Abstract
Background
Heat-treated expressed breastmilk is recommended by WHO as an option to reduce vertical HIV transmission in resource poor regions. Flash-heat (FH) is a low technology pasteurization method developed for home use, but its effect on quantity and quality of breastmilk immunoglobulins is unknown.
Objective
To evaluate FH's effect on breastmilk immunoglobulin levels and antigen binding capacity.
Design/Methods
Fifty HIV+ mothers in South Africa provided breastmilk. Part of each sample served as an unheated (UH) control; the remainder was Flash-heated. Total and antigen-specific IgA and IgG were measured by ELISA. Paired t-test was performed on log transformed data.
Results
FH significantly decreased total IgA and IgG concentrations [geometric mean (geometric sd) 318.0 (1.9) vs. 398.2 (1.9) mcg/mL and 89.1 (2.7) vs. 133.3 (2.5) mcg/mL, p<0.001 each]. Similar decreases in anti-HIV-1 gp120 IgG, anti-pneumococcal polysaccharide and anti-poliovirus IgA occurred (p<0.001 each). Although the latter was most affected, FH retained 66% of the antigen binding ability. In contrast, binding capacity of IgA and IgG to influenza increased after FH (p=0.029 and 0.025 respectively).
Conclusions
Most breastmilk immunoglobulin activity survives FH, suggesting Flash-heated breastmilk is immunologically superior to breastmilk substitutes. Clinical significance of this decreased immunoglobulin activity needs evaluation in prospective trials.
Introduction
Prolonged breastfeeding accounts for up to 40% of maternal to child transmission (MTCT) of HIV in resource poor regions of the world. [1] Multiple studies, however, document that HIV-free infant survival is not improved in many of these areas by use of breastmilk substitutes. [2-5] When infants are not breastfed in these regions an increase in malnutrition [6, 7] and morbidity and mortality from diarrhea [8-10] result. Accordingly, ways to decrease MTCT during breastfeeding could potentially improve HIV-free child survival.
The World Health Organization recommends pasteurization of breastmilk as a modification to breastfeeding in this setting. [11, 12] We have previously described a low ‘tech’ method of pasteurization, Flash-heat, which mothers can use in their homes, and documented that this method can successfully inactivate cell-free HIV in naturally infected human milk [13] as well as in high-titer ‘spiked’ breastmilk. [14] Before subjecting this novel pasteurization method to clinical trial, it was necessary to ascertain the effect on breastmilk immunoglobulins in order to ensure the milk would continue to offer passive immunoprotection.
Flash-heat was designed to mimic commercial flash-pasteurization, a high-temperature, short-time (HTST) pasteurization method. As a general principal, HTST methods more effectively kill micro-organisms while better preserving nutritional food value when compared to low-temperature, long-time pasteurization (LTLT) methods. [15-17]
Effects of LTLT methods on IgA and IgG in milk have been extensively studied, [18-20] but minimal work has examined effects of HTST methods on breastmilk immunoglobulins. [21] Furthermore, Flash-heat raises and lowers the milk's temperature more slowly than does its high ‘tech’ counterpart, which rapidly heats liquid to 72°C for 15 seconds, and therefore can potentially cause greater harm. The objective of this study was to evaluate the effects of Flash-heat treatment on concentrations of breastmilk IgA and IgG and on their binding capacity to selected relevant microbial antigens.
Methods
Samples
Fifty breastmilk samples were collected from HIV-infected women in Durban, South Africa between October-December, 2004. Clinical and demographic characteristics of these women and breastmilk collection procedures have been previously described. [22] Briefly, mean [sd (range)] maternal age was 25.9 [4.9 (19-40)] years, body mass index was 27.5 [4.3 (20.0-37.5)] kg/m2, and CD4+ cell count was 527 [255 (27-1173)]; mean infant age was 15 [11 (6-68)] weeks.
