Abstract
The epithelium of the mammary gland exists in a highly dynamic state, undergoing dramatic morphogenetic changes during puberty, pregnancy, lactation, and regression. The recent identification of stem and progenitor populations in mouse and human mammary tissue has provided evidence that the mammary epithelium is organized in a hierarchical manner. Characterization of these normal epithelial subtypes is an important step toward understanding which cells are predisposed to oncogenesis. This review summarizes progress in the field toward defining constituent cells and key molecular regulators of the mammary epithelial hierarchy. Potential relationships between normal epithelial populations and breast tumor subtypes are discussed, with implications for understanding the cellular etiology underpinning breast tumor heterogeneity.
Keywords: Stem cell, luminal progenitor, mammary gland, development, breast cancer, BRCA1
Breast cancer is a very heterogeneous disease at both the histological and molecular levels. At least six distinct subtypes have been described on the basis of gene expression profiling, with the most important determinants of these subtypes being the presence or absence of expression of the estrogen receptor (ER) or the progesterone receptor (PgR), or the amplification/overexpression of the HER2/ERBB2 locus (Perou et al. 2000; Sorlie et al. 2001; Sotiriou et al. 2003; Herschkowitz et al. 2007). Despite the ability of these subtypes to predict outcome, patient response to chemotherapy or targeted therapy remains variable. The prevailing concept in the field has been that these different subtypes originate in distinct breast epithelial cells that serve as the “cell of origin.” A better understanding of breast tumor heterogeneity and the nature of tumor-propagating cells requires delineation of the mammary epithelial subtypes that reside within normal human breast tissue. Eventual lineage tracing of specific mammary epithelial cells will be required to definitively identify “cells of origin” for the different tumor types.
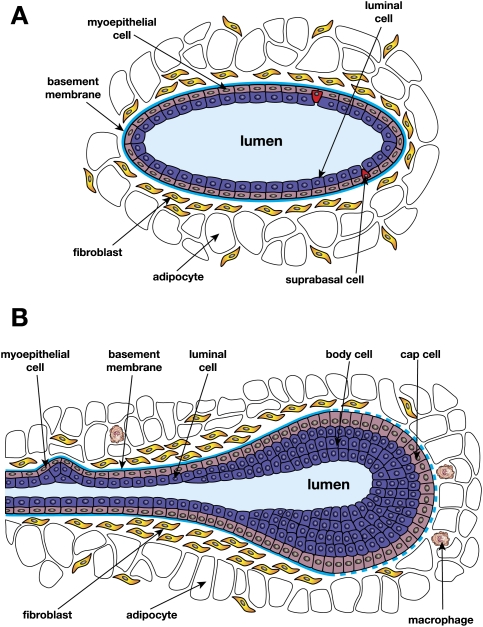
Analogous to the paradigm established by the hematopoietic compartment, there is increasing evidence for the existence of a differentiation hierarchy in the adult mammary gland. Mammary stem cells (MaSCs) are presumed to be important for both organ development and maintaining tissue homeostasis. These cells give rise to mature epithelium of either the luminal or myoepithelial lineage via a series of lineage-restricted intermediates. The luminal lineage can be further subdivided into ductal and alveolar luminal cells that line the ducts and constitute the alveolar units that arise during pregnancy, respectively. In contrast, myoepithelial cells are specialized, contractile cells located at the basal surface of the epithelium adjacent to the basement membrane (Fig. 1). The profound expansion of mammary epithelium that occurs during puberty and pregnancy further implicates a stem-like cell with remarkable regenerative capability.
Figure 1.
Schematic representations of a duct (A) and a TEB (B). A suprabasal cell sits on the myoepithelial layer but does not reach the lumen.
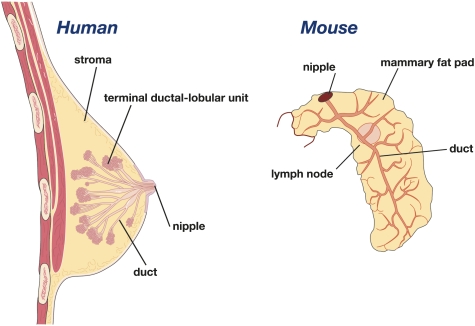
It is notable that there are morphological differences between mouse and human mammary tissue. The human breast is characterized by a branching network of ducts that end in clusters of small ductules that constitute the terminal ductal lobular units (TDLUs), with the vast majority of breast cancers arising within the TDLUs (Fig. 2). In contrast, the mouse mammary epithelial tree does not possess TDLUs, but comprises alveolar buds that are formed during each estrous cycle. Ductal branching and elongation occur from prominent terminal end buds (TEBs) in the mammary gland during puberty (Fig. 1). Furthermore, the mouse mammary gland has less fibrous connective tissue than the human breast, but significantly more adipocytes. Despite differences in the architecture of the ductal tree between species, emerging evidence points to striking parallels in their cellular hierarchies (described below). Significant insights into breast cancer have also come from genetically engineered mouse models of mammary tumorigenesis, further emphasizing functional similarities between mouse and human mammary tissue.
Figure 2.
Schematic representations of the human and mouse mammary glands.
A short history of mammary fat pad transplantation assays
The development and optimization of in vivo mammary reconstitution and tissue dissociation techniques over the last 60 years has allowed the recent prospective isolation of MaSCs. The in vivo transplantation method pioneered by De Ome et al. (1959) represents the “gold-standard” assay for mammary gland reconstitution in mice. This assay involves de-epithelialization of the prepubertal mammary gland, resulting in a cleared fat pad into which donor explants or cells can be transplanted. The nonepithelial or stromal elements in the mammary gland comprise fibroblasts, endothelial cells, macrophages, and adipocytes, and are collectively referred to as the mammary fat pad (Neville et al. 1998).
Classical transplantation studies in the mouse revealed that mammary epithelial outgrowths could be generated in cleared mammary fat pads implanted with either explants (small fragments) or cell suspensions (Hoshino and Gardner 1967; Daniel et al. 1968; Smith 1996). Explants taken from different regions of the mammary gland were demonstrated to reconstitute fully functional outgrowths, indicating the presence of repopulating cells throughout the ductal epithelial tree. Furthermore, these could be serially transplanted for up to seven generations before the onset of senescence. Precursor cells were also shown to exist throughout the life span of the mammary gland, but neither the reproductive history nor developmental state of the gland had significant impact on the longevity of the mammary transplants (Daniel and Young 1971; Smith and Medina 1988). The clonality of mammary outgrowths was demonstrated using MMTV-infected donor tissue fragments, suggesting that a single stem cell was capable of repopulating the entire mammary epithelium (Kordon and Smith 1998).
Delineation of mouse MaSCs
Prospective isolation of the mouse MaSC
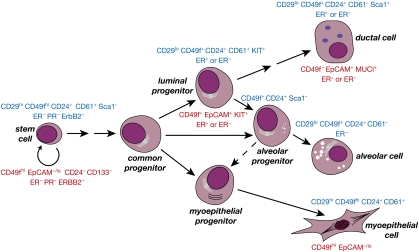
One of the primary challenges in developing a rigorous assay for MaSC activity has been the requirement to dissociate solid tissue into a suspension of single cells for fractionation studies. Epithelial cells in solid organs are tightly associated with one another and/or the surrounding extracellular matrix, and depend on these interactions for their normal function. Nevertheless, using a series of minimal enzymatic digestions, it has been possible to achieve viable single-cell suspensions that can then be used for flow cytometry to sort cells on the basis of cell surface marker expression (Shackleton et al. 2006; Sleeman et al. 2006; Stingl et al. 2006). Notably, the focus has been on freshly dissociated mammary tissue to avoid the potential deleterious effects associated with the in vitro culture of mammary epithelial cells. Thus far, combinations of cell surface markers have been applied for the isolation of discrete epithelial subpopulations (markers are shown in Fig. 3; Table 1). As additional markers are discovered to allow further purification, it is anticipated that they, too, will be required in a combinatorial manner. Although there is a strong consensus in the described cell surface phenotypes of stem and progenitor cells by different groups (see below), some markers, such as CD24, have been reported differently due to the use of antibodies conjugated to varying fluorochromes. Other variabilities in FACS profiles have underscored the importance of antibody titration and the use of controls for establishing robust gates.
