Abstract
Purpose
Previous studies have demonstrated that estrogen prevents colon cancer in postmenopausal women, indicating a role in CRC carcinogenesis and tumor progression. We investigated the interactions between sex, age, ethnicity, and year of diagnosis on overall survival in patients with MCRC.
Experimental Design
We screened 52,882 patients with MCRC from 1988–2004, using the Surveillance, Epidemiology, and End Results (SEER) registry. Age at diagnosis, sex, ethnicity, tumor location, year of diagnosis, overall survival (OS) and cancer specific survival (CSS) were evaluated using Cox proportional hazards model. The models were adjusted for marital status, tumor site, tumor differentiation, and treatment with radiation and/or surgery.
Results
We observed that younger women (18–44 years old) with MCRC lived longer than younger men (17 months vs. 14, p<0.0001, log-rank test). In contrast, older women (55 and older) had significantly worse OS than older men (7 months vs. 9, p<0.0001, log-rank test). In multivariate analysis, we found that gender discrepancies have widened in recent years; young women diagnosed after 2000 have improved CSS, compared to men (HR 0.778, 95% C.I. 0.669 –0.904), but those diagnosed before 2000 benefit less (HR 0.931, 95% C.I. 0.821 –1.056).
Conclusion
As one of the largest data sets analyzed to establish that younger women with MCRC survive longer than younger men, hormonal status appears to play an important role not only in the development and pathogenesis of colorectal cancer, but may be of prognostic significance. These data warrant further studies to determine the role of estrogen in colorectal cancer.
INTRODUCTION
The impact of gender on colorectal cancer (CRC) incidence is well established. At all ages, women are less likely to develop CRC than men(1, 2). Indeed, their risk is comparable to men between 4–8 years younger in age(3). Variable environmental exposures and co-morbidities may contribute to these findings; however an alternate explanation is emerging.
In the Women’s Health Initiative trial, post-menopausal hormone use was associated with a 40% decrease in colorectal cancer(4). This association between hormone replacement therapy and colon cancer prevention is consistent with previous reports(5, 6). Hormones also protect pre-menopausal women, as oral contraceptive use reduces the risk of developing CRC by approximately 20%(7, 8). The mechanism by which this occurs is unclear, but these findings parallel reports that menopause increases a woman’s risk of developing colon cancer over pre-menopausal women of the same age(9).
Gender differences in CRC extend to environmental sequelae, tumor biology, and therapeutic response. For example, obesity(10) and a sedentary lifestyle(11) increase the risk of colon cancer mostly in men. There is also evidence that tumorigenesis may be gender specific as women are more likely to develop right-sided tumors with the MSI phenotype(12, 13). In addition, polymorphisms in methylenetetrahydrofolate reductase (MTHFR) and epidermal growth factor receptor (EGFR) genes have gender-specific prognostic significance in MCRC (14, 15). Many reports suggest that women derive more benefit from adjuvant chemotherapy(13), while others suggest that women have a greater incidence of 5-fluorouracil toxicity when treated for colorectal cancer(16).
Whether female gender is associated with improved survival in CRC is under investigation. Although epidemiologic studies suggest women present at a more advanced stage of disease(17), female gender is a predictor for improved survival after resection in a number of studies(18, 19). Recent reports also suggest that women with CRC may have lower cancer specific mortality than men(20, 21).
Gender differences impact CRC incidence and gene expression patterns, yet the significance of gender as a prognostic or predictive factor for overall survival (OS) in MCRC is unclear. We used the Surveillance Epidemiology and End Results (SEER) database to investigate the interactions between sex, age, and ethnicity on overall survival and to evaluate the progression of these associations by year of diagnosis in a large cohort of patients with MCRC.
STUDY DESIGN
Data source
The SEER public use database 1973–2004 was employed for the current analysis. SEER registry, sponsored by the National Cancer Institute, collects information on cancer incidence and survival from 17 population-based cancer registries covering approximately 26% of the United States population.
Study Population
All patients with primary, histologically confirmed, MCRC were eligible for the study. The disease was defined by International Classification of Diseases for Oncology codes: C18.0–C18.9, C19.9, and C20.9. We identified patients with metastasic disease defined by SEER Extent of Disease code: 85. We restricted eligibility to adults (18 years and older) who were diagnosed with MCRC between 1988 and 2004; as extent of disease was unavailable for accurate staging prior to 1988. We excluded those diagnosed at death or autopsy, missing follow-up records, and lacking documentation on age at the diagnosis or race/ethnicity. A total of 52,882 MCRC patients were included in the final sample for the current analysis.
