleomorphic | Total, non-WHO | |||||||||||||
| No. | IR | IRR | (95% CI) | No. | IR | IRR | (95% CI) | No. | IR | IRR | (95% CI) | No. | IR | IRR | (95% CI) | |
| Site | ||||||||||||||||
| Parotid | 736 | 1.32 | 1.00 | (referent) | 486 | 0.92 | 1.00 | (referent) | 134 | 0.25 | 1.00 | (referent) | 824 | 1.58 | 1.00 | (referent) |
| Submandibular | 17 | 0.03 | 0.02 | (0.01–0.04) | 94 | 0.18 | 0.19 | (0.15–0.24) | 31 | 0.06 | 0.23 | (0.15–0.35) | 140 | 0.27 | 0.17 | (0.14–0.20) |
| Sublingual | 1 | ~ | ~ | 2 | ~ | ~ | 0 | ~ | ~ | 3 | ~ | ~ | ||||
| Other/unspecified | 16 | 0.03 | 0.02 | (0.01–0.04) | 59 | 0.11 | 0.12 | (0.09–0.16) | 9 | 0.02 | 0.07 | (0.03–0.13) | 54 | 0.10 | 0.07 | (0.05–0.09) |
| Race | ||||||||||||||||
| Whites | 637 | 1.47 | 1.00 | (referent) | 537 | 1.27 | 1.00 | (referent) | 140 | 0.33 | 1.00 | (referent) | 872 | 2.06 | 1.00 | (referent) |
| Blacks | 56 | 0.90 | 0.61 | (0.45–0.81) | 49 | 1.00 | 0.79 | (0.57–1.06) | 15 | ~ | ~ | 73 | 1.53 | 0.74 | (0.57–0.94) | |
| API | 65 | 1.07 | 0.73 | (0.55–0.95) | 49 | 0.87 | 0.69 | (0.50–0.93) | 15 | ~ | ~ | 65 | 1.17 | 0.57 | (0.43–0.73) | |
| Other/unspecified | 12 | ~ | ~ | 6 | ~ | ~ | 4 | ~ | ~ | 11 | ~ | ~ | ||||
| Age (years) | ||||||||||||||||
| <50 | 387 | 0.90 | 1.00 | (referent) | 106 | 0.26 | 1.00 | (referent) | 45 | 0.11 | 1.00 | (referent) | 156 | 0.37 | 1.00 | (referent) |
| 50+ | 383 | 2.65 | 2.94 | (2.54–3.39) | 535 | 3.73 | 14.61 | (11.84–18.17) | 129 | 0.89 | 8.31 | (5.88–11.95) | 865 | 6.09 | 16.5 | (13.86–19.66) |
| Age and gender | ||||||||||||||||
| <50 years | ||||||||||||||||
| Females | 238 | 1.12 | 1.00 | (referent) | 49 | 0.23 | 1.00 | (referent) | 21 | 0.10 | 1.00 | (referent) | 54 | 0.26 | 1.00 | (referent) |
| Males | 149 | 0.69 | 0.62 | (0.50–0.76) | 57 | 0.28 | 1.18 | (0.79–1.76) | 24 | 0.11 | 1.11 | (0.59–2.10) | 102 | 0.48 | 1.89 | (1.35–2.68) |
| 50+ years | ||||||||||||||||
| Females | 219 | 2.75 | 1.00 | (referent) | 196 | 2.40 | 1.00 | (referent) | 62 | 0.76 | 1.00 | (referent) | 298 | 3.62 | 1.00 | (referent) |
| Males | 164 | 2.54 | 0.92 | (0.75–1.14) | 339 | 5.48 | 2.28 | (1.90–2.74) | 67 | 1.04 | 1.37 | (0.95–1.98) | 567 | 9.77 | 2.70 | (2.34–3.12) |
Abbreviations: No., number of cases; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; NOS, not otherwise specified; ~,IRs and IRRs not calculated for other/unspecified race or fewer than 16 cases.