Figure 3.
Model of the differentiation hierarchy within mammary epithelium. Primary cell surface markers used in the isolation of mouse and human epithelial cell subsets are shown in blue and red, respectively. (ER) ERα. The common progenitor is also referred to as a bipotent progenitor cell. There may be a hierarchy of stem and bipotent progenitor cells. During pregnancy, the alveolar progenitor may exhibit bipotential capacity.
Table 1.
Markers used in the fractionation of epithelial cells in mouse and human mammary tissues
The availability of an organ-specific in vivo reconstitution assay has allowed the evaluation of mammary repopulating activity in defined cell subsets when transplanted at limiting dilution into cleared fat pads. Mouse MaSCs are highly enriched in the CD49fhiCD29hiCD24+Sca1− subset (referred to as MaSC-enriched) and can generate extensive ductal outgrowths upon transplantation (Shackleton et al. 2006; Sleeman et al. 2006; Stingl et al. 2006). The formation of milk-producing alveolar units during pregnancy underscores the multidifferentiative capability of these stem cells. The regenerated outgrowths were shown to comprise daughter cells with the same in vivo repopulating activity as the original transplanted stem cell. Thus, MaSCs display the defining stem cell characteristics of in vivo multilineage differentiation and self-renewal. Moreover, a single genetically tagged MaSC could regenerate an entire mammary epithelial tree (Shackleton et al. 2006), thus demonstrating that an epithelial organ could be reconstituted from a single stem cell. Compatible with these data, mixing experiments using limiting numbers of freshly isolated genetically tagged cells and wild-type cells in a 1:1 ratio produced few chimeric structures (Shackleton et al. 2006; Stingl et al. 2006). Previous studies using large numbers of cells and/or cultured cells have indicated that mammary epithelial progenitor cells can form chimeric structures in a polyclonal manner (Smith 1996; Brisken et al. 1998; Boulanger et al. 2005). Nonetheless, cooperation between different epithelial cells seems almost implicit, as asymmetric division of a single transplanted stem cell would yield such progeny.
It is important to note that the CD49fhiCD29hiCD24+Sca1− subset comprises a small pool of MaSCs (<5%). This heterogeneous subset also contains mature myoepithelial cells and other likely intermediates yet to be identified, such as a bipotential progenitor or committed basal progenitor cell. Estimates of the number of MaSCs in the steady-state mammary gland have varied widely (1000–14,000 MaSCs per young adult gland) due to loss of cells during the dissociation procedure. MaSCs can be distinguished from luminal epithelium by lower (but not negative) levels of CD24 expression (Sleeman et al. 2006). Although cells expressing the highest level of CD49f were shown to be enriched for mammary repopulating capacity, it has proven difficult to segregate myoepithelial and stem cells, as they exhibit a common cell surface phenotype and gene expression profile (Stingl et al. 2006). The similarities between stem and myoepithelial cells may in part reflect their shared basal position.
Given that most MaSCs express high levels of both α6 (CD49f) and β1 (CD29) integrins (Asselin-Labat et al. 2008), it is tempting to speculate that the α6/β1 heterodimeric complex has an important role in anchoring these stem cells to the extracellular matrix. In the mammary gland, β1 integrin plays an essential role in stem cell maintenance, as well as governing the balance of the basal and luminal lineages (Taddei et al. 2008). Notably, β1 integrins in Drosophila melanogaster gonadal stem cells play a role in the stem cell niche and organization of the extracellular matrix (Tanentzapf et al. 2007). The down-regulation of β1 and α6 integrins that occurs during mammary tumor progression suggests that disengagement of MaSCs and/or myoepithelial cells from their normal microenvironment may be an integral part of the tumorigenic process (Lin et al. 2003; Vaillant et al. 2008).
Mouse MaSCs appear to be cycling
Although the majority of MaSCs in the mouse are cycling (Stingl et al. 2006), there is enrichment of label-retaining cells (LRCs) in the MaSC-enriched fraction (Shackleton et al. 2006), suggesting a pool of quiescent stem cells. Such a pool may be activated during puberty or pregnancy to allow epithelial cell expansion. Long-term label-retaining epithelial cells that divide asymmetrically and retain their template DNA strands have also been described (Smith 2005). Although repopulating cells have been demonstrated throughout different stages of mammopoiesis, they may not be identical. The subpopulations of LRCs that express steroid hormone receptors in both mice (Booth and Smith 2006) and humans (Clarke et al. 2005) are distinct from the mouse MaSC-enriched subset defined by in vivo repopulation, but may represent a short-term repopulating cell, reminiscent of that occurring in the hematopoietic compartment. Furthermore, the parity-identified mammary epithelial cell (PI-MEC) population (Wagner and Smith 2005) that persists following involution may correspond to a short-term repopulating cell. Serial transplantation assays using uncultured cells will be essential to address a potential hierarchy of stem cells with differing self-renewing capabilities.
Mammary epithelium and pregnancy
A central question in the mammary gland and breast cancer fields is how pregnancy elicits permanent changes in the mammary gland and by what mechanism an early pregnancy decreases the risk of breast cancer (MacMahon et al. 1970). While it is recognized that the post-pregnancy mammary gland morphologically resembles a virgin gland, permanent alterations in gene expression patterns have been demonstrated (Russo et al. 2005). Recent findings using mice have suggested that an early pregnancy is associated with a small decrease in MaSC number, although their capacity to repopulate the fat pad was unaffected (Siwko et al. 2008). A later pregnancy was shown to have no effect on the MaSC pool (Britt et al. 2009). It therefore will be important to recapitulate these studies by direct comparison of an early versus late pregnancy using purified cellular subsets. Although the stroma may predominantly mediate the protective effects of an early pregnancy on breast cancer (Abrams et al. 1998), the developmental state of the epithelium may also be altered (perhaps via epigenetic modifications), thus permanently affecting the response of these cells to carcinogens throughout life. While multiparity reduces the long-term risk of breast cancer, there is an increased short-term risk of developing cancer for a few years following pregnancy (Lambe et al. 1994). The increased risk may reflect expansion of a stem or transit-amplifying pool that is predisposed to targeting by oncogenic events. In addition, the microenvironment is thought to play an instrumental role in promoting tumorigenesis after remodeling of the mammary gland to its prepregnant state (Schedin 2006).
Prospective isolation of human MaSCs
In human breast tissue, the observation of identical chromosomal alterations in contiguous regions of human breast epithelium has implied the presence of MaSCs (Deng et al. 1996; Lakhani et al. 1996; Tsai et al. 1996). The persistence of a long-lived cell in breast tissue is also consistent with the increased risk of breast cancer associated with ionizing radiation exposure in teenage women that is not evident for many years following exposure (Land and McGregor 1979). Moreover, extensive in vitro clonogenic assays using human breast epithelial cells have provided support for a hierarchical model of human breast epithelium (Stingl et al. 2001; Gudjonsson et al. 2002; Dontu et al. 2003; Villadsen et al. 2007). Only recently, however, has it been possible to explore the in vivo regenerative potential of epithelial cells in human mammary tissue. In important studies preceding isolation of the human MaSC, Kuperwasser et al. (2004) “humanized” the mammary fat pads of immunocompromised NOD/SCID mice by preinjection of immortalized human fibroblasts to generate a stromal environment more characteristic of human breast tissue. Using this assay, Ginestier et al. (2007) identified a subpopulation of cells with stem/progenitor cell activity that exhibited high aldehyde dehydrogenase 1 (ALDH1) activity. The ALDH1+ epithelial cell subset was shown to be enriched for cells that could generate mammary epithelial structures in vivo, but their self-renewal properties were not defined. Curiously, the ALDH1+ cells were restricted to the luminal epithelial rather than the basal layer, and did not express typical luminal or myoepithelial lineage markers.