Variable Definitions
The primary endpoint in this study was OS, defined as the period from diagnosis to death. For the patients still alive at the last follow-up, OS was censored at date of last follow-up or December 31, 2004, which ever came first. Colorectal cancer-specific survival (CSS) was a secondary endpoint. CSS measures survival from diagnosis to death from colorectal cancer (SEER Cause of Death Recode: 21040, 21050). Patients who died of causes other than colorectal cancer were considered to be censored (SEER website). Information on age at diagnosis, sex, race and ethnicity, marital status, treatment type, primary site, tumor grade and differentiation, survival time were available in SEER database. We chose the range for age groups based on previous studies (18–44, 45–54, 55 and older). Patients were divided into five race/ethnicity groups, non-Hispanic white, non-Hispanic Blacks, non-Hispanic Asians/Pacific Islanders, Hispanics (identified to have Spanish/Hispanic surname or of Spanish) and Native Americans. Subjects were categorized into not married (including never married, separated, divorced, widowed, and unknowns) and married (including common law). Treatment type was coded using surgery and radiation records and classified as colectomy or proctectomy, local surgery, radiation therapy only, untreated (for patients who did not have surgery nor radiation therapy), and unknown (for missing information on surgery and radiation). Records on chemotherapy were not available in the SEER public use data. Primary site was coded as proximal colon, distal colon, rectosigmoid, rectum, and overlapping or unspecified. Tumor grade and differentiation was coded as well/moderately differentiated, poorly differentiated, undifferentiated, and unknown.
Statistical Analysis
We compared men and women using descriptive statistics. Survival time was censored at 3 years for all analyses. Univariate survival analysis was performed using Kaplan-Meier curves, and log-rank tests. We constructed Cox proportional hazards models to evaluate associations between patient characteristics and survival in men and female separately. All multivariable models included year of diagnosis and registry as stratification variables, marital status, treatment, primary site, and tumor grade and differentiation as covariates. Hazard Ratios (HRs) and 95% confidence intervals were generated, with HR’s less than 1.0 indicating survival benefit. Pairwise interactions (i.e., age and sex, age and race, and sex and race) were examined using stratified models and were tested by comparing corresponding likelihood ratio statistics between the baseline and nested Cox proportional hazards models that included the multiplicative product terms(22). Departure of the proportional hazard assumption of Cox models will be examined graphically such as log-log survival curves or smoothed plots of weighted Schoenfeld residuals(23) and by including a time-dependent component individually for each predictor. All analyses were conducted using P<.05 as the significance level and Statistical analyses were performed with the use of SAS software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
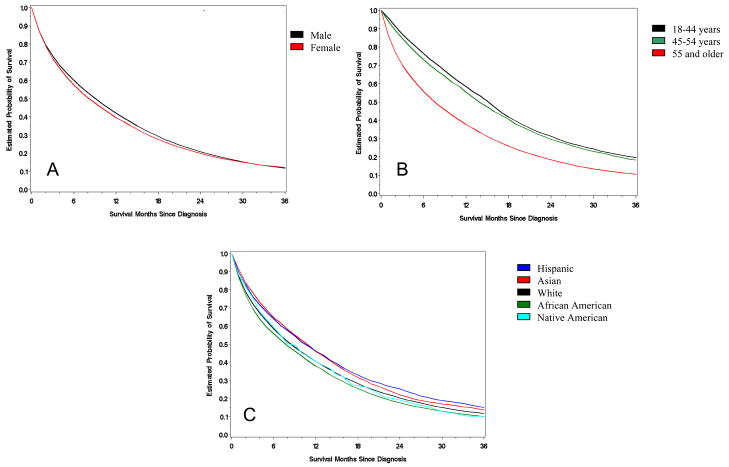
We studied 52,882 patients with metastatic colorectal cancer diagnosed from 1998 – 2003. 27,427 (52%) MCRC patients were men with a median OS of 10 months; 25,455 (48%) were women and their survival was 9 months (Figure 1A). In our cohort, the average age of MCRC patients was 68 years: 5.6% of the patients were less than 45, 11.4% were between 45–54, and 83% were greater than 55 years old. Across all age groups, the OS significantly decreased as patients aged (Figure 1B).
Figure 1. Kaplan-Meier curves of OS in MCRC.
Stratified by sex (A), age (B), and ethnicity (C). Log-rank tests were used to calculate significance.
