Abstract
Emotions have powerful effects on pain perception. However, the brain mechanisms underlying these effects remain largely unknown. In this study, we combined functional cerebral imaging with psychophysiological methods to explore the neural mechanisms involved in the emotional modulation of spinal nociceptive responses (RIII-reflex) and pain perception in healthy participants. Emotions induced by pleasant or unpleasant pictures modulated the responses to painful electrical stimulations in the right insula, paracentral lobule, parahippocampal gyrus, thalamus, and amygdala. Right insula activation covaried with the modulation of pain perception, consistent with a key role of this structure in the integration of pain signals with the ongoing emotion. In contrast, activity in the thalamus, amygdala, and several prefrontal areas was associated with the modulation of spinal reflex responses. Last, connectivity analyses suggested an involvement of prefrontal, parahippocampal, and brainstem structures in the cerebral and cerebrospinal modulation of pain by emotions. This multiplicity of mechanisms underlying the emotional modulation of pain is reflective of the strong interrelations between pain and emotions, and emphasizes the powerful effects that emotions can have on pain.
Keywords: brain, nociceptive flexion (RIII) reflex, fMRI, insula
Pain and emotions are intimately related experiences with robust reciprocal interactions (1). In general, negative emotions increase pain, whereas positive ones decrease it (2). In a recent study, Rhudy et al. (3) have demonstrated that emotions induced by emotional pictures (4) activate descending pain-modulatory pathways affecting the amplitude of a spinal nociceptive reflex (see ref. 4; nociceptive flexion reflex or RIII reflex, see ref. 5). However, the modulation of spinal responses typically accounts for only part of the modulation of pain experiences assessed by self-reports. The goal of this study is to identify the brain generators of cerebrospinal modulatory effects and to investigate additional brain mechanisms underlying the modulation of pain by emotions.
Descending pain-modulatory pathways originate from various cerebral structures involved in emotions (6–8) and sensorimotor functions (9). These regions are thought to affect spinal nociception through their projections to several brainstem structures, including the periaqueductal gray matter (PAG), rostroventral medulla (RVM), dorsolateral pontine tegmentum (DLPT), and nucleus cuneiformis (NCF) (10). All of these structures are thus potential cerebral sources of the descending modulation of pain by emotions. In turn, the modulation of spinal activity is expected to affect the transmission of nociceptive signals and the response of their target brain regions through the multiple ascending pathways. However, the important interconnectivity between emotional brain networks and areas implicated in the affective dimension of pain suggests that additional supraspinal mechanisms might also contribute to the emotional modulation of pain experiences. Among the potential cortical candidates, the anterior cingulate cortex (ACC) (11) and the insula (12) are well positioned to contribute to the emotional modulation of pain. Whereas the ACC appears to be involved in the motoric and motivational aspects of pain and emotions, the insula is thought to generate subjective interoceptive feelings as a result of the gradual posterior-to-anterior integration of primary interoceptive information with contextual emotional and cognitive information (12).
The cerebral correlates of the emotional modulation of pain perception have been explored in two brain imaging studies. In one study, pain-related activation in the entorhinal cortex was increased by expectation-induced anticipatory anxiety of a highly painful stimulation (13). Also, the increased activation in the entorhinal cortex was correlated with activity in other pain-related areas such as the cingulate cortex and the insula. These results were interpreted in light of the Gray–McNaughton theory of anxiety (14), which postulates that in situations of conflict, the hippocampal formation amplifies the neural representation of the aversive event. Consistent with the proposed model of emotion modulation, the hippocampal system might be involved in the facilitation of nociceptive processes at the spinal and/or cerebral level.
In the other study, unpleasant odors, compared with pleasant ones, increased both pain perception and activations in the ACC, medial thalamus, and primary and secondary somatosensory cortices (SI/SII) (15). However, in this case, pain-related activations were confounded with activations related to odors due to the simultaneous application of odors and painful stimulation. This is especially problematic in the emotional/limbic regions such as the ACC, which are commonly activated to both types of stimuli. Therefore, the results of these two studies indicate that emotions can modulate activity in pain-responsive regions of the brain, but the underlying mechanisms remain to be determined. We hypothesized that the modulation of pain-related brain activations resulted at least in part from a modulation of spinal nociception and also possibly from supraspinal interregional interactions between pain and emotion brain networks.