A subset of human breast cells defined by high expression of CD49f and negligible (or low) expression of the epithelial cell adhesion molecule EpCAM has been demonstrated recently to have mammary regenerative capacity in vivo, using either an orthotopic or a nonorthotopic transplantation site (Eirew et al. 2008; Lim et al. 2009). Coimplantation of epithelial subsets with immortalized human breast fibroblasts into the cleared fat pads of NOD/SCID/IL2Rγ−/− recipient mice revealed that only the CD49fhiEpCAM− subpopulation had regenerative and self-renewal capacity, although the latter proved to be limited (Lim et al. 2009). The regenerated human mammary structures contained lobular regions reminiscent of TDLUs that characterize normal breast tissue and were capable of terminal differentiation. The low repopulating frequency observed presumably reflects inadequate humanization of this orthotopic site, despite the use of supporting human mammary fibroblasts and estrogen implants. Suboptimal humanization also likely accounts for the low rate of engraftment of human breast tumors in mice. Although serial transplantation remains a major challenge for human stem cell work, improvement of the fat pad microenvironment may allow more definitive proof of the self-renewing ability of human MaSCs. The importance of the host strain and microenvironment in xenotransplantation assays is exemplified by recent work showing that the tumor-initiating capability of melanoma cells could be increased by >100,000-fold using combined modifications (Quintana et al. 2008). The inclusion of Matrigel, however, for assaying normal stem cell function may not always be optimal, since the extracellular matrix can lead to morphological changes (Bissell and Labarge 2005).
To circumvent some of the inherent challenges associated with implantation into the mammary fat pad, Eaves and colleagues (Eirew et al. 2008) developed an assay that allows quantitation of human MaSCs under the highly vascular renal subcapsule. This assay extended on previous observations that human mammary tissue could be maintained after implantation into this site using irradiated mouse fibroblasts (Parmar et al. 2002). Suspension of CD49fhiEpCAMlo cells in collagen gels and subsequent xenografting under the renal capsule revealed their mammary reconstituting capability and allowed quantification of MaSCs in reduction mammoplasty tissue, with estimates of one MaSC per 103–104 total mammary epithelial cells. Pertinently, this system provides a readout for self-renewal and has the potential for assessing the sensitivity of MaSCs to cytokines and inhibitors. In another study (Villadsen et al. 2007), the phenotype of the subset (CD49fhiEpCAM+) that generated budding TDLU-like structures in vitro differs from that of the MaSC subset defined in vivo (CD49fhiEpCAMlo), for reasons that remain unclear at this stage.
Identification of luminal progenitor populations in mammary epithelium
Progenitor cells committed to a luminal cell fate have been identified in the mouse mammary gland on the basis of CD61 (β3 integrin) expression, and negligible or low levels of CD133 (prominin-1) and Sca1 (Asselin-Labat et al. 2007; Sleeman et al. 2007). CD61 and CD133 expression do not resolve identical luminal subsets, but the CD61+CD29loCD24+ subset may be contained within the CD133−CD24+Sca1− population (Kendrick et al. 2008). CD61 marks ∼30% of ductal luminal cells in the virgin mammary gland (Asselin-Labat et al. 2007). Notably, these progenitor cells are restricted to a luminal cell fate and do not have any regenerative capacity in vivo. Differentiation of mouse epithelium along the luminal lineage is accompanied by a profound decrease in CD61 levels and increased expression of CD133 and Sca1, yielding CD61−CD133+Sca1+ mature luminal cells. While a substantial fraction of mature ductal cells express ERα, only a small fraction (<10%) of mouse luminal progenitors are ERα-positive.
Functionally distinct luminal progenitor cells are likely to reside within mammary tissue. CD61 appears to delineate a common luminal-restricted progenitor that can commit to either a ductal or an alveolar cell fate, dependent on the hormonal milieu. This notion is largely based on findings from analysis of Gata-3-deficient mammary glands (see below). The proportion of CD61+ progenitor cells is highest during puberty, concomitant with extensive ductal branching and elongation, indicating that these cells correspond to ductal progenitors. Their level declines markedly during mid–late pregnancy, when alveolar differentiation occurs. It seems probable that a discrete population of alveolar-restricted progenitors also exists, and these would be anticipated to expand during the early phase of pregnancy. Likely candidates include the CD24+Sca1− subset, which expresses milk protein but not ERα genes (Sleeman et al. 2007), and the recently defined Sca1−CD49b+ER− luminal progenitor cell (Li et al. 2009). The “mature” luminal cell subset (CD29loCD24+CD61−) also contains a small fraction of clonogenic cells that may have alveolar progenitor activity (Asselin-Labat et al. 2007).
In human breast tissue, both unipotent and bipotent progenitors have been identified. Bipotent progenitor and stem cells display a phenotype of EpCAM−/loCD49f+ (also MUC1−CD24−CD133−Thy1+CD10+), but cannot be distinguished in these cell-based assays. Through serial passaging, myoepithelial-restricted progenitor cells were shown to lie downstream from bipotent progenitors (Stingl et al. 2001). On the other hand, luminal-restricted progenitors exhibit an EpCAM+CD49f+MUC1+CD24+CD133+Thy1−CD10− phenotype, with abundant expression of EpCAM also occurring on mature luminal epithelial cells (Stingl et al. 2001; Eirew et al. 2008; Raouf et al. 2008; Lim et al. 2009). These committed luminal progenitor cells are analogous to the CD61+CD29loCD24+ cells delineated in the mouse mammary gland. Interestingly, EpCAM may convey a proliferative function on these cells, since it can be activated by release of its intracellular domain, which then translocates to the nucleus and enhances proliferation via the Wnt signaling pathway (Maetzel et al. 2009). In addition to luminal progenitor cells expressing typical luminal-specific cytokeratins, they contain a substantial population of cytokeratin 5/6-positive cells (Lim et al. 2009). The latter finding indicates that cytokeratin 5/6 is not a specific marker of the basal cell lineage, and suggests that breast cancers expressing these cytokeratins could have perturbations in either basal or immature luminal cells. KIT has emerged recently as a defining marker of committed luminal progenitor cells in human tissue and could be used for further fractionation studies (Lim et al. 2009). CD44, a molecule of great interest in the context of breast cancer stem cells, has been used to fractionate cells that express putative stem cell markers and have an activated transforming growth factor β (TGFβ) pathway (Shipitsin et al. 2007). Recent studies, however, have revealed that CD44 is expressed on the majority of cells in both the basal and luminal lineages (Raouf et al. 2008).
Parallels between the human and mouse epithelial hierarchies
The delineated epithelial subsets in human and mouse mammary tissue appear to exhibit highly conserved functions. Although the mouse and human MaSC-enriched populations express high levels of CD49f, there are species-specific differences in the expression of cell surface markers. For example, CD24 is a pan-epithelial marker in the mouse mammary gland, but not in human breast tissue where it serves exclusively as a luminal marker (Shackleton et al. 2006; Stingl et al. 2006; Raouf et al. 2008; Lim et al. 2009). Interestingly, the committed luminal progenitor cells in mice and humans share similar growth factor requirements in vitro, but express differing levels of ERα, such that the human progenitors have substantially higher levels of ERα relative to their murine counterparts (Asselin-Labat et al. 2007; Lim et al. 2009). Importantly, though, both the human and mammary MaSC populations lack expression of the steroid hormone receptors.