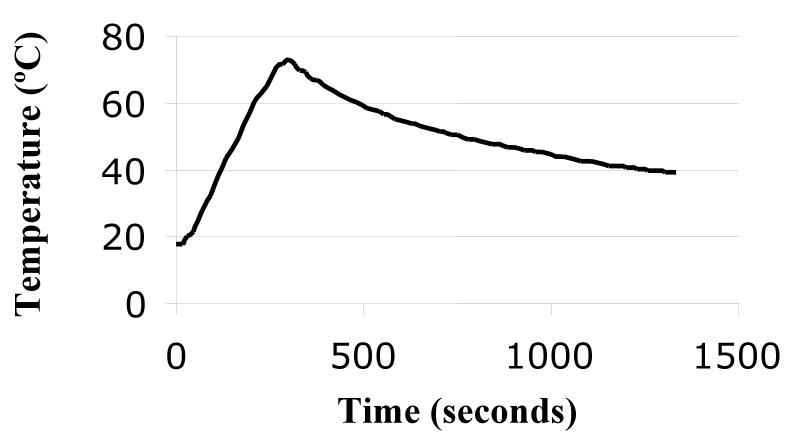
After aliquotting an unheated control, the remainder of the fresh milk was Flash-heated in the laboratory under conditions designed to mimic those in the field. Briefly, 50 mL of milk was placed in an uncovered 16-oz (455 mL) glass food jar which was then placed in 450 mL of water in a 1:1 Hart brand 1 quart aluminum pan. The water and milk were heated together over a butane stove burner, used to imitate the intense heat of a fire, until the water reached 100°C and was at a rolling boil. The jar of breastmilk was then immediately removed from the water bath and allowed to cool to 37.0°C. Time-temperature curve of the milk is shown in Figure 1. The breastmilk typically reached a peak temperature of 72.9°C and was above 56.0°C for 6 minutes 15 seconds. Samples were stored at -70°C until analysis.
Figure 1. Typical time-temperature curve of Flash-heated breastmilk.
Immunoglobulin Measures
Total and antigen-specific IgA and IgG levels were measured in treated and untreated milk samples by ELISA. High binding capacity polystyrene 96-microwell ELISA plates (Nalge Nunc) were coated overnight either with 1 μg/ml of F(ab')2 fragment of goat IgG specific for human IgA or IgG isotypes (Jackson ImmunoResearch Laboratories), or with the following microbial antigens: 1 μg/ml rgp120 of HIV-1 [prepared and purified at University of Alabama (UAB)]; inactivated trivalent influenza viruses purified subvirion antigens at 0.3 μg/ml hemagglutinin of each influenza virus types A (H3N2 and H1N1) and B (Flushield vaccine/Wyeth); inactivated poliovirus (UAB) at 2 μg/ml; 23-valent pneumococcal polysaccharide vaccine (Merck) diluted 1:100; and Salmonella typhosa lipopolysaccharide (Sigma) at 5 μg/ml. Plates were blocked with 5% goat serum in phosphate-buffered saline containing 0.05% Tween 20 for 2 h at room temperature. After washing the plates, serial two-fold dilutions of samples, standards [a pool of human sera calibrated for immunoglobulin isotype levels (The Binding Site)] and positive controls (human sera with known positivity for each of the antigens analyzed) were incubated overnight at 4°C. The captured antibodies were then detected by sequential addition of: a) biotin-conjugated F(ab')2 fragment of goat IgG specific for human IgA or IgG antibodies (BioSource); b) horseradish peroxidase (HRP)-labeled ExtrAvidin (Sigma); c) the chromogenic substrate for HRP: ortho-phenylene-diamine (OPD) and 0.0075% hydrogen peroxide (Sigma). The color reaction was stopped with 1 M sulfuric acid, and absorbance at 490 nm was read in a Bio-Kinetics reader (Bio-Tek Instruments).
Results were calculated for total and antigen-specific IgA and IgG by interpolating the optical densities (ODs) of samples on calibration curves constructed from standardized sera using Delta Soft computer program (BioMettalics). Data were log transformed to achieve normal distribution and analyzed using paired t-test.