In this study, we combined the recording of a spinal nociceptive reflex (RIII reflex), as an index of spinal nociception, with fMRI in a paradigm investigating the emotional modulation of pain. This combination of spinal and cerebral measures allowed pain-related regions whose modulation by emotions are linked to spinal nociception to be distinguished from those that are not. Also, to separate pain- and emotion-related activation, we used a mixed blocked/event-related design in which participants received painful electric shocks while they viewed blocks of pleasant, unpleasant, or neutral pictures (see Fig. 1A). This particular design was essential to dissociate changes in pain-responses induced by the emotional context (i.e., modulation of shock-related activations) from the effects of the emotional pictures (i.e., blocks of emotional pictures). Last, we used connectivity analyses to reveal networks of brain regions associated with the effects of emotions on pain-related brain activation.
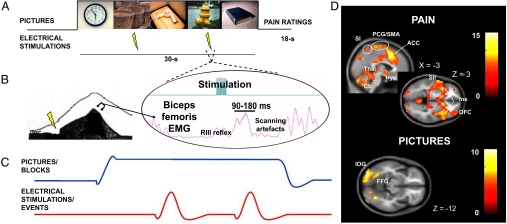
Fig. 1.
Experimental paradigm and brain responses to painful shocks and pictures. (A) Each trial consists of a block of five 6-s pictures (neutral, pleasant, or unpleasant), within which two painful electrical stimulations are delivered 2,700 ms after the onset of the 2nd and 4th pictures. At the end of the trial, participants were asked to rate the mean pain elicited by the two painful stimulation on a VAS. (B) Electrical stimulations were delivered over the right sural nerve (ankle) and RIII reflexes were extracted from the EMG of the biceps femoris in a window of 90–180 ms after the onset of the electrical stimulation. (C) A mixed block/event-related model was used to analyze the data. Pictures were modeled as blocks and electrical stimulations as events. (D) Electrical stimulations elicited activations in the thalamus (Thal), hypothalamus (Hyp), SI/SII, ACC, insula (Ins), precentral gyrus (PCG), SMA, OFC, and cerebellum (Cb). Pictures elicited activation in visual areas such as the inferior occipital gyrus (IOG) and fusiform gyrus (FFG) (see Table S1).
Results
Pain Ratings and RIII Reflex.
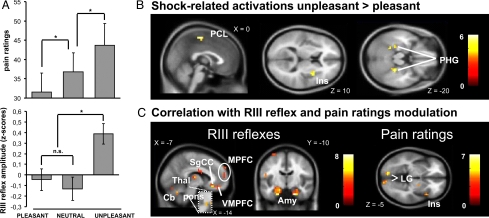
Mean pain ratings and RIII reflex amplitudes are reported for each experimental condition in Fig. 2A. There was a significant effect of picture content on pain ratings [F(3, 33) = 13.88, P < 0.001, η2 = 0.59]. Planned follow-up contrasts showed that pain ratings were higher during unpleasant than neutral pictures [t(11) = 5.11, P < 0.001, d = 2.57] and lower during pleasant than neutral pictures [t(11) = 2.30, P < 0.05, d = 0.91]. RIII reflex amplitudes were also significantly influenced by the experimental condition [F(3, 27) = 8.74, P < 0.01, η2 = 0.67]. Planned follow-up contrasts showed that the RIII reflex amplitude was increased during the viewing of unpleasant pictures compared with pleasant and neutral pictures [unpleasant vs. pleasant: t(9) = 2.64, P < 0.05, d = 1.16; unpleasant vs. neutral: t(9) = 4.18, P < 0.05, d = 1.82]. However, there was no significant difference between the amplitude of the reflex during the presentation of pleasant vs. neutral pictures conditions [t(9) = 0.94, P = not significant (n.s.), d = 0.41]. The absence of a significant effect of pleasant pictures on the RIII might be due to the inclusion of pictures depicting outdoors sports scenes where danger is impending (parachuting, kayaking, etc.), which in some cases may produce opposite effects than those expected by their valence (16). Nevertheless, the significant difference in RIII reflex amplitudes between the pleasant and unpleasant pictures condition clearly shows that, for equal levels of arousal, emotional valence influenced spinal nociception.
Fig. 2.
Modulation of pain-related responses by the emotional conditions. (A) Effects of the experimental condition on pain ratings (Upper). Effects of the experimental condition on the standardised R-III reflex amplitudes (Lower). *, P < 0.05. (B) Regions where shock-related activity was higher during the viewing of unpleasant compared with pleasant pictures included the PCL, right Ins, and bilateral PHG. (C) Regions where the amplitude of the modulation of brain responses between unpleasant and pleasant pictures correlated with the corresponding modulation in RIII reflex or pain ratings. (Left) Correlations with RIII reflex modulation included the left medial Thal, left pons, bilateral amygdalae (Amy), SgCC, VMPFC and MPFC, and Cb. (Right) Correlations with pain modulation (pain ratings) included the right Ins and bilateral lingual gyri (LG). Activation maps are thresholded at P < 0.005 for display purposes (see Tables S2 and S3).