Although the linear relationships between epithelial cells in the mammary gland remain somewhat conjectural, a simple model that accommodates much of the mounting data is presented in Figure 3. The stem cell gives rise to committed progenitor cells for either the myoepithelial or luminal epithelial lineages (ductal and alveolar sublineages), but the precise number and nature of the intermediates remain elusive. The luminal progenitor subpopulation can commit to either a ductal or alveolar cell fate, dependent on the developmental stage (puberty or pregnancy), but there are likely to be distinct luminal sublineages that include ductal- and alveolar-restricted progenitors. There may be a degree of plasticity built into the luminal lineages, to allow rapid expansion of the epithelium in response to hormonal cues; differentiated alveolar cells could derive from either a common luminal or an alveolar-restricted progenitor cell. A bipotential cellular intermediate and, perhaps, a short-term repopulating MaSC may lie upstream of the lineage-restricted progenitor cells, analogous to that in the hematopoietic compartment.
An alternative model invoking bipotential progenitors for the ductal and alveolar luminal lineages must also be considered. In this model, bifurcation of the ductal and alveolar lineages occurs before luminal versus myoepithelial cell fate decisions. The observation of epithelial outgrowths with either ductal-only or alveolar-only characteristics that contain both luminal and myoepithelial elements following mammary cell transplantation lends support to this model (Smith 1996). However, there is no evidence for distinct myoepithelial lineages. While alveolar-associated and ductal-associated myoepithelium differ in their size and shape in the mammary gland, these differences likely reflect the number of myofilaments, consistent with those changes occurring in myoepithelial cell shape during involution (Emerman and Vogl 1986). Moreover, the immediate environment and hormonal status (e.g., estrus cycle phase) conceivably alter the degree of branching or alveologenesis evident following transplantation, and could lead to either ductal- or alveolar-restricted structures arising from a bipotent progenitor cell.
Despite estrogen and progesterone acting as critical mitogens for mammary epithelial cells, the murine and human MaSC-enriched populations have been demonstrated to lack expression of the ERα and PgR (Asselin-Labat et al. 2006; Lim et al. 2009). Moreover, these MaSCs do not express detectable levels of ErbB2/HER2, reminiscent of the triple-negative receptor phenotype that typifies many basal cancers (Carey et al. 2007). In addition, they express high levels of epidermal growth factor receptor (EGFR), cytokeratin 5/6, and the myoepithelial marker p63, all of which are hallmarks of triple-negative basal-like tumors. These data suggested that the MaSC might be the cell of origin for the basal cancer subtype, and that these tumor cells have become independent of the influences of estrogen and progesterone signaling.
A role for estrogen or progesterone in regulating the normal activity of MaSCs cannot be excluded, as estrogen is known to mediate its effects in the adult mammary gland through paracrine signaling (Scully et al. 1997; Mallepell et al. 2006). In the ducts of the adult mammary gland, it is well recognized that ERα expression and the cell cycling status tend to be mutually exclusive (Clarke et al. 1997). Despite no change in the size of the MaSC-enriched population in ovariectomized mice, the repopulating capacity of these cells is impaired, suggesting that MaSCs respond to paracrine signaling by hormones (Asselin-Labat et al. 2006; ML Asselin-Labat, unpubl.). Although the factors that mediate these effects are yet to be established, amphiregulin, an agonist of the EGFR, represents a promising candidate. Amphiregulin is a regulator of mammary epithelial proliferation and ductal elongation, and was found recently to be a direct target of estrogen signaling through its receptor (Ciarloni et al. 2007).
What is the nature of the MaSC niche?
The maintenance and function of MaSCs are dependent on combinatorial interactions between various epithelial cells and the mammary stroma. Many studies have documented the importance of the mammary stroma in ductal development (for review, see Silberstein 2001). The different epithelial subtypes can be generated by asymmetric division of the MaSCs in the mammary stroma, even following implantation of a single stem cell. The lack of impact of supporting mammary cells on the capacity of individual MaSCs to generate extensive epithelial outgrowths underscores the importance of tissue-specific signals derived from the parenchymal cells (Shackleton et al. 2006). Recent data have indicated that the mammary gland stroma is instructive and can reprogram stem cells from other organs, such as testicular cells and neural stem cells, to produce progeny committed to a mammary epithelial cell fate (Boulanger et al. 2007; Booth et al. 2008). Although the nature of the MaSC niche is yet to be defined, macrophages may be one component, as they have an integral role in supporting MaSC function (Gyorki et al. 2009).
MaSC activity is likely to be subject to both positive and negative stromal signals. Stem cells are thought to be enriched in TEBs of the ductal tree and to distribute at sites from which lateral branches will subsequently emanate. Thus, concurrent with the activation of MaSCs in TEBs, the proliferation of stem cells located along mature ducts must be inhibited to prevent the development of excessive lateral branches during puberty. Interestingly, the inhibitory effects of TGF-β1 on MaSC proliferation appear to be mediated by stromal intermediates (Silberstein et al. 1992; Pierce et al. 1993; Boulanger et al. 2005).
Detailed histological and ultrastructural analyses have identified specific cell populations in mouse and rat mammary epithelium, including small pale cells located in a basal position with no lumenal contact (Chepko and Smith 1997). These cells occur at a frequency of 1%–3% in the epithelium and have been proposed to be MaSCs. Other studies have indicated that stem cells in the mouse mammary gland have a basal location (Shackleton et al. 2006; Stingl et al. 2006; Taddei et al. 2008). Interestingly, multiscale in situ analysis of the mouse mammary gland has shown that large ducts contain a reservoir of very long-term LRCs (Fernandez-Gonzalez et al. 2009), while stem-like cells in the human breast have also been proposed to have an asymmetric distribution, primarily restricted to the ducts rather than lobules (Villadsen et al. 2007).
Molecular regulators of MaSC activity, lineage commitment, and differentiation
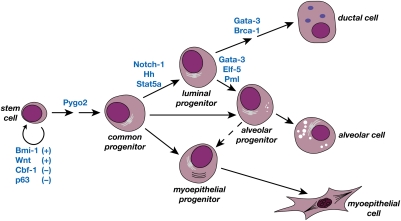
Many transcriptional regulators have been demonstrated to control different aspects of mammary development through the analysis of targeted mice (for review, see Hennighausen and Robinson 2005). The purification and characterization of discrete mammary epithelial cell subsets provide an indispensable framework for defining regulators of mammary stem and progenitor cell function. Different strategies can be employed to pinpoint where genes are acting along the epithelial hierarchy. These include the separation of mammary epithelial subpopulations from targeted mice or retroviral/lentiviral-mediated transduction of normal epithelial subsets to allow their genetic manipulation ex vivo, prior to transplantation (Bouras et al. 2008; Welm et al. 2008). These complementary approaches have proved useful in providing insight into the cellular functions of several genes, summarized in Figure 4. Although the Wnt pathway almost certainly regulates the normal self-renewal program of MaSCs, the physiological role of this pathway in mammary epithelium has not yet been established.
Figure 4.
Transcriptional regulators and molecular pathways that influence discrete cell types and stages along the mammary epithelial hierarchy. These have been demonstrated to affect MaSC self-renewal, lineage commitment, or luminal differentiation. Plus (+) and minus (−) signs refer to positive or negative effects on self-renewal.