Results
In the 50 samples analyzed, Flash-heat induced a statistically significant decrease in total IgA [geometric mean (sd) 318.0 (1.9) vs. 398.2 (1.9) mcg/mL, p<0.001] and IgG [89.1 (2.7) vs. 133.3 (2.5) mcg/mL, p<0.001] concentrations which corresponded to a 20% (95% CI 15, 25) and 33% (27, 39) reduction, respectively. Similar decreases were observed in levels of HIV-1 gp120-specific IgG [26% (18, 33), p<0.001], and in anti-pneumococcal polysaccharide and anti-poliovirus IgA [30% (21, 38) and 34% (26, 41), p<0.001 each]; the anti-poliovirus IgA being the most affected of all the antibodies measured. In contrast, the amount of IgA and IgG binding to influenza viruses increased after Flash-heat by 13% (2, 26), p=0.029 and 15% (2, 31), p=0.025 respectively (Table 1). The increase in anti-Salmonella lipopolysaccharide IgA of 9% (-2,21) did not reach statistical significance, p=0.13.
Table 1. Concentration and Antigen Binding Capacity of IgA and IgG in Flash-heated and Unheated Breastmilk from HIV-Positive Mothers (n=50).
| Immunoglobulin | Unheated Median Concentration (range) |
Flash-heated Median Concentration (range) |
Unheated Geometric Mean (sd) |
Flash- heated Geometric Mean (sd) |
p-value Paired t- test |
% of Concentration in Unheated Milk Detected Post- heat |
|---|---|---|---|---|---|---|
| Total IgA (ug/ml) |
378.2 (155.6, 13164.5) |
323.5 (118.5, 7027.2) |
398.2 (1.9) |
318.0 (1.9) |
<.001 | 79.9 |
| Total IgG (ug/ml) |
117.2 (23.8, 7306.3) |
82.8 (15.6, 6953.5) |
133.3 (2.5) |
89.1 (2.7) |
<.001 | 66.9 |
| HIV-1 gp120 IgG (ng/ml) |
1174.4 (20.1, 126413.2) |
957.5 (21.6, 70010.6) |
1118.0 (3.9) |
827.6 (3.8) |
<.001 | 74.0 |
| Influenza virus IgG (ng/ml) |
246.0 (38.4, 3697.5) |
284.2 (79.6, 3916.5) |
240.8 (2.2) |
272.3 (1.9) |
0.025 | 113.1 |
| Influenza virus IgA (ng/ml) |
465.7 (93.2, 5496.5) |
610.6 (247.1, 4999.5) |
575.6 (2.3) |
663.8 (1.9) |
0.029 | 115.3 |
| Pneumococcal polysaccharide IgA (ng/ml) |
797.3 (289.8, 32198.9) |
608.7 (71.0, 38209.2) |
906.5 (2.1) |
635.7 (2.4) |
<.001 | 70.1 |
| Salmonella lipopolysaccharide IgA (ng/ml) |
270.8 (101.1, 5546.7) |
253.5 (65.2, 4996.3) |
284.0 (1.9) |
308.7 (2.0) |
0.13 | 108.7 |
| Poliovirus IgA (ng/ml) |
729.3 (138.9, 11336.2) |
525.5 (94.5, 10508.5) |
914.8 (2.6) |
604.2 (2.6) |
<.001 | 66.1 |
Discussion
The majority of IgA and IgG activity in these samples survived the Flash-heat treatment and, in the case of anti-influenza immunoglobulins, the antigen-binding capacity of both increased. These results suggest that infants receiving Flash-heated breastmilk would still receive substantial passive immunoprotection. The IgA concentration measured after Flash-heating was nearly identical to that reported by Goldblum et al.[21] who observed a decrease in IgA concentration from 0.37 to 0.30 mg/mL (19%) after heating breastmilk to 72°C for 15 seconds; this difference was not statistically significant in that study, possibly due to the fewer samples analyzed. These results suggest that our ‘low-tech’ version of HTST requiring longer time to reach 72°C did not substantively increase the immunoglobulin denaturation compared to the ‘high-tech’ version. A similar proportion of IgA survives the traditional Holder pasteurization used in breastmilk banks (62.5°C for 30 minutes). Gibbs et al. [20] detected a 21% loss of IgA with Holder pasteurization of drip milk and Ford noted 20% reduction in IgA titer with this method. [19] However, very little IgA has been noted to survive higher temperatures, with greater than 80% reduction at 87°C for 1 second [21] and essentially total destruction with boiling. [23] IgG is apparently more heat sensitive than IgA, as noted by 33% vs. 20% destruction by the Flash-heat method in our study; greater destruction of IgG relative to IgA was also noted by Evans [24] when heating to 73°C for 30 minutes.