Pain and Picture Activations.
We first looked at the general patterns of activation related to the electric shocks and the blocks of pictures across experimental conditions (Fig. 1D; Table S1). The electrical stimulation induced the typical pattern of pain-related activation comprising the thalamus, SI/SII, insula, and mid and ACCs. Additional activation peaks were found in the hypothalamus, parahippocampal gyrus (PHG), pons, cerebellum, and bilateral middle and medial frontal gyri. Significant activation to the blocks of pictures was found primarily in the visual cortices and in the FFG.
Main Effect of Emotions on Pain-Evoked Activation.
We then examined the effects of emotions on pain-related brain activation. For this analysis, we focused on the contrast of shock-evoked responses observed during unpleasant vs. pleasant pictures, so as to maximize the valence effects while controlling for nonspecific arousal effects induced by the emotional pictures. Fig. 2B shows larger brain activations to the painful shocks during unpleasant compared with pleasant pictures in the paracentral lobule (PCL), bilateral PHG, and right ipsilateral insula (Table S2).
Correlations with Pain Ratings and RIII Reflex Modulation.
To reveal brain structures associated with the effects of emotions on pain ratings or spinal nociception, we correlated the effects of unpleasant vs. pleasant pictures on shock-evoked brain activation with the magnitude of the modulation of pain ratings and RIII reflexes. For this analysis, the intraindividual differences in pain ratings and RIII reflexes for the unpleasant vs. pleasant pictures conditions were used as the magnitude scores for the effects of emotions on pain. The correlations of these scores with the effects of emotions on pain-related brain activation are displayed in Fig. 2C (see also Table S3). The effects of emotion on pain ratings correlated with activity in the right (ipsilateral) anterior insula, whereas the effects of emotions on the RIII reflex correlated with activity in the left (contralateral) medial thalamus, bilateral amygdalae, left (contralateral) pons, subgenual cingulate gyrus, ventromedial and medial prefrontal cortices (VMPFC/MPFC). Together, these results clearly demonstrate that emotions modulated spinal nociceptive processes, pain perception, and the corresponding brain responses.
Emotional Pictures Activations.
Unpleasant pictures elicited stronger activation than neutral pictures in visual areas and in the left PHG, left amygdala, posterior thalamus, midbrain, VMPFC, right dorsolateral prefrontal cortex (DLPFC), and left lateral orbitofrontal cortex (OFC) (Fig. S1 and Table S4). More robust activation was also found compared with pleasant pictures in the MPFC, retrosplenial cingulate cortex, and right DLPFC. In contrast, pleasant pictures elicited larger activation compared with neutral pictures only in visual areas, and showed no significant activation peak in comparison with unpleasant pictures.
Connectivity Analyses.
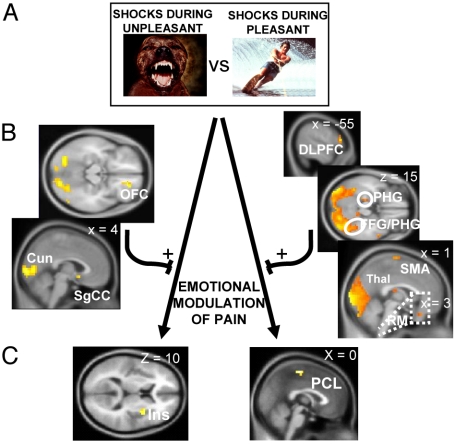
As a complementary step in exploring brain regions predicting the effects of emotional pictures on pain activations, we conducted psychophysiological interactions (PPI) analyses to examine functional connectivity based on emotion-dependent interactions with pain-related regions (17). For these analyses, the contrast of unpleasant vs. pleasant pictures in the event-related brain responses to the electrical shocks was chosen as the effect of interest. The right insula and the PCL (sensorimotor cortex) were selected as seed regions for the PPI because of their established role in pain processing and their clear modulation in the unpleasant vs. pleasant contrast (Fig. 2B; Table S2). The results of the PPI analyses are displayed in Fig. 3(see also Table S5) and represent the regions that correlated more with the right insula or PCL seed regions for the shocks delivered during unpleasant vs. pleasant pictures. The results obtained using the right insula as the seed region included areas related to emotional processing, such as the right OFC and subgenual cingulate cortex (SgCC). The pattern of enhanced connectivity with the PCL was more widespread and included the left PHG, supplementary motor area, left DLPFC, left posterior thalamus, and left rostral medulla (RM). Because activation of these regions predicted the effects of unpleasant vs. pleasant emotions on pain activation in the seed regions, the regions revealed by the PPI analyses can be seen as potential moderators of the effects of emotions on pain.