Incidence rates are age-adjusted to the 2000 U.S. standard population and expressed per 1,000,000 person-years. IRRs are based on unrounded rates.
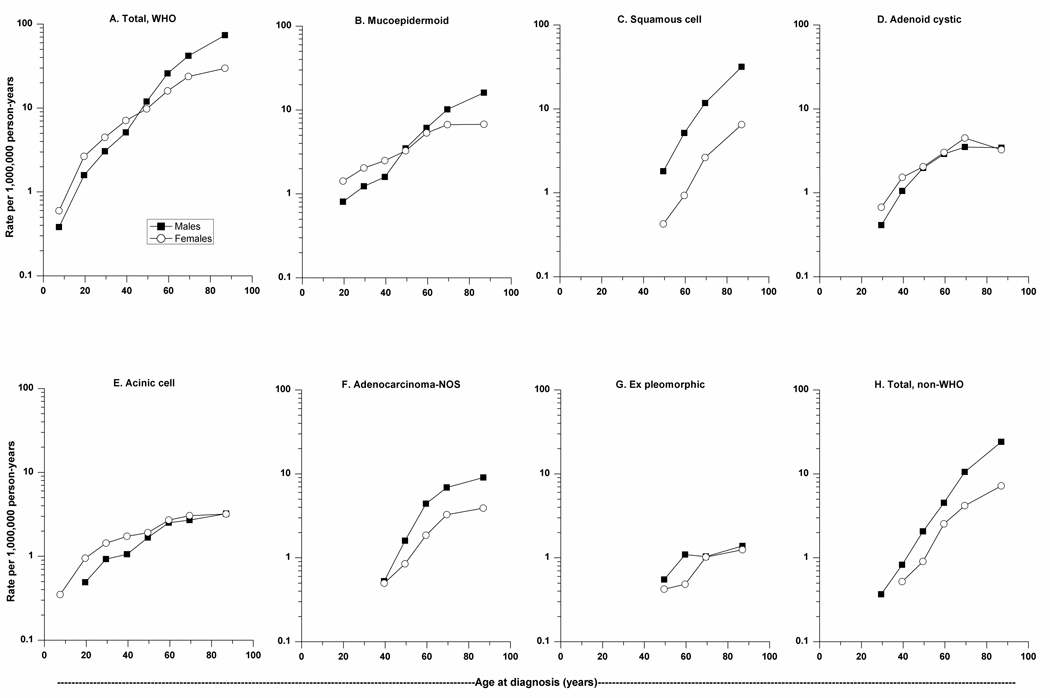
We further explored age-specific differences by gender as depicted in Figure 1. Mucoepidermoid, adenoid cystic, and acinic cell carcinomas tended to have an earlier age at onset than adenocarcinoma-NOS, squamous cell carcinoma, and carcinoma ex pleomorphic adenoma. Mucoepidermoid, adenoid cystic, and acinic cell carcinomas were more common among females through approximately age 50 years; thereafter incidence of adenoid cystic and acinic cell carcinomas was nearly equal among females and males at older ages, in contrast to mucoepidermoid carcinoma which had higher IRs among older males than among older females. Mucoepidermoid carcinoma was the only histologic subtype evaluated that was uniquely characterized by a crossing age-specific IR pattern by gender, which was also reflected in the total WHO category. For adenoid cystic and acinic cell carcinomas, IRs increased more prominently at younger ages with a more moderate rise in incidence thereafter among males and females. These patterns contrast with the exponential rise in IRs of squamous cell carcinoma and total, non-WHO M-SGCs. Distinct from these age-specific patterns, adenocarcinoma-NOS, which generally had higher IRs among males, was characterized by a steep rise in incidence through midlife with a subsequent plateau at older ages.
Figure 1.
(A–H). Age-specific incidence rates of carcinomas of the major salivary glands diagnosed in SEER-13 according to histology and gender, 1992–2006.