Most importantly, the antibody activity of IgA and IgG, measured as antigen binding capacity, was also predominantly preserved with the Flash-heat method, with post-heat activity ranging from 66% of that measured pre-heat for anti-polio virus IgA to 115% for anti-influenza virus IgA. It is unclear why the influenza virus-specific antibodies slightly increased in our samples after pasteurization, and if this would correlate clinically to enhanced protection. Chen and colleagues reported that 61.1% of E. coli-specific IgA activity was retained after HTST pasteurization of human milk, [25] a percentage slightly lower than that of polio-specific IgA in our study, the most diminished by Flash-heat. Again, this suggests that there is no greater impact on antibody activity when the milk is heated more slowly during the Flash-heat method vs. ‘high-tech’ methodologies. It is reassuring to note that Carbornare and colleagues in their study of Holder pasteurization found that although concentration and anti-enteropathogenic E. coli activity of IgA in colostrum were reduced, the remaining IgA was sufficient to effectively inhibit bacterial adhesion to HEp-2 cells. [26]
IgA concentration in breastmilk is known to decline as the infant ages. The geometric mean concentration of IgA in our unheated milk samples from the HIV-infected women was 0.40 mg/mL (arithmetic mean was 0.46 mg/mL) at a mean postpartum time of 15 weeks. Previously reported means are somewhat variable: 0.71 mg/mL at 10 weeks postpartum, [27] 0.50 mg/mL at 12 weeks, [28] and 0.57 mg/mL at a mean of 24 weeks.[29] As malnourished women have less IgA in their colostrum, [30] it is not surprising that the samples from our cohort had slightly lower levels of IgA than the samples from the above reports. [27-29] While no women in our study were underweight, they were from an impoverished region and may have had micronutrient deficiencies. HIV-infection may also contribute to alterations in IgA content. To the best of our knowledge, effects of HIV infection per se on breastmilk composition has not yet been studied. Alternatively, the differences may be due to laboratory methodologies, as the original reports were all performed more than 25 years ago and used radial immunodiffusion rather than ELISA to measure immunoglobulin levels. In contrast to the lower concentrations of IgA, IgG levels in the milk from HIV-infected mothers in this study were substantially higher than those previously reported in mature milk (30 mg/mL), [31] presumably because of the hypergammaglobulinemia frequently found in HIV-infected individuals.[32]
The current study was limited to laboratory measurement of IgA and IgG concentrations and binding capacities for five representative antigens. Antibody activity for other antigens may have been more or less affected. Also, this study reports the impact of Flash-heat on immunoglobulins only whereas many other breastmilk components provide anti-infective activity and immunoprotection. Work is currently underway to evaluate Flash-heat's effect on bioactivity of key breastmilk proteins.
We anticipate that because Flash-heated milk retains most antibody-specificity for the microbial antigens tested, it will confer similar protection from infection for the infant as would unheated milk. Correlation with clinical data from field trials will be necessary. In conclusion, this study documents that the majority of breastmilk IgA and IgG survive Flash-heat treatment and retain the ability to bind specific antigens, suggesting that this method would be immunologically superior to boiling milk or using breastmilk substitutes. The clinical significance of the observed decrease in antibody activity to some antigens, will need further evaluation in prospective clinical trials. Flash-heat may be a safe and affordable method for home pasteurization for HIV+ mothers in developing countries, of particular value during times of greater risk for HIV transmission, such as during episodes of infant oral thrush or maternal mastitis or upon addition of complementary foods.
Acknowledgments
The authors would like to acknowledge the mothers who participated in this study and the Cato Manor Clinic staff for their time and dedication. Funding for this project was provided by the National Institute of Child Health and Human Development (Grant #HD051473-01), the University of California, Davis Children's Miracle Network, the Thrasher Research Fund and the James B. Pendleton Charitable Trust.