74.9% of our CRC patients were White, 11.8% African American, 6.3% Asian, 6.5% Hispanic, and 0.5% were Native American. Among ethnicities, Asians and Hispanics had the longest median overall survival (11 months) followed by Whites ( 9 months), Native Americans (9 months), and African Americans (8 months) (P<0.0001, Table 1, Figure 1C).
Table 1.
Overall survival of men and women with metastatic colorectal cancer by demographic characteristics, SEER data 1988–2004.
| Male (n=27,427) |
Female (n=25,455) |
|||
|---|---|---|---|---|
| Characteristics | N | Median OS (95%CIs) | N | Median OS (95%CIs) |
| Age, yrs | ||||
| 18–44 | 1,458 (5%) | 14 (13–15) | 1,501 (6%) | 17 (16–17) |
| 45–54 | 3,291 (12%) | 14 (13–14) | 2,761 (11%) | 15 (14–16) |
| >55 | 22,678 (83%) | 9 (8–9) | 21,193 (83%) | 7 (7–8) |
| Race | ||||
| White | 20,551 (75%) | 9 (9–10) | 19,050 (75%) | 8 (8–9) |
| African American | 3,034 (11%) | 8 (8–9) | 3,193 (13%) | 8 (8–9) |
| Asian | 1,852 (7%) | 11 (10–11) | 1,498 (6%) | 12 (11–13) |
| Hispanic | 1,862 (7%) | 11 (10–12) | 1,587 (6%) | 11 (10–12) |
| Native American | 128 (0.5%) | 10 (8–15) | 127 (0.5%) | 8 (6–10) |
| Primary site | ||||
| Proximal | 10,859 (40%) | 8 (8–8) | 12,177 (48%) | 8 (7–8) |
| Distal | 7,324 (27%) | 11 (11–12) | 6,323 (25%) | 12 (11–12) |
| Rectosigmoid | 2,779 (10%) | 13 (12–13) | 2,111 (8%) | 11 (11–13) |
| Rectal | 4,770 (17%) | 11 (11–11) | 3,163 (12%) | 10 (9–10) |
| Overlapping or NOS | 1,695 (6%) | 3 (3–3) | 1,681 (7%) | 3 (3–3) |
| Year of diagnosis | ||||
| 1988–1999 | 15,751 (57%) | 9 (9–9) | 14,547 (57%) | 8 (8–8) |
| 2000–2003 | 11,676 (43%) | 10 (10–11) | 10,908 (43%) | 9 (9–10) |
Based on log-rank test and survival time was censored at 3 years
Gender and MCRC
The characteristics of men and women with MCRC are shown in Table 1. The distribution of MCRC across age groups is fairly consistent between genders. The average age of diagnosis for men and woman was 67 and 69 years, respectively. However, 40% of the women with MCRC were diagnosed > 75 years old as compared to 30% of the men.
There were no gender differences in MCRC prevalence across ethnicities. White, African American, and Native American men survived longer than women. However, Asians were the sole ethnicity where women had better survival then men (Table 1).
Tumor location was also examined with respect to gender. We found significant differences in the frequency of right and left-sided colon lesions among men and women. Women were more likely to have right-sided or proximal lesions. 47.8% of MCRC lesions in women were located in the proximal or right colon. Only 39.6% of MCRC cancers from men were right sided. 45.6% of women presented with left-sided lesions, which include the descending colon, sigmoid and rectal locations, whereas 54.2% of men presented with left-sided lesions (Table 1).
Gender, Age, and MCRC
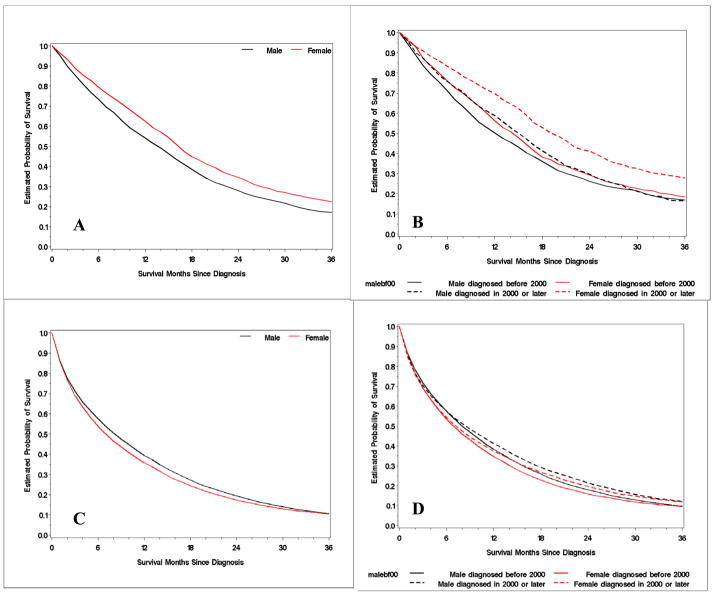
In the aggregate, there was little difference in overall survival between men and women. However when age was accounted for we found a significant divergence between the genders. OS for women less than 45 years old was 17 months compared to 14 months for similarly aged men (p<0.0001, log-rank test, Figure 2). Conversely, OS for woman older than 55 years was 7 months compared to 9 months for men (p<0.0001, log-rank test, Figure 2). These gender differences persisted when we evaluated for CSS. CSS for women less than 45 years of age was greater than men (18 months vs 16 months p<0.0001, log-rank test). Older men had poorer CSS than women (11 months vs 10, p<0.0001, log-rank test). These findings were consistent across all ethnicities (data not shown).