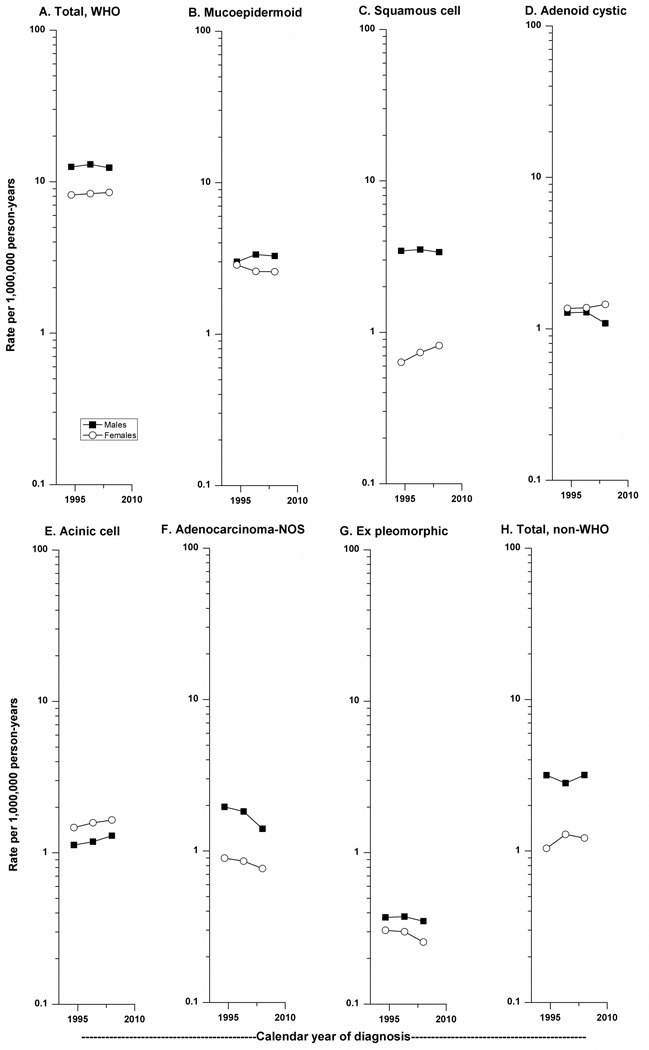
Over the 15-year period of study, there was little change in incidence of M-SGC among males and females for all WHO subtypes combined (Figure 2), with an APC of - 0.10 (P=0.84) and 0.43 (P=0.37), respectively. The most notable change in incidence was observed for adenocarcinoma-NOS (APC=−2.78, P=<0.01) which declined more notably among males (APC=−3.01, P=0.03) than among females (APC=−1.85, P=0.18). Temporal patterns were also evaluated according to age group (<65, 65+ years) (data not shown) and only adenocarcinoma-NOS changed significantly, with APC of −3.51 (P=0.03) and −2.19 (P=0.02) among those <65 and 65+ years, respectively. IRs of carcinoma ex pleomorphic adenoma also decreased among males and females, although APC was not significant for either (males: APC=−0.42; females: APC=−1.26). The greatest increase in IR during 1992–2006 was noted for squamous cell carcinoma among females (APC=2.44, P=0.14), in contrast to the slight decrease in IR observed among males (APC=−0.18, P=0.80), particularly in the more recent calendar period. Acinic cell carcinoma was the only M-SGC subtype with slight but progressive rise in IR over time among males and females (males: APC=0.83, P=0.42; females: APC=0.97, P=0.47).
Figure 2.
(A–H). Trends in age-adjusted incidence rates of carcinomas of the major salivary glands diagnosed in SEER-13 according to histology and gender, 1992–2006.