References
- 1.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 2.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of Early, Abrupt Weaning for HIV-free Survival of Children in Zambia. N Engl J Med. 2008 doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha A, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1- infected women: A randomized clinical trial. JAMA. 2001;286:2413–2420. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becquet R, Bequet L, Ekouevi DK, Viho I, Sakarovitch C, Fassinou P, et al. Two-Year Morbidity-Mortality and Alternatives to Prolonged Breast-Feeding among Children Born to HIV-Infected Mothers in Cote d'Ivoire. PLoS Med. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becquet R, Leroy V, Ekouevi DK, Viho I, Castetbon K, Fassinou P, et al. Complementary feeding adequacy in relation to nutritional status among early weaned breastfed children who are born to HIV-infected mothers: ANRS 1201/1202 Ditrame Plus, Abidjan, Cote d'Ivoire. Pediatrics. 2006;117:e701–710. doi: 10.1542/peds.2005-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson W, Alons C, Fidalgo L, Piwoz E, Kahn S, Macombe S, et al. The challenge of providing adequate infant nutrition following early breastfeeding cessation by HIV-positive, food-insecure Mozambican mothers. XVI International AIDS Conference; Toronto, Canada. 2006. [Google Scholar]
- 8.Creek T, Arvelo W, Kim A, Lu L, Bowen A, Finkbeiner T, et al. Role of infant feeding and HIV in a severe outbreak of diarrhea and malnutrition among young children, Botswana, 2006. XIV Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 9.Creek T, Arvelo W, Kim A, Lu L, Bowen A, Mach O, et al. A large outbreak of diarrhea among non-breastfed children in Botswana 2006 - Implications for HIV prevention strategies and child health. XIV Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 10.Kourtis AP, Fitzgerald G, Hyde L, Tien H, Chavula C, Mumba N, et al. Diarrhea in uninfected infants of HIV-infected mothers who stop breastfeeding at 6 months: the BAN study experience. XIV Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 11.WHO, UNICEF. HIV and infant feeding: A guide for health-care managers and supervisors. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 12.WHO. WHO HIV and Infant Feeding Technical Consultation. Geneva: 2006. Consensus Statement: Held on behalf of the Inter-Agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and their Infants. [Google Scholar]
- 13.Israel-Ballard K, Donovan R, Chantry C, Coutsoudis A, Sheppard H, Sibeko L, Abrams B. Flash-heat inactivation of HIV-1 in human milk: a potential method to reduce postnatal transmission in developing countries. J Acquir Immune Defic Syndr. 2007;45:318–323. doi: 10.1097/QAI.0b013e318074eeca. [DOI] [PubMed] [Google Scholar]
- 14.Israel-Ballard K, Chantry C, Dewey K, Lonnerdal B, Sheppard H, Donovan R, et al. Viral, Nutritional, and Bacterial Safety of Flash-Heated and Pretoria-Pasteurized Breast Milk to Prevent Mother-to-Child Transmission of HIV in Resource-Poor Countries: A Pilot Study. J Acquir Immune Defic Syndr. 2005;40:175–181. doi: 10.1097/01.qai.0000178929.15904.95. [DOI] [PubMed] [Google Scholar]
- 15.Dhar J, Fichtali J, Skura BJ. Pasteurization Efficiency of a HTST System for Human Milk. J Food Sci. 1996;61:569–573. [Google Scholar]
- 16.Morgan JN, Lin FJ, Eitenmiller RR, Barnhart HM, Toledo RT. Thermal destruction of Escherichia coli and Klebsiella pneumoniae in human milk. J Food Pro. 1988;51:132–136. doi: 10.4315/0362-028X-51.2.132. [DOI] [PubMed] [Google Scholar]