Transcriptional regulators of MaSCs
In the mouse MaSC compartment, down-regulation of the canonical Notch effector Cbf-1 leads to increased stem cell repopulating activity in vivo and aberrant ductal morphogenesis, providing evidence that this pathway normally plays a role in restricting expansion of the MaSC pool (Bouras et al. 2008). It is not clear which Notch member performs this function in vivo, but higher levels of Notch-2 and Notch-3 mRNA are evident in the mouse MaSC pool. Intriguingly, there appear to be differences in the expression of Notch receptors between the analogous mouse and human epithelial subsets, although the functional outcome of Notch activity in a given cell type would be expected to be conserved. Notch-4, for example, is most abundant in the human MaSC-enriched population (Raouf et al. 2008), but not its mouse counterpart. It is possible that Notch-4 mediates the enhanced mammosphere-forming capacity of basal cells induced by Notch ligand (Dontu et al. 2004), but this would differ from findings in the mouse where canonical Notch signaling plays a repressive role. The disparate functions ascribed to the Notch pathway in mammary epithelial cells appear to reflect cellular context and whether in vivo or in vitro systems were explored, highlighting the importance of physiological systems.
The polycomb group protein Bmi-1 has emerged as a critical regulator of stem cell self-renewal in many tissue types, presumably reflecting its fundamental role as an epigenetic silencer that preserves chromatin patterns and cell identity (Buszczak and Spradling 2006; Sparmann and van Lohuizen 2006). Similarly, MaSC activity in the developing mammary gland was shown to be dependent on Bmi-1 (Pietersen et al. 2008), consistent with findings that BMI-1 promotes human mammosphere formation and is overexpressed in breast cancer (Liu et al. 2006). In addition, Bmi-1 maintains the proliferation of committed mammary progenitor cells, with precocious alveolar differentiation occurring in Bmi-1-deficient tissues. Interestingly, MaSCs appear to be less dependent on Bmi-1 than hematopoietic stem cells, in which more profound effects on self-renewal were evident.
Hedgehog (Hh) signaling has also been implicated in regulating the self-renewal of stem cells in specific tissues (Molofsky et al. 2004). In the mammary gland, however, Hh signaling (via constitutive activation of the smoothened receptor) appears to have an opposing role and leads to diminished MaSC activity (Moraes et al. 2007). Instead, the proliferation of committed progenitor cells was enhanced, contributing to ductal dysplasia. Loss of a single copy of the negative regulator of Hh, Patched-1, also augmented mammary progenitor formation by MaSCs (Li et al. 2006), compatible with findings based on mammosphere cultures (Liu et al. 2006). Interestingly, Hh appears to promote restriction of MaSCs to progenitor cells by mediating differential p63 promoter selection, such that ΔNp63 and TA-p63 are preferentially expressed in the MaSC and progenitor pools, respectively (Li et al. 2006).
Regulators of cell fate decisions
Notch signaling plays a key role in binary cell fate decisions in the mammary gland, reminiscent of findings in other cellular compartments (Artavanis-Tsakonas et al. 1999; Chiba 2006). In both humans and mice, the Notch pathway is important for promoting the commitment of MaSCs to the luminal cell lineage at the expense of the myoepithelial lineage (Bouras et al. 2008; Raouf et al. 2008). In human breast tissue, transcriptome and functional analyses have indicated that Notch-3 is a primary regulator of luminal cell fate determination, while in the mouse, Notch-1 can orchestrate this function. Indeed, Notch activity was shown to be substantially higher in the mouse luminal epithelial subsets, with notable expression of the active form of Notch-1 and its target genes in luminal cells in vivo. During pregnancy, canonical Notch signaling also plays a role in controlling the balance of lineages within alveoli (Buono et al. 2006).
Loss of the nuclear protein PML leads to aberrant differentiation of ductal and alveolar structures in the mammary gland, implicating PML in regulating the balance of the two luminal progenitor cell subsets by affecting their proliferation (Li et al. 2009). PML may therefore influence lineage fate decisions in the mammary gland, but this is yet to be proven. Interestingly, the defect in alveologenesis that accompanies the loss of Stat5a/5b in the mammary gland reflects a role for Stat5a in the establishment or maintenance of luminal progenitor cells, rather than promoting their differentiation (Yamaji et al. 2009).
Regulators of luminal cell differentiation
Of the several genes that have been implicated in regulating alveolar morphogenesis, both Gata-3 and Elf-5 have emerged as key regulators of luminal cell differentiation within the epithelial hierarchy. Gata-3 controls differentiation along the ductal and alveolar luminal lineages, with the accumulation of luminal progenitor cells in Gata-3-deficient glands (Asselin-Labat et al. 2007). Moreover, introduction of Gata-3 into a stem cell-enriched population induced maturation along the luminal lineage, suggesting that it is a “master” regulator of differentiation. The perturbation of the luminal progenitor population in heterozygous mice underscores the importance of the absolute level of Gata-3 in the mammary gland. Of relevance, the level of Gata-3 in luminal breast cancers is a determinant of patient survival, with the highest expression occurring in the more favorable “Luminal” subtypes of breast tumors (Perou et al. 2000; Sorlie et al. 2001; Sotiriou et al. 2003; Voduc et al. 2008). The higher levels of Gata-3 may drive cells into a more differentiated state, thus conferring a better prognosis. Other genes that interact at a molecular level with Gata-3 to influence differentiation include ERα and its target genes, FOXA1 and TFF1, all of which are abundantly expressed by differentiated luminal cells (Lim et al. 2009). Interestingly, Gata-3 can function as a repressor of the CDK inhibitor p18INK4C, which in turn has been shown to suppress the development of ERα-positive luminal tumors in the mammary gland by restraining luminal progenitor proliferation (Pei et al. 2009). In addition to its apparent role in tumor initiation, Gata-3 can suppress the metastasis of luminal tumor cells (Kouros-Mehr et al. 2008).
Unlike Gata-3, mammary glands deficient in the ETS transcription factor Elf-5 do not exhibit defects in ductal growth and morphogenesis. During pregnancy, however, a pronounced defect in alveolar morphogenesis is evident in the absence of a single Elf-5 allele (Zhou et al. 2005). These glands harbor an expanded pool of luminal progenitor cells, whereas Elf-5 overexpression results in precocious alveolar differentiation, even in the virgin state (Oakes et al. 2008). Elf-5 therefore appears to be a central component of the alveolar switch. This gene could play an earlier role in the hierarchy, as it is a marker of the luminal progenitor population in both human and mouse mammary tissue (Lim et al. 2009). Intriguingly, there are distinctive longitudinal cells that span the luminal to basal layer of ducts that are Elf-5+ and ERα− (Oakes et al. 2008). These columnar cells are reminiscent of c-KIT+ progenitor cells evident in the human ductal tree. Only a few luminal cells in the ductal network directly contact the basement membrane, and these may correspond to a specialized type of progenitor cell.
The tumor suppressor BRCA1 has been implicated in a plethora of functions, including the DNA damage response, X-chromosome inactivation, and transcriptional control. In mammary epithelium, conditional deletion of BRCA1 results in impaired alveolar development (Xu et al. 1999), while knockdown of BRCA1 in primary human breast epithelial cells leads to an increase in ALDH1+ cells and failure of immature luminal cells to differentiate into mature ERα+ cells (Liu et al. 2008). Notably, breast tissue from multiple BRCA1 mutation carriers (BRCA1+/−) harbors an expanded luminal progenitor population with aberrant growth characteristics (Lim et al. 2009). Moreover, parallel observations were made in the case of mouse mammary glands lacking both copies of Brca1, indicating that luminal progenitor function is also affected by loss of both Brca1 alleles. This defect in the luminal lineage is compatible with the higher expression of BRCA1 in the luminal versus basal cell subsets. Indeed, the expansion of ALDH1+ cells observed in BRCA1 mutation carriers could reflect the presence of Aldefluor-positive cells in the luminal progenitor subset. Loss of BRCA1 not only limits the differentiation potential of luminal progenitor cells, but perturbs the differentiation pathway. In BRCA1 mutant preneoplastic tissue, elevated PgR expression and markedly higher levels of cytokeratin 5/6 were noted in the mature luminal subset (Lim et al. 2009). The expression of certain basal markers such as cytokeratin 14 in BRCA1 tumors is also consistent with luminal progenitor cells having an altered differentiation program, and the observation that many basal-like tumors express luminal-specific genes (Palacios et al. 2005).