Discussion
This is among the first studies to evaluate patterns of M-SGC incidence in a U.S. population during 1992–2006 according to the WHO-2005 classification that presents a detailed evaluation of more than 6,000 cases by age, gender, race, calendar year, and site. New information includes the observation that the highest IRs among males were observed for squamous cell carcinoma, mucoepidermoid carcinoma, and adenocarcinoma-NOS while the predominant histologic subtypes among females were mucoepidermoid, acinic cell, and adenoid cystic carcinomas. Male-to-female IRRs varied markedly, with 14–23% lower incidence for acinic cell and adenoid cystic carcinoma and nearly five-fold male-to-female IRRs for squamous cell carcinoma. Mucoepidermoid and adenoid cystic carcinomas IRs were similar among Whites, Blacks, and APIs whereas most other histologic subtypes evaluated generally had higher IRs among Whites. Except for adenoid cystic carcinoma which developed equally in the partotid and submandibular glands, other subtypes occurred primarily in the parotid gland. Age-specific IRs varied by histologic subtype among males and females, and an age-gender interaction was suggested for mucoepidermoid carcinoma. Incidence of adenocarcinoma-NOS decreased significantly over the 15-year time period of study, with no significant changes in other histologic subtypes. Taken together, we demonstrate tremendous heterogeneity in M-SGC incidence patterns by WHO-2005 histologic subtypes, suggesting that this rare tumor includes distinct entities associated with etiologic and/or biologic diversity.
In contrast to other head and neck cancers which are largely squamous cell histology (1, 2), M-SGC includes numerous histologic subtypes with a classification schema that has evolved over time (4, 8). M-SGC is further distinguished from other head and neck malignancies by its lack of international variation (16), although comparisons across international population-based and clinical series are limited by differences in study inclusion criteria (e.g., benign +/− malignant tumors, major +/− minor salivary glands, varying histologic categories). Futhermore, prior population-based studies have reported frequency distributions of M-SGC according to histologic subtype (13–15, 18, 19), but none have described corresponding incidence rates.
There are substantial differences across studies regarding frequencies of M-SGC by histologic subtypes. Many series describe mucoepidermoid carcinoma to be the most commonly occurring M-SGC (8, 11, 12, 24, 25); however, others report a predominance of adenoid cystic carcinoma or nearly equal frequencies of adenoid cystic and mucoepidermoid carcinomas (14, 15, 18, 26). In one of the largest series of M-SGC to date (8), the Armed Forces Institute of Pathology (AFIP) reported mucoepidermoid carcinoma as the most common histology followed in turn by acinic cell carcinoma, adenoid cystic carcinoma, adenocarcinoma-NOS, PLGA, and carcinoma ex-pleomorphic adenoma. This M-SGC histology distribution differs from that observed in the SEER population. Although the AFIP series included only data from civilian laboratories in an effort to minimize potential bias related to cases diagnosed among male military personnel (8), the possibility of referral bias of more difficult cases can not be excluded. In the SEER population, the average age of several M-SGCs, including mucoepidermoid, adenocarcinoma-NOS, acinic cell, and squamous cell carcinomas, was generally older than that reported in the AFIP series, raising the possibility that M-SGC diagnosed among younger individuals may be preferentially sent for external pathology review. Notably, PLGA was rare in our study, likely reflecting its general occurrence in the minor salivary glands and the absence of an ICD-O histology code until 2000.
Population-based studies of M-SGC that have considered age-specific IR patterns according to gender are rare (17). Findings from another SEER-based study (1973–1992) suggested the presence of an age/gender interaction with predominance of M-SGC among females at younger ages and among males at older ages (17). Considering only histologies within the WHO classification, we show that overall IRs of M-SGC are higher among females compared to males prior to approximately 50 years of age, and that IRs among males exceed those among females at older ages. The resulting age-specific crossing pattern was especially marked for mucoepidermoid carcinoma and is consistent with a qualitative or age-gender interaction (27). These findings raise the possibility that hormonal influences may be important to the development of mucoepidermoid carcinoma, and similarly may be central to the development of acinic and adenoid cystic carcinomas which were more common among females at younger ages. Although M-SGC has been linked with reproductive risk factors in at least one study (28), reports have been inconsistent (29).