- 17.Terpstra FG, Rechtman DJ, Lee ML, Van Hoeij K, Berg H, Van Engelenberg F, Van't Wout AB. Antimicrobial and antiviral effect of high-temperature short-time (HTST) pasteurization applied to human milk. Breastfeeding Medicine. 2007;2:27–33. doi: 10.1089/bfm.2006.0015. [DOI] [PubMed] [Google Scholar]
- 18.Braga L, Palharas D. Effect of evaporation and pasteurization in the biochemical and immunological composition of human milk. J Pediatr (Rio J) 2007;83:59–63. doi: 10.2223/JPED.1578. [DOI] [PubMed] [Google Scholar]
- 19.Ford JE, Law BA, Marshall VM, Reiter B. Influence of the heat treatment of human milk on some of its protective constituents. J Pediatr. 1977;90:29–35. doi: 10.1016/s0022-3476(77)80759-2. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs JH, Fisher C, Bhattacharya S, Goddard P, Baum JD. Drip breast milk: it's composition, collection and pasteurization. Early Hum Dev. 1977;1:227–245. doi: 10.1016/0378-3782(77)90037-8. [DOI] [PubMed] [Google Scholar]
- 21.Goldblum RM, Dill CW, Albrecht TB, Alford ES, Garza C, Goldman AS. Rapid high-temperature treatment of human milk. J Pediatr. 1984;104:380–385. doi: 10.1016/s0022-3476(84)81099-9. [DOI] [PubMed] [Google Scholar]
- 22.Israel-Ballard K, Abrams B, Coutsoudis A, Sibeko L, Cheryk L, Chantry C. Vitamin Content of Breastmilk from HIV-1 Infected Mothers Before and After Flash-heat Treatment. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e31817beb8d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh JK, May JT. Anti-infective properties of breast milk. J Pediatr. 1979;94:1–9. doi: 10.1016/s0022-3476(79)80340-6. [DOI] [PubMed] [Google Scholar]
- 24.Evans TJ, Ryley HC, Neale LM, Dodge JA, Lewarne VM. Effect of storage and heat on antimicrobial proteins in human milk. Arch Dis Child. 1978;53:239–241. doi: 10.1136/adc.53.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen HY, Allen JC. Human milk antibacterial factors: the effect of temperature on defense systems. Adv Exp Med Biol. 2001;501:341–348. [PubMed] [Google Scholar]
- 26.Carbonare SB, Palmeira P, Silva ML, Carneiro-Sampaio MM. Effect of microwave radiation, pasteurization and lyophilization on the ability of human milk to inhibit Escherichia coli adherence to HEp-2 cells. J Diarrhoeal Dis Res. 1996;14:90–94. [PubMed] [Google Scholar]
- 27.Mickleson KN, Moriarty KM. Immunoglobulin levels in human colostrum and milk. J Pediatr Gastroenterol Nutr. 1982;1:381–384. doi: 10.1097/00005176-198201030-00018. [DOI] [PubMed] [Google Scholar]
- 28.Goldman AS, Garza C, Nichols BL, Goldblum RM. Immunologic factors in human milk during the first year of lactation. J Pediatr. 1982;100:563–567. doi: 10.1016/s0022-3476(82)80753-1. [DOI] [PubMed] [Google Scholar]
- 29.Liebhaber M, Lewiston NJ, Asquith MT, Olds-Arroyo L, Sunshine P. Alterations of lymphocytes and of antibody content of human milk after processing. J Pediatr. 1977;91:897–900. doi: 10.1016/s0022-3476(77)80885-8. [DOI] [PubMed] [Google Scholar]
- 30.Miranda R, Saravia NG, Ackerman R, Murphy N, Berman S, McMurray DN. Effect of maternal nutritional status on immunological substances in human colostrum and milk. Am J Clin Nutr. 1983;37:632–40. doi: 10.1093/ajcn/37.4.632. [DOI] [PubMed] [Google Scholar]
- 31.Weaver LT, Arthur HM, Bunn JE, Thomas JE. Human milk IgA concentrations during the first year of lactation. Arch Dis Child. 1998;78:235–239. doi: 10.1136/adc.78.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagase H, Agematsu K, Kitano K, Takamoto M, Okubo Y, Komiyama A, Sugane K. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin Immunol. 2001;100:250–259. doi: 10.1006/clim.2001.5054. [DOI] [PubMed] [Google Scholar]