The WNT pathway and mammary oncogenesis
Deregulated self-renewal may contribute to preneoplasia in the mammary gland, as inferred by the profoundly enhanced serial transplantability of hyperplastic mammary tissue (Daniel et al. 1968). The Wnt, Notch, and Hedgehog signaling pathways are conserved among many different adult stem cell types, and their deregulation is linked to oncogenesis (Reya and Clevers 2005). Of these pathways, there is evidence that inappropriate WNT signaling in the mammary gland can result in deregulated self-renewal. Activation of Wnt-1, originally identified as a frequent site of integration by the mouse mammary tumor virus, seems to target at least two cell types in preneoplastic mammary tissue. MMTV-Wnt1 mammary tissue harbors a substantially increased number of MaSCs and an aberrant population of progenitor cells that have in vivo regenerative activity (Shackleton et al. 2006; Vaillant et al. 2008). The latter progenitor cells from Wnt-1 transgenic glands express high levels of the basal marker cytokeratin 14, suggesting that Wnt-1 hyperactivity may elicit dedifferentiation to a more stem-like state. Previous studies have suggested that the oncogenic effects of Wnt-1 on mammary epithelium are initiated in mammary progenitor cells (Li et al. 2003; Liu et al. 2004; Teuliere et al. 2005), and Wnt-1/β-catenin has also been implicated in mediating the radiation resistance of mouse mammary progenitor cells (Woodward et al. 2007). Interestingly, loss of the plant homeodomain protein Pygo2 prevents the formation of hyperplastic outgrowths by Wnt/β-catenin induction, and this epigenetic activator plays a role in controlling the expansion of mammary progenitor cells (Gu et al. 2009). In human breast tissue, the Wnt/β-catenin pathway was discovered recently to be activated following pTEN knockdown. Inhibition of AKT activity, up-regulated upon loss of pTEN, was found to be effective in targeting ALDH1+ tumor-initiating cells (Korkaya et al. 2009).
Multiple molecular subtypes of breast cancer
Breast cancer has been stratified into six distinct subtypes on the basis of gene expression profiling. These include the luminal A or B, basal-like, claudin-low, HER2/ERBB2-overexpressing, and normal-breast-like subtypes. The differences in tumor subtypes are hypothesized to reflect different mutation profiles, as well as differences in the cell of origin (Perou et al. 2000; Gusterson et al. 2005). The longevity of many adult stem cells makes them likely candidates for accumulating genetic mutations. On the other hand, restricted progenitors can serve as targets of oncogenesis. For example, the β-catenin pathway confers self-renewal on granulocyte–macrophage progenitors in chronic myeloid leukemia (Jamieson et al. 2004).
Most breast cancers, including the common types of invasive ductal and invasive lobular carcinomas, display evidence of luminal cell differentiation. While the luminal A and B subtypes are generally associated with a good prognosis, those tumors that overexpress HER2 (or exhibit amplification) usually display luminal features, but are associated with poor overall survival (Slamon et al. 1987). The basal-like subtype is very heterogeneous and comprises 15%–20% of breast cancers (for review, see Gusterson 2009). This group of tumors is among the most clinically aggressive and tends to exhibit a triple-negative phenotype (i.e., lack expression of ER, PgR, and HER2/ERBB2). In combination with EGFR and cytokeratin 5/6, these markers provide high specificity in identifying basal-like tumors (Cheang et al. 2008). Poorly differentiated basal-like adenocarcinomas have also been reported to overexpress embryonic stem cell genes such as NANOG, SOX2, OCT4, and MYC (Ben-Porath et al. 2008). The claudin-low subtype of receptor-negative cancers expresses low levels of genes involved in tight junctions, cell–cell adhesion, and luminal genes, including potential Gata-3 target genes (Herschkowitz et al. 2007). Interestingly, this subclass is also characterized by expression of endothelial and lymphocytic markers and has mesenchymal features. Metaplastic breast cancers, a subtype of the basal-like group that is largely chemoresistant, were reported recently to have a distinct molecular profile that most closely resembles that of the claudin-low subgroup and is enriched for epithelial–mesenchymal transition (EMT) signature genes (Hennessy et al. 2009). Whether these triple-negative tumors have undergone an EMT in vivo remains to be proven. Notably, the expression of genes including vimentin, smooth muscle actin, slug, and N-cadherin does not necessarily imply that tumor cells have undergone an EMT, since these genes are normally expressed by basal cells in breast tissue. Interestingly, the expression profiles of the metaplastic and claudin-low tumors share similarities with the breast cancer stem cell subset that was identified to have a CD44+CD24−/low phenotype (Al-Hajj et al. 2003).
Breast cancer subtypes and cellular origins
While the normal epithelial hierarchy serves as a useful framework to understand the cellular origins of the different molecular subtypes of breast cancer, the findings remain correlative until formally proven by assessing tumorigenic capacity. It is possible that the different cell populations may exhibit differentiation plasticity during tumor progression, and that the molecular signatures of the different tumor subtypes might not necessarily reflect the properties of the cell of origin. Lineage tracing or clonality studies will ultimately be required to prove the cellular target of transformation for a specific cancer type, analogous to the elegant lineage tracing experiments described recently (Barker et al. 2009). In this study, colon crypt stem cells were shown to be the cell of origin for neoplasia associated with loss of APC.
Reclassification of basal-like tumors as ‘luminal progenitor’ tumors?
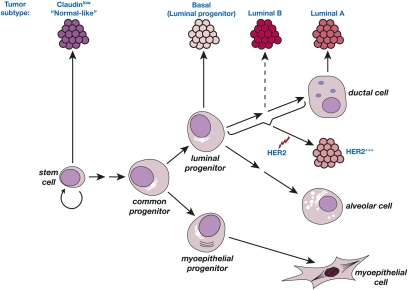
The recent derivation of specific gene signatures for human MaSC-enriched, luminal progenitor, mature luminal, and stromal populations has provided insight into potential target cells for the different breast tumor subtypes (Fig. 5). It seems important to profile uncultured cells, as differences have been observed between the gene expression portraits of freshly sorted cells (Lim et al. 2009) versus those passaged for a short time (Raouf et al. 2008). Interrogation of the breast cancer subtype gene sets with the different mammary epithelial signatures unexpectedly revealed that the basal-like group shares a striking similarity with the luminal progenitor gene signature. This finding has profound implications for the basal subtype of cancer, as the stem cell has been presumed to be the cell of origin for these breast cancers. The marked similarity in molecular signatures suggests that luminal progenitor subtype is a more apt description than “basal-like” subtype.
Figure 5.
Schematic model of the human breast epithelial hierarchy and potential relationships with breast tumor subtypes. The six different tumor types are shown together with their closest normal epithelial counterpart based on gene expression analyses. The luminal progenitor subtype may be a more appropriate name for basal tumors. The HER2 subtype could originate through amplification of the HER2 locus in a target cell restricted to the luminal cell lineage.