Figure 2. Kaplan Meier curves of OS by sex and year of diagnosis.
A - young MCRC patients (≤44 years) B - young patients by year of diagnosis C - old MCRC patients (> 55 years) D - old patients by year of diagnosis
Gender, Age, and Year of Diagnosis
As expected, patients diagnosed with MCRC from 2000 – 2004 did significantly better than those diagnosed between 1988–1999 (Table 1). However, when we examined gender and age by year of diagnosis, the survival differences in the youngest age group became more pronounced. In those diagnosed from 2000–2004, women <45 years of age had an OS of 20 months vs. 15 months for men (Figure 2). Whereas among those diagnosed from 1988–1999, women < 45 years of age had an OS of 15 months vs. 13 months for men. For patients above the age of 55, year of diagnosis did not affect survival in a clinically appreciable way.
Tumor Location and MCRC
We also examined the relationships between tumor location, gender, ethnicity, and age. When age was considered we found that older patients, both men and women, with MCRC were more likely to have proximal cancers. Among those with MCRC at age < 45, women had 39.6% proximal tumors and men 34.8%. Of those with MCRC at age 55 and older, women had 49.1% proximal tumors and men 40.4%. As men and women aged, this “rightward shift” was consistent across all age groups (Table 2).
Table 2.
Tumor Location, Gender, and Age in MCRC, SEER data 1988–2004
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years |
N | Proximal | Distal | Recto- Sigmoid |
Rectum | NOS | N | Proximal | Distal | Recto- Sigmoid |
Rectum | NOS |
| 18–44 | 1,458 | 507 (34.8%) | 364 (25.0%) | 161 (11.0%) | 343 (23.5%) | 83 (5.7%) | 1,501 | 595 (39.6%) | 494 (32.9%) | 144 (9.6%) | 216 (14.4%) | 52 (3.5%) |
| 45–54 | 3,291 | 1190 (39.2%) | 832 (25.3%) | 381 (11.6) | 703 (21.4%) | 185 (5.6%) | 2,761 | 1171 (42.4%) | 837 (30.3%) | 248 (9.0%) | 382 (13.8%) | 123 (4.5%) |
| ≥55 | 22,678 | 9162 (40.4%) | 6128 (27.0%) | 2237 (9.9%) | 3724 (16.4%) | 1427 (6.3%) | 25,455 | 10411 (49.1%) | 4992 (23.6%) | 1719 (8.1%) | 2565 (12.1%) | 1506 (7.1%) |
| Total | 27,427 | 10,859 (39.6%) | 7324 (26.7%) | 2779 (10.1%) | 4770 (17.4%) | 1695 (6.2%) | 25,455 | 12,177 (47.8%) | 6323 (24.8%) | 2111 (8.3%) | 3163 (12.4%) | 1681 (6.6%) |
NOS – not otherwise specified, includes overlapping tumors
There are significant ethnic variations in tumor location as well. African Americans had the highest rate of proximal tumors (48.1%), followed by Whites (44.3%), Hispanics (37.7%), Native Americans (36.9%), and Asians (33.4%). Cancers of the rectum were most common in Native Americans (23.5%), then Hispanics (19.0%), Asians (18.1%), Whites (14.9%), and African Americans (11.5%).