Ionizing radiation is a well-established risk factor for mucoepidermoid M-SGC in irradiated populations (6, 7). However, radiation likely accounts for only a small fraction of M-SGC reported in our general population study. The evidence linking M-SGC to occupational exposures is sparse, although elevated risks have been noted among male woodworkers, rubber industry workers, and among persons exposed to nickel compounds or silica dust (6). It is plausible that the more prominent rise in incidence of M-SGC at older ages among males compared to females, in particular for mucoepidermoid carcinoma, may reflect occupational exposures in male-dominated jobs, although studies have not assessed risk according to histologic subtypes.
Squamous cell carcinoma accounted for 15% of M-SGC overall and 20% of M-SCG among males. In contrast to our findings, squamous cell carcinomas of the salivary gland generally comprise fewer than 10% of cases in other series (8, 10–12, 14, 15). Varying study inclusion criteria may account for some of the differences observed; however, retrospective reviews of cases initially diagnosed as primary squamous cell carcinoma of the salivary gland found that only approximately 20% of cases were consistent with the original diagnosis (30, 31). In addition, clinical studies evaluating metastases to the parotid glands noted that squamous cell carcinoma accounted for the vast majority of such cases (32, 33). Importantly, a diagnosis of primary squamous cell carcinoma of the salivary gland should be considered only after high-grade mucoepidermoid carcinoma and metastases to the parotid gland from cutaneous, oropharyngeal, or other primary site have been excluded (8, 30, 31, 34).
Although our study can not determine the extent of possible misclassification of squamous cell carcinomas, the age-specific IR patterns we describe were distinct from those of other specified M-SGC histologic subtypes. The male predominance and the exponential rise in IR of squamous cell carcinoma with advancing age exemplifies IR patterns of cancer sites where the long-term, multistep process of carcinogenesis is deemed important, e.g., lung cancer (35). When all histologic types are considered jointly, some studies have suggested an association of M-SGC with tobacco use (36, 37), whereas others have found an equivocal relationship (38, 39).
A paucity of data exists on race and M-SGC. Studies from Africa have described a predominance of both mucoepidermoid (25) and adenoid cystic carcinomas (26). A previous SEER-based study reported 12–16% lower IRs of M-SGC among Blacks compared to Whites (40). We found approximately 20% lower IRs for M-SGC overall among Blacks and APIs compared to Whites, however no racial predilection was observed for mucoepidermoid and adenoid cystic carcinomas. Although small numbers of cases precluded histology-, gender-, and age-specific analyses by race, our findings suggest that there are racial differences in susceptibility to M-SGC according to histologic subtype.
Similar to results from other series, we found that the majority of M-SGCs occurred in the parotid gland (12, 15, 18). The notable exception was adenoid cystic carcinoma, which occurred with equal incidence in the parotid and submandibular glands. These findings further illustrate the diversity that characterizes M-SGC and support the possibility of distinct risk factors and/or biology by histologic subtype.
With the exception of adenocarcinoma-NOS, significant changes in temporal trends were not observed overall or for the other histologic subtypes described. It is possible that the significant decline in incidence for adenocarcinoma-NOS may reflect reclassification to another histologic subtype or a true decline in incidence. Rising temporal trends have been previously reported mainly among older persons (13), suggesting a possible diagnostic bias among the elderly, although findings were limited to males and based on small numbers. In the 15-year time period of our study, we did not find IRs to differ significantly by age (<65 vs. 65+ years).
The strengths of our population-based study include the absence of biases inherent to clinical series and the large numbers of histologically confirmed cases of M-SGC. Our analysis is limited by the lack of central pathology review and standardization of histopathologic diagnosis for all reported M-SGC cases. Furthermore, we can not exclude the possibility of histologic misclassification or misclassified metastatic carcinoma to the salivary gland.