Conversely, the gene signature of the MaSC subset had the greatest overlap with the claudin-low and normal breast-like subtypes. The latter association may reflect a combination of mesenchymal and basal components in these two tumor types, although the nature of the normal breast-like subtype is not entirely clear. The gene signature of human breast stroma, devoid of ductal epithelium, was most highly enriched in the claudin-low subtype, almost certainly reflecting the mesenchymal features in the heterogeneous claudin-low subtype. As predicted, the signature of differentiated luminal cells in breast tissue showed a profound similarity with that of the luminal A and B subtypes. Given that the majority (70%) of breast cancers is ERα+, many of these may arise from progenitors within the luminal sublineages, although limited differentiation of ERα− cells to generate ERα+ cells cannot be excluded. The HER2 subgroup of tumors has no clear association with the normal epithelial cell types identified thus far, but presumably derives from a cell with a luminal predisposition. Importantly, MMTV-erbB2/neu mouse mammary tumors were highly enriched for committed luminal progenitor cells (Vaillant et al. 2008), indicating that this mouse strain does not accurately recapitulate HER2-overexpressing cancers arising in women.
Aberrant luminal progenitors as the cellular target in BRCA1 mutation carriers
BRCA1-associated breast tumors have a distinctive pathology and usually express markers of the basal subtype (Foulkes 2004; Turner et al. 2004). However, no alterations in the growth properties of the basal MaSC-enriched subset were evident in preneoplastic tissue from BRCA1 carriers: The size of the MaSC-enriched population was markedly diminished and Brca1-deficient mouse mammary glands contained fewer functional stem cells (Lim et al. 2009). Rather, a perturbed luminal progenitor population was evident. Together with the significant molecular links that exist between the gene expression profiles of the normal luminal progenitor, the basal subtype of cancer, and preneoplastic tissue from BRCA1 carriers, these findings indicate that the luminal progenitor is a likely target of transformation in BRCA1 mutation carriers (Fig. 5).
It is pertinent that a substantial proportion of human luminal progenitor cells lack expression of ERα, but do express cytokeratin 5/6—both features of the basal-like subtype of breast tumors. However, ERα is expressed on about one-third of luminal progenitor cells; furthermore, not all BRCA1 tumors are negative for ERα, with up to 30% expressing variable levels of this receptor (Lakhani et al. 2005). Thus, ERα may directly mediate the partial efficacy provided by prophylactic ovariectomy in the prevention of basal breast tumors in BRCA1 mutation carriers (Kauff et al. 2008), and possibly in tamoxifen chemoprophylaxis (Narod 2006).
Why are breast epithelial cells more susceptible to loss of function of BRCA1 than other cell types? This may reflect the repeated cycles of estrogen-driven cell proliferation occurring in women, together with the critical roles of BRCA1 in regulating cellular differentiation and the DNA damage response (for review, see Venkitaraman 2002; Narod and Foulkes 2004). The higher degree of genomic instability found in BRCA1 tumors provides evidence in support of this notion. In rapidly dividing luminal progenitor cells, the loss of a single BRCA1 allele may generate a pool of genetically unstable cells. In BRCA1-associated tumors, p53 mutations are common and may lead to increased survival and subsequent expansion of BRCA1-deficient cells. It is noteworthy that deletion of both BRCA1 and p53 in mouse models leads to highly proliferative mammary tumors that are ERα- and PgR-negative and that express basal epithelial markers, thus recapitulating tumors that arise in BRCA1 mutation carriers (Liu et al. 2007).
Luminal progenitors as targets in other breast tumors
The Notch pathway plays a central role in breast oncogenesis. Indeed, both Notch-1 and Notch-4 were identified as frequent sites of proviral insertional activation in mouse mammary tumors, and overexpression of Notch-1 or Jagged-1 is often observed in breast tumors (Stylianou et al. 2006). Interestingly, high Notch-1 levels have been found in basal (i.e., luminal progenitor) breast cancers, and this correlates with a decrease in patient survival (Lee et al. 2008). Moreover, constitutive Notch signaling in the mouse mammary gland was found to specifically target luminal-restricted progenitor cells for expansion and self-renewal (an activity normally restricted to MaSCs), leading to hyperplasia and eventual tumorigenesis. These data implicate the luminal progenitor as a potential cell of origin for tumors in which the Notch pathway has been activated inappropriately (Bouras et al. 2008).
Concluding remarks
The definition and isolation of functionally distinct epithelial subsets from the mammary gland at increasing purities will pave the way for elucidating new cellular intermediates and molecular pathways that regulate the self-renewal and progressive differentiation of stem and progenitor cells. The refined populations should allow fundamental issues to be addressed, such as where the MaSC is localized in situ, whether there is a quiescent MaSC pool, and what signals mediate cross-talk between specific epithelial cells and the stroma. Multiplex genome-wide RNAi screening in primary mammary epithelial subsets ex vivo may prove useful in illuminating key regulators of proliferation and survival. In this context, it would be valuable to have improved cellular assays for stem cell activity. Although human mammospheres can be cultured in a clonal manner (Dontu et al. 2003), MaSC versus progenitor cell activities are not always clearly distinguishable, and this assay has not yet been demonstrated to enrich for stem cells on serial passage.
The hierarchy also provides an essential framework for understanding potential cells of origin in breast cancer. The unexpected molecular similarities between the luminal progenitor cell and basal-like cancers have suggested that the luminal progenitor cell is a potential target for carcinogenesis rather than the “basal” stem cell. Further delineation of novel luminal precursors may reveal the cellular target for the HER2 subtype. Future lineage tracing studies to prove the cell of origin will require gene promoters that enable cre-mediated excision in a specific mammary epithelial population, but at present this remains a challenge. In a complementary approach, lentivirus-mediated tagging of individual cells has the potential to reveal those cells predisposed to carcinogenesis, provided clonal expansion of the transduced cells does not occur (Kustikova et al. 2005). The next generation of experiments is likely to define key oncogenic events occurring in the different cells of origin for the distinct tumor subtypes, and to further address the heterogeneity evident within basal tumors.
Establishing relationships between tumor subtypes and normal epithelial subsets has profound implications for the development of clinically useful diagnostic and prognostic markers, as well as targeted therapies. Candidate targets derived from the luminal progenitor signature such as C-KIT need to be evaluated for their ability to eradicate or modulate the altered luminal progenitor subset in BRCA1-associated and other basal cancers (Lim et al. 2009). These inhibitors could be used alone or in combination with agents such as PARP inhibitors (Fong et al. 2009) to specifically target the basal group of tumors for which few noncytotoxic-based therapies currently exist.
Acknowledgments
I am grateful to G. Lindeman for critical reading of the manuscript, and to P. Maltezos for preparation of the figures. My apologies to those authors whose papers could not be cited due to space constraints. J.E.V. is supported by the Victorian Breast Cancer Research Consortium, Inc., and the National Health and Medical Research Council, Australia.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1849509.