Multivariate Model
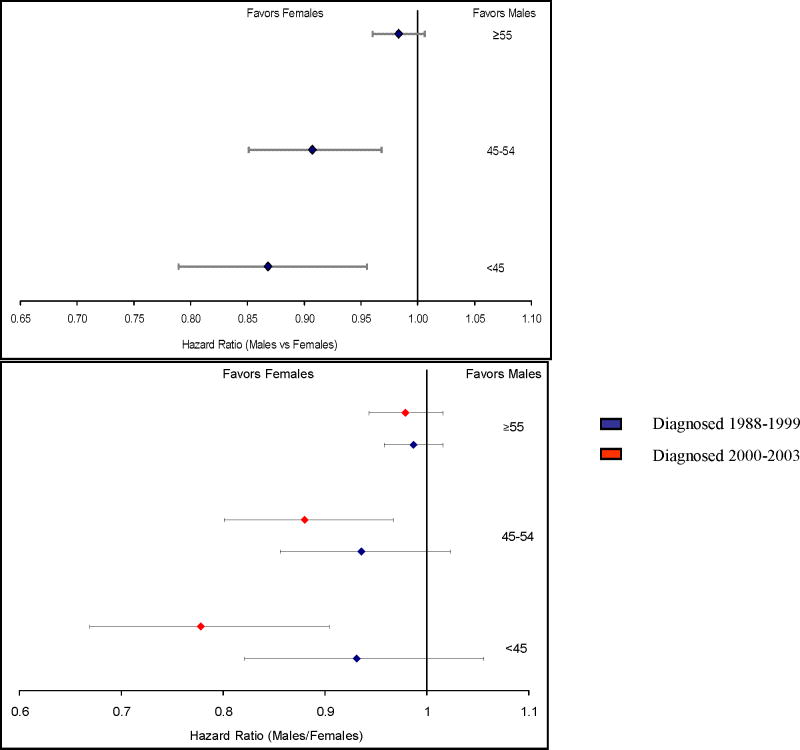
In multivariate analysis of cancer specific survival, we adjusted for marital status, tumor site, treatment with radiation and/or surgery, and tumor differentiation and stratified by SEER registry site and year of diagnosis. We found that young (< 45 years old) women have a lower risk of dying from MCRC than men (HR 0.868, 95% CI: 0.789–0.955, Figure 3 – top panel). As women age, this risk reduction becomes more diminutive until the risk between men and women becomes equivalent (> 55 years old, HR 0.983, 95% CI: 0.96–1.01, P value for interaction=0.011). When we accounted for year of diagnosis we found that these differences have become more pronounced in recent years. For example, the HR of women aged less than < 45 years has gone from 0.931 (95% CI: 0.821–1.056) in those diagnosed from 1988–1989 to 0.778 (95% CI: 0.669– 0.904) for those diagnosed between 2000–2004 (figure 3 – bottom panel). We also examined the hazard ratios of men and women across all ethnicities and found no association between gender specific survival and ethnicity (P value for interaction=0.29).
Figure 3. Hazard Ratio (females/males) of cancer specific survival comparing females with males across age all groupings (top panel).
The bottom panel is a sub- group analysis by year of diagnosis. Cox proportional hazards models were developed to evaluate association between patient characteristics and survival in men and female separately. All multivariable models included year of diagnosis and registry as stratification variable, marital status, treatment, primary site, and tumor grade and differentiation as covariates.
DISCUSSION
Our data suggests that sex, age, and ethnicity have a significant impact on overall survival in MCRC patients. This supports previous findings that as patients age they have poorer survival, and that ethnicity significantly impacts prognosis. We found in one of the largest cohort of CRC patients analyzed that gender in combination with age has an important influence on overall survival, and cancer specific survival in patients with MCRC. We also note for the first time that the benefits among younger women are more pronounced in recent years which may result from the interaction of hormonal status and treatment options recently available.
Menopause is commonly defined as 12 months of amenorrhea in women over the age of 45. Woman typically reach menopause between 45–55 years of age, with an average age of 50.5(24). Only 5% of women become menopausal between 40–45 years of age and another 5% occur after 55 years of age(25). Based on this data, we used the age of 45 as our proxy for a cohort of pre-menopausal women. This is consistent with reports and studies that have used age as a surrogate for menopause.
In our study, younger women (< 45 years of age) have better OS than younger men. At 17 months, their OS was 20% higher than the male cohort, and corresponded with a hazard ratio of 0.865 in our multivariate model. Yet this benefit did not extend to older women. Indeed, as women aged their prognosis declined with respect to similarly aged men, and we found that women > 55 years of age do even worse than older men (figure 2). These findings suggest that pre-menopausal women with metastatic colorectal cancer may have an improved prognosis. As they age through menopause, they lose this advantage and their risk eventually becomes equivalent to men. Our findings are similar to those reported recently by Koo et al., where similar gender discrepancies are described in a prospective cohort of CRC patients of all stages(20).