In summary, this investigation is among the first to describe IRs of M-SGC according to histologic categories specified in the most recent WHO-2005 classification scheme (3). We demonstrate differences in IR patterns of various histologic subtypes of M-SGC according to age, gender, race, and site, and these findings suggest that histologic subtypes of M-SGC are characterized by etiologic heterogeneity and/or differences in disease biology. The marked diversity that exists in this group of carcinomas may account, in part, for the limited understanding of the etiology of these cancers to date. Future studies assessing risk factors and host susceptibility in M-SGC will likely benefit from stratification according to histologic subtype, gender, and race.
Acknowledgements
The authors thank David Check, Biostatistics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, for help with the figures.
Financial support: This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; and by the Department of Veterans Affairs.
References
- 1.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 2.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451–457. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Barnes LB, Eveson JW, Reichart P, Sidransky D, editors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 4.Foote FW, Jr, Frazell EL. Tumors of the major salivary glands. Cancer. 1953;6:1065–1133. doi: 10.1002/1097-0142(195311)6:6<1065::aid-cncr2820060602>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8:229–240. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 6.Mayne ST, Morse DE, Winn DM. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. Cancers of the Oral Cavity and Pharynx; pp. 674–696. [Google Scholar]
- 7.Land CE, Saku T, Hayashi Y, Takahara O, Matsuura H, Tokuoka S, Tokunaga M, Mabuchi K. Incidence of salivary gland tumors among atomic bomb survivors, 1950–1987. Evaluation of radiation-related risk. Radiat Res. 1996;146:28–36. [PubMed] [Google Scholar]
- 8.Ellis GL, Auclair PL. Tumors of the Salivary Glands. Washington, D.C: Armed Forces Institute of Pathology; 2008. [Google Scholar]
- 9.Eneroth CM. Salivary gland tumors in the parotid gland, submandibular gland, and the palate region. Cancer. 1971;27:1415–1418. doi: 10.1002/1097-0142(197106)27:6<1415::aid-cncr2820270622>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51–58. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 11.Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44:407–417. doi: 10.1016/j.oraloncology.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–184. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 13.Horn-Ross PL, West DW, Brown SR. Recent trends in the incidence of salivary gland cancer. Int J Epidemiol. 1991;20:628–633. doi: 10.1093/ije/20.3.628. [DOI] [PubMed] [Google Scholar]
- 14.Luukkaa H, Klemi P, Leivo I, Koivunen P, Laranne J, Makitie A, Virtaniemi J, Hinkka S, Grenman R. Salivary gland cancer in Finland 1991--96: an evaluation of 237 cases. Acta Otolaryngol. 2005;125:207–214. doi: 10.1080/00016480510003174. [DOI] [PubMed] [Google Scholar]
- 15.Ostman J, Anneroth G, Gustafsson H, Tavelin B. Malignant salivary gland tumours in Sweden 1960–1989--an epidemiological study. Oral Oncol. 1997;33:169–176. doi: 10.1016/s0964-1955(96)00077-2. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan EM. International variation in the incidence of oral and pharyngeal cancer. Community Dent Health. 2008;25:148–153. [PubMed] [Google Scholar]
- 17.Sun EC, Curtis R, Melbye M, Goedert JJ. Salivary gland cancer in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:1095–1100. [PubMed] [Google Scholar]
- 18.Wahlberg P, Anderson H, Biorklund A, Moller T, Perfekt R. Carcinoma of the parotid and submandibular glands--a study of survival in 2465 patients. Oral Oncol. 2002;38:706–713. doi: 10.1016/s1368-8375(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 19.Muir C, Weiland L. Upper aerodigestive tract cancers. Cancer. 1995;75:147–153. doi: 10.1002/1097-0142(19950101)75:1+<147::aid-cncr2820751304>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER-13 Regs Public-Use, Nov 2008 Sub (1992–2006) National Cancer Institute; DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009. ( www.seer.cancer.gov), based on the November 2008 submission.