References
- Abrams TJ, Guzman RC, Swanson SM, Thordarson G, Talamantes F, Nandi S. Changes in the parous rat mammary gland environment are involved in parity-associated protection against mammary carcinogenesis. Anticancer Res. 1998;18:4115–4121. [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Shackleton M, Bouras T, Lindeman GJ, Visvader JE. Delineating the epithelial hierarchy in the mouse mammary gland. Cold Spring Harb Symp Quant Biol. 2008;73:469–478. doi: 10.1101/sqb.2008.73.020. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Smith GH. Estrogen receptor-α and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 2006;8:R49. doi: 10.1186/bcr1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-β1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt KL, Kendrick H, Regan JL, Molyneux G, Magnay FA, Ashworth A, Smalley MJ. Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells. Breast Cancer Res. 2009;11:R20. doi: 10.1186/bcr2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Spradling AC. Searching chromatin for stem cell identity. Cell. 2006;125:233–236. doi: 10.1016/j.cell.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- Chepko G, Smith GH. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 1997;29:239–253. doi: 10.1016/s0040-8166(97)80024-9. [DOI] [PubMed] [Google Scholar]
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Young LJ. Influence of cell division on an aging process. Life span of mouse mammary epithelium during serial propagation in vivo. Exp Cell Res. 1971;65:27–32. doi: 10.1016/s0014-4827(71)80046-0. [DOI] [PubMed] [Google Scholar]
- Daniel CW, De Ome KB, Young JT, Blair PB, Faulkin LJ., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: A serial transplantation study. Proc Natl Acad Sci. 1968;61:53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ome KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, Eaves CJ. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14:1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Vogl AW. Cell size and shape changes in the myoepithelium of the mammary gland during differentiation. Anat Rec. 1986;216:405–415. doi: 10.1002/ar.1092160310. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Illa-Bochaca I, Welm BE, Fleisch MC, Werb Z, Ortiz-de-Solarzano C, Barcellos-Hoff MH. Mapping mammary gland architecture using multi-scale in situ analysis. Integ Biol. 2009;1:80–89. doi: 10.1039/b816933k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Foulkes WD. BRCA1 functions as a breast stem cell regulator. J Med Genet. 2004;41:1–5. doi: 10.1136/jmg.2003.013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, Agrawal A, Rheaume C, Bilanchone V, Veltmaat JM, et al. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol. 2009;185:811–826. doi: 10.1083/jcb.200810133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes & Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. Do ‘basal-like’ breast cancers really exist? Nat Rev Cancer. 2009;9:128–134. doi: 10.1038/nrc2571. [DOI] [PubMed] [Google Scholar]
- Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7:143–148. doi: 10.1186/bcr1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11:R62. doi: 10.1186/bcr2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Gardner WU. Transplantability and life span of mammary gland during serial transplantation in mice. Nature. 1967;213:193–194. doi: 10.1038/213193a0. [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, Isaacs C, Evans DG, Lynch H, Eeles RA, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, Smalley MJ. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591. doi: 10.1186/1471-2164-9-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustikova O, Fehse B, Modlich U, Yang M, Dullmann J, Kamino K, von Neuhoff N, Schlegelberger B, Li Z, Baum C. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science. 2005;308:1171–1174. doi: 10.1126/science.1105063. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Slack DN, Hamoudi RA, Collins N, Stratton MR, Sloane JP. Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab Invest. 1996;74:129–135. [PubMed] [Google Scholar]
- Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- Land CE, McGregor DH. Breast cancer incidence among atomic bomb survivors: Implications for radiobiologic risk at low doses. J Natl Cancer Inst. 1979;62:17–21. [PubMed] [Google Scholar]
- Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, Hsieh CC, Altieri DC. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10:R97. doi: 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li H, Cherukuri P, Farzan S, Harmes DC, DiRenzo J. TA-p63-γ regulates expression of ΔN-p63 in a manner that is sensitive to p53. Oncogene. 2006;25:2349–2359. doi: 10.1038/sj.onc.1209270. [DOI] [PubMed] [Google Scholar]
- Li W, Ferguson BJ, Khaled WT, Tevendale M, Stingl J, Poli V, Rich T, Salomoni P, Watson CJ. PML depletion disrupts normal mammary gland development and skews the composition of the mammary luminal cell progenitor pool. Proc Natl Acad Sci. 2009;106:4725–4730. doi: 10.1073/pnas.0807640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat M-L, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Holstege H, van der Gulden H, Treur-Mulder M, Zevenhoven J, Velds A, Kerkhoven RM, van Vliet MH, Wessels LF, Peterse JL, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci. 2007;104:12111–12116. doi: 10.1073/pnas.0702969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci. 2008;105:1680–1685. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, Allred DC, Lewis MT. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- Narod SA. Modifiers of risk of hereditary breast cancer. Oncogene. 2006;25:5832–5836. doi: 10.1038/sj.onc.1209870. [DOI] [PubMed] [Google Scholar]
- Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- Neville MC, Medina D, Monks J, Hovey RC. The mammary fat pad. J Mammary Gland Biol Neoplasia. 1998;3:109–116. doi: 10.1023/a:1018786604818. [DOI] [PubMed] [Google Scholar]
- Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, Kazlauskas M, Pritchard MA, Chodosh LA, Pfeffer PL, et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes & Dev. 2008;22:581–586. doi: 10.1101/gad.1614608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J, Honrado E, Osorio A, Cazorla A, Sarrio D, Barroso A, Rodriguez S, Cigudosa JC, Diez O, Alonso C, et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat. 2005;90:5–14. doi: 10.1007/s10549-004-1536-0. [DOI] [PubMed] [Google Scholar]
- Parmar H, Young P, Emerman JT, Neve RM, Dairkee S, Cunha GR. A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology. 2002;143:4886–4896. doi: 10.1210/en.2002-220570. [DOI] [PubMed] [Google Scholar]
- Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY, Ho IC, Perou CM, Xiong Y. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15:389–401. doi: 10.1016/j.ccr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pierce DF, Jr, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, Daniel CW, Hogan BL, Moses HL. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-β 1. Genes & Dev. 1993;7:2308–2317. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- Pietersen AM, Evers B, Prasad AA, Tanger E, Cornelissen-Steijger P, Jonkers J, van Lohuizen M. Bmi1 regulates stem cells and proliferation and differentiation of committed cells in mammary epithelium. Curr Biol. 2008;18:1094–1099. doi: 10.1016/j.cub.2008.06.070. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, Iscove N, Jones S, McKinney S, Emerman J, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3:109–118. doi: 10.1016/j.stem.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11:931s–936s. [PubMed] [Google Scholar]
- Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG. Role of estrogen receptor-α in the anterior pituitary gland. Mol Endocrinol. 1997;11:674–681. doi: 10.1210/mend.11.6.0019. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Silberstein GB. Postnatal mammary gland morphogenesis. Microsc Res Tech. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Regulation of mammary morphogenesis: Evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-β 1. Dev Biol. 1992;152:354–362. doi: 10.1016/0012-1606(92)90142-4. [DOI] [PubMed] [Google Scholar]
- Siwko SK, Dong J, Lewis MT, Liu H, Hilsenbeck SG, Li Y. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells—Implications for pregnancy-induced protection against breast cancer. Stem Cells. 2008;26:3205–3209. doi: 10.1634/stemcells.2008-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci. 1988;90:173–183. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. β1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze'ev A, Thiery JP, Glukhova MA. Targeted activation of β-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS. Contiguous patches of normal human mammary epithelium derived from a single stem cell: Implications for breast carcinogenesis. Cancer Res. 1996;56:402–404. [PubMed] [Google Scholar]
- Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/β3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ, Petersen OW. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voduc D, Cheang M, Nielsen T. GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev. 2008;17:365–373. doi: 10.1158/1055-9965.EPI-06-1090. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Smith GH. Pregnancy and stem cell behavior. J Mammary Gland Biol Neoplasia. 2005;10:25–36. doi: 10.1007/s10911-005-2538-1. [DOI] [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJP, Bledau AS, Welm AL, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell. 2008;2:90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Henninghausen L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes & Dev. 2009;20:2382–2387. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chehab R, Tkalcevic J, Naylor MJ, Harris J, Wilson TJ, Tsao S, Tellis I, Zavarsek S, Xu D, et al. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 2005;24:635–644. doi: 10.1038/sj.emboj.7600538. [DOI] [PMC free article] [PubMed] [Google Scholar]