These results are consistent across all ethnicities and not confounded by treatment with radiation and/or surgery or the site of disease. Access to care(26), treatment disparities(27), and sex-specific comorbidites(28) have often been associated with gender disparities in colorectal cancer survival, but are unlikely explanations for our findings. MCRC is uniformly treated with chemotherapy and perhaps less prone to treatment disparities that have been described in the adjuvant setting. In addition, we analyzed cancer specific mortality to limit the effects of comorbidities that are not reported on the SEER database. Our analysis, after censoring patients whose cause of death was not attributed to their colorectal cancer, did not alter our findings. Cardiovascular disease was the cause of death in 2.92% of our patients and was not associated with the gender differences found (data not shown).
A possible explanation of these gender disparities in younger patients is forthcoming when we attempt to reconcile the more pronounced disparity among those diagnosed after 1999. Women < 45 years of age, diagnosed from 2000–2004, had a 33% increase in OS when compared to those diagnosed from 1988–1999. Comparably aged men only had an increase in survival of 15%. Similar improvements based on year of diagnosis were absent from the older group, supporting recent reports of the underutilization of combination chemotherapy in elderly patients(29).
After decades of using 5-flurouracil alone in the metastatic setting, in 2000 irinotecan based combination chemotherapy became the new first-line treatment for patients with MCRC(30). Over the next few years, oxaliplatin(31), bevacizumab(32), and cetuximab(33) were approved and the OS of patients with MCRC improved considerably. Our findings suggest that younger women may benefit more from these current therapies than younger men. Whether this reflects a differential response or toxicity to modern chemotherapy or represents an emerging pattern is unclear. Regardless, these data support the growing notion that estrogen is an impediment to CRC development and progression.
Although the exact role of estrogen in CRC tumorigenesis is unclear, current investigations point to estrogen receptor β as the most likely mediator. As women age and their nascent levels of estrogen decline, the expression of estrogen receptor B is down-regulated (34). Loss of estrogen receptor β is emerging as a common step in hormone-dependent tumor progression in several cancer models(35). In colon cancer, it has been shown that ERβ is selectively lost in malignant tissue(36), and ERβ knockout mice demonstrate increased proliferation and decreased differentiation and apoptosis of the colonic mucosa(37). Recent data have shown that functional polymorphisms in ERβ are associated with sex-specific survival in MCRC(38).
Estrogen may affect tumor biology and alter the effectiveness of screening and treatment. For example, one report has shown that loss of estrogen receptor ERβ expression is associated with left-sided colon cancers and a more advanced presentation(39). Hormone status may also affect the underlying genetic underpinning of CRC cancer, predisposing one to chromosomal instability (CIN), microsatelllite instability (MSI)(13, 40), or CpG Island Methylator phenotypes(41).
Estrogen has also been shown to prevent carcinogenesis by down-regulating inflammation. In a mouse model of hepatocellular cancer, estrogen mediated inhibition of interleukin 6 was shown to be protective for the development of HCC(42), perhaps explaining in part the gender disparities in HCC. The link between chronic inflammation and colon cancer has been demonstrated by the increased rates of CRC in patients with chronic inflammatory bowel disease(43), and the risk reduction in those taking ASA(44). Estrogen regulation of inflammatory cytokines may be a promising area of investigation in CRC.
Our study also confirmed reports that women(45) and the elderly(46) are more likely to have right sided tumors. Gender differences in sidedness were not age dependent and both men and women were more likely to develop proximal tumors as they aged. Furthermore, in our cohort proximal tumors were a poorer prognostic marker than distal or rectal tumors across all ages and ethnicities. As such, sidedness does not explain the differences in survival between younger men and women.
We found that ethnicity significantly impacts CRC survival. As previously reported(47, 48), African Americans and Native Americans had the worse survival among all ethnic groups. Notably, Whites had worse overall survival than Hispanics and Asian Americans. This may reflect differences in “western “ and “eastern” diets(49), red-meat consumption(50), NSAID use, amount of exercise(11) and other behaviors that have been shown to be associated with CRC incidence and recurrence. Additionally, environmental variations in estrogen exposure - either endogenous or exogenous - may contribute to these ethnic differences.
Our data was retrospective and therefore this report is subject to errors that commonly arise from such analyses. Specifically, our proxy for hormone status was age, as the databases did not query menopausal status, history of hormone replacement therapy, or contraceptive use. The SEER database does not record chemotherapy use and therefore limited our ability to investigate its interaction with our findings.