- 21.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 22.Percy C, Van Holten V, Muir C, editors. International Classification of Diseases for Oncology. Geneva (Switzerland): World Health Organization; 1990. [Google Scholar]
- 23.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 24.Spitz MR, Batsakis JG. Major salivary gland carcinoma. Descriptive epidemiology and survival of 498 patients. Arch Otolaryngol. 1984;110:45–49. doi: 10.1001/archotol.1984.00800270049013. [DOI] [PubMed] [Google Scholar]
- 25.Kolude B, Lawoyin JO, Akang EE. Salivary gland neoplasms: a 21year review of cases seen at University College Hospital, Ibadan. Afr J Med Med Sci. 2001;30:95–98. [PubMed] [Google Scholar]
- 26.Onyango JF, Awange DO, Muthamia JM, Muga BI. Salivary gland tumours in Kenya. East Afr Med J. 1992;69:525–530. [PubMed] [Google Scholar]
- 27.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 28.Horn-Ross PL, Morrow M, Ljung BM. Menstrual and reproductive factors for salivary gland cancer risk in women. Epidemiology. 1999;10:528–530. [PubMed] [Google Scholar]
- 29.Spitz MR, Tilley BC, Batsakis JG, Gibeau JM, Newell GR. Risk factors for major salivary gland carcinoma. A case-comparison study. Cancer. 1984;54:1854–1859. doi: 10.1002/1097-0142(19841101)54:9<1854::aid-cncr2820540915>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Flynn MB, Maguire S, Martinez S, Tesmer T. Primary squamous cell carcinoma of the parotid gland: the importance of correct histological diagnosis. Ann Surg Oncol. 1999;6:768–770. doi: 10.1007/s10434-999-0768-y. [DOI] [PubMed] [Google Scholar]
- 31.Ying YL, Johnson JT, Myers EN. Squamous cell carcinoma of the parotid gland. Head Neck. 2006;28:626–632. doi: 10.1002/hed.20360. [DOI] [PubMed] [Google Scholar]
- 32.Bergersen PJ, Kennedy PJ, Kneale KL. Metastatic tumours of the parotid region. Aust N Z J Surg. 1987;57:23–26. doi: 10.1111/j.1445-2197.1987.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 33.Nuyens M, Schupbach J, Stauffer E, Zbaren P. Metastatic disease to the parotid gland. Otolaryngol Head Neck Surg. 2006;135:844–848. doi: 10.1016/j.otohns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Eveson JW. Troublesome tumours 2: borderline tumours of salivary glands. J Clin Pathol. 1992;45:369–377. doi: 10.1136/jcp.45.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 36.Hayes RB, Bravo-Otero E, Kleinman DV, Brown LM, Fraumeni JF, Jr, Harty LC, Winn DM. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10:27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 37.Sadetzki S, Oberman B, Mandelzweig L, Chetrit A, Ben-Tal T, Jarus-Hakak A, Duvdevani S, Cardis E, Wolf M. Smoking and risk of parotid gland tumors: a nationwide case-control study. Cancer. 2008;112:1974–1982. doi: 10.1002/cncr.23393. [DOI] [PubMed] [Google Scholar]
- 38.Muscat JE, Wynder EL. A case/control study of risk factors for major salivary gland cancer. Otolaryngol Head Neck Surg. 1998;118:195–198. doi: 10.1016/S0194-5998(98)80013-2. [DOI] [PubMed] [Google Scholar]
- 39.Spitz MR, Fueger JJ, Goepfert H, Newell GR. Salivary gland cancer. A case-control investigation of risk factors. Arch Otolaryngol Head Neck Surg. 1990;116:1163–1166. doi: 10.1001/archotol.1990.01870100057012. [DOI] [PubMed] [Google Scholar]
- 40.Canto MT, Devesa SS. Oral cavity and pharynx cancer incidence rates in the United States, 1975–1998. Oral Oncol. 2002;38:610–617. doi: 10.1016/s1368-8375(01)00109-9. [DOI] [PubMed] [Google Scholar]