As one of the largest data sets analyzed to establish that younger women with MCRC survive longer than younger men, hormonal status appears to play an important role not only in the development and pathogenesis of colorectal cancer, but also has prognostic significance. In CRC, estrogens have been shown to consistently improve clinical outcomes in women who take oral contraceptives or hormone replacement. Our report adds this body of evidence and warrants further studies to determine the role of age and estrogen in colorectal cancer development, growth and progression.
Acknowledgments
Funding
This work was supported by University of Southern California/Norris Comprehensive Cancer Center Support Grant P30 CA14089, the San Pedro Guild Research Fund and the Dhont Foundation.
Footnotes
Statements
All authors of this research paper have directly participated in the planning, execution, or analysis of the study.
All authors of this paper have read and approved the final version submitted. The contents of this manuscript have not been copyrighted or published previously. An abstract of this work was accepted at ASCO 2008 Annual Meeting and included in the poster discussion for Colorectal Cancer abstracts.
The contents of this manuscript are not now under consideration for publication elsewhere and will not be copyrighted, submitted, or published elsewhere while acceptance by the Journal is under consideration.
Statement of Translational Relevance: This manuscript demonstrates the impact of gender on survival in MCRC. Younger women have an increased overall survival when compared to their male counterparts. Conversely, older women have a comparatively decreased overall survival. We also report for the first time that the benefits among younger women have increased with newly available treatments. We postulate an interaction of hormonal status and chemotherapy options recently approved for colorectal cancer. These findings add to the burgeoning principle that hormones, specifically estrogen, may play an important role in colorectal cancer tumorigenesis. Additionally, these findings emphasize the importance of investigating menopausal status as a predictive and prognostic marker in CRC.
The interplay between hormones and carcinogenesis is complex. In the Women’s Health Initiative, combined estrogen and progesterone hormone replacement was both chemo-preventative and carcinogenic. Defining these interactions is important to understanding the gender predilection of certain malignancies and will lead to improved treatment and prevention.
Conflicts of Interest
Andrew Hendifar: none
Dongyun Yang: none
Felicitas Lenz: none
Georg Lurje: none
Alexandra Pohl: none
Cosima Lenz: none
Yan Ning:none
Wu Zhang:none
Heinz-Josef Lenz: none
References
- 1.Farquhar CM, Marjoribanks J, Lethaby A, Lamberts Q, Suckling JA. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2005:CD004143. doi: 10.1002/14651858.CD004143.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RL, Dollear T, Freels S, Persky V. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer. 1997;80:193–7. doi: 10.1002/(sici)1097-0142(19970715)80:2<193::aid-cncr4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–31. doi: 10.1038/sj.bjc.6603628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 5.Newcomb PA, Storer BE. Postmenopausal hormone use and risk of large-bowel cancer. J Natl Cancer Inst. 1995;87:1067–71. doi: 10.1093/jnci/87.14.1067. [DOI] [PubMed] [Google Scholar]
- 6.Mandelson MT, Miglioretti D, Newcomb PA, Harrison R, Potter JD. Hormone replacement therapy in relation to survival in women diagnosed with colon cancer. Cancer Causes Control. 2003;14:979–84. doi: 10.1023/b:caco.0000007970.04094.76. [DOI] [PubMed] [Google Scholar]
- 7.Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study. BMJ. 2007;335:651. doi: 10.1136/bmj.39289.649410.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez E, La Vecchia C, Balducci A, Chatenoud L, Franceschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722–7. doi: 10.1054/bjoc.2000.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschi S, Gallus S, Talamini R, Tavani A, Negri E, La Vecchia C. Menopause and colorectal cancer. Br J Cancer. 2000;82:1860–2. doi: 10.1054/bjoc.1999.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19:939–53. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashktorab H, Smoot DT, Carethers JM, et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin Cancer Res. 2003;9:1112–7. [PubMed] [Google Scholar]
- 13.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–50. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Press OA, Haiman CA, et al. Association of methylenetetrahydrofolate reductase gene polymorphisms and sex-specific survival in patients with metastatic colon cancer. J Clin Oncol. 2007;25:3726–31. doi: 10.1200/JCO.2007.11.4710. [DOI] [PubMed] [Google Scholar]
- 15.Press OA, Zhang W, Gordon MA, et al. Gender-related survival differences associated with EGFR polymorphisms in metastatic colon cancer. Cancer Res. 2008;68:3037–42. doi: 10.1158/0008-5472.CAN-07-2718. [DOI] [PubMed] [Google Scholar]
- 16.Sloan JA, Goldberg RM, Sargent DJ, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002;20:1491–8. doi: 10.1200/JCO.2002.20.6.1491. [DOI] [PubMed] [Google Scholar]
- 17.Woods SE, Basho S, Engel A. The influence of gender on colorectal cancer stage: the state of Ohio, 1996–2001. J Womens Health (Larchmt) 2006;15:877–81. doi: 10.1089/jwh.2006.15.877. [DOI] [PubMed] [Google Scholar]
- 18.McArdle CS, McMillan DC, Hole DJ. Male gender adversely affects survival following surgery for colorectal cancer. Br J Surg. 2003;90:711–5. doi: 10.1002/bjs.4098. [DOI] [PubMed] [Google Scholar]
- 19.Wichmann MW, Muller C, Hornung HM, Lau-Werner U, Schildberg FW. Gender differences in long-term survival of patients with colorectal cancer. Br J Surg. 2001;88:1092–8. doi: 10.1046/j.0007-1323.2001.01819.x. [DOI] [PubMed] [Google Scholar]
- 20.Koo J, Jalaludin B, Wong S, Kneebone A, Connor S, Leong R. Improved survival in young women with colorectal cancer. Am J Gastroenterol. 2008;103:1488–95. doi: 10.1111/j.1572-0241.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 21.Angell-Andersen E, Tretli S, Coleman M, Langmark F, Grotmol T. Colorectal cancer survival trends in Norway 1958–1997. Eur J Cancer. 2004;40:734–42. doi: 10.1016/j.ejca.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Rothman K. Modern epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 23.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:512–26. [Google Scholar]
- 24.Nichols HB, Trentham-Dietz A, Hampton JM, et al. From menarche to menopause: trends among US Women born from 1912 to 1969. Am J Epidemiol. 2006;164:1003–11. doi: 10.1093/aje/kwj282. [DOI] [PubMed] [Google Scholar]
- 25.McKinlay S, Brambilla D, Posner J. The normal menopause transition. Maturitas. 1992;14:103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 26.Ananthakrishnan AN, Schellhase KG, Sparapani RA, Laud PW, Neuner JM. Disparities in colon cancer screening in the Medicare population. Arch Intern Med. 2007;167:258–64. doi: 10.1001/archinte.167.3.258. [DOI] [PubMed] [Google Scholar]
- 27.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–11. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 28.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–34. [PubMed] [Google Scholar]
- 29.Renouf D, Kennecke H, Gill S. Trends in chemotherapy utilization for colorectal cancer. Clin Colorectal Cancer. 2008;7:386–9. doi: 10.3816/CCC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 30.Saltz L, Cox J, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 31.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 32.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 34.Murillo-Ortiz B, Perez-Luque E, Malacara JM, Daza-Benitez L, Hernandez-Gonzalez M, Benitez-Bribiesca L. Expression of Estrogen Receptor Alpha and Beta in Breast Cancers of Pre- and Post-menopausal Women. Pathol Oncol Res. 2008 doi: 10.1007/s12253-008-9088-y. [DOI] [PubMed] [Google Scholar]
- 35.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–51. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60:245–8. [PubMed] [Google Scholar]
- 37.Wada-Hiraike O, Imamov O, Hiraike H, et al. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103:2959–64. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Press OA, Zhang W, Yang D, Gordon MA, Mallik N, Lenz HJ. Survival differences related to estrogen receptor beta (ERβ) polymorphism and age in female patients with metastatic colon cancer. journal of clinical oncology. 2004;22:3618. [Google Scholar]
- 39.Jassam N, Bell SM, Speirs V, Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol Rep. 2005;14:17–21. [PubMed] [Google Scholar]
- 40.Slattery M, Potter J, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61:126–30. [PubMed] [Google Scholar]
- 41.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–74. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 42.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 43.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 44.Dube C, Rostom A, Lewin G, et al. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–75. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 45.Meguid R, Slidell M, Wolfgang C, Chang D, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–94. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper G, Yuan Z, Landefeld C, Johanson J, Rimm A. A national population-based study of incidence of colorectal cancer and age. Implications for screening in older Americans. Cancer. 1995;75:775–81. doi: 10.1002/1097-0142(19950201)75:3<775::aid-cncr2820750305>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 47.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 48.Chien C, Morimoto L, Tom J, Li C. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104:629–39. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 49.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 50.Chao A, Thun MJ, Connell CJ, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–82. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]