Fig. 3.
Results of the PPI analyses. (A) The psychological variable for the interaction is the contrast between the electrical stimulations presented during unpleasant vs. pleasant pictures. (B) Right OFC, SgCC, and cuneus (Cun) exhibited higher connectivity with the right insula for the electrical stimulations presented during the viewing of unpleasant vs. pleasant pictures (Left). Left DLPFC, FFG, and PHG, left SMA, left Thal, and RM showed higher connectivity with the PCL for the electrical stimulations presented during the viewing of unpleasant vs. pleasant pictures (Right). (C) The right Ins and the PCL, where activation to electrical stimulations were shown to be higher during unpleasant than pleasant pictures, served as the physiological variables (“seeds”) for the PPIs. Activation maps were thresholded at P < 0.005 for display purposes (see Table S5).
Discussion
This study revealed that the pain-related activity of several brain regions was modulated by emotions. The recording of the RIII reflex as an index of spinal nociception also allowed brain regions that are modulated as a function of descending modulatory pathways to be distinguished from those associated more closely with supraspinal mechanisms of pain modulation. Also, the mixed block/event-related design allowed the identification of the brain regions activated by the blocks of emotional pictures separately from those reflecting phasic pain-related responses. Last, connectivity analyses were used to reveal the brain regions involved in the effects of emotions on pain-related activations.
The observation of numerous regions correlating with the effects of emotions on RIII reflex is consistent with the involvement of descending modulatory mechanisms in the effects of emotions on pain. Indeed, many of the regions correlating with the modulation of the RIII, such as the left (contralateral) thalamus, left pons, and bilateral amygdalae, are cerebral targets of ascending spinal nociceptive pathways. The thalamus is at the endpoint of the spinothalamic tract and is involved, in its more medial part, in the transmission of nociceptive signals to prefrontal cortical areas, contributing to the affective dimension of pain. The amygdala is also a main cerebral target of spinal nociceptive afferents. It receives nociceptive inputs from the dorsal horn of the spinal cord through the parabrachial nucleus, a structure located close to a peak of activation found in the pons and which correlated with the effects of emotions on RIII reflex amplitude. Here, the response of the amygdala to the painful shocks might reflect the emotional arousal associated with the detection of brief and sudden aversive stimuli (18). This first automatic step of the affective processing of acute noxious stimuli is generally followed by more controlled and elaborate processes of affective evaluation in the medial and/or orbital parts of the frontal lobes. Thus, the activation observed in the subgenual cingulate gyrus, VMPFC, and MPFC could reflect these later processes of affective evaluation and meaning attribution.
The main contrast between pain-evoked activation during the presentation of unpleasant vs. pleasant pictures also revealed a locus of activation in the PCL, in a region implicated in the primary sensorimotor representation of the foot (19). Thus, the enhanced activation of this structure in the context of unpleasant emotions could reflect a modulation of the sensory and motor aspects of the pain responses to the electrical stimulations. This is consistent with two recent studies (20, 21) showing that early somatosensory evoked-potentials elicited by painful electrical stimulation are modulated by the presentation of emotional pictures. Moreover, this result also replicates the observation by Villemure and Bushnell (15) that SI activity can be modulated by the emotional context. Thus, emotions seem to influence the transmission of nociceptive information to somatosensory regions.
In contrast, the effects of emotional pictures on pain-related activation in the right anterior insula appear to reflect a higher-order mechanism of pain modulation. Recent theories of emotional processing suggest that this region is involved in the integration of interoceptive information with ongoing emotional states represented in frontal and limbic regions of the brain (12). According to this model, the activity of the right anterior insula would reflect the formation of a higher-order subjective representation of a “global emotional moment,” which in the case of this particular study would be a representation of the subjective feeling of pain within the general emotional context. Our findings of right insula activation correlating with the modulation of subjective pain ratings strongly support this interpretation.
Increases in pain-evoked activation in bilateral PHG were also observed in the context of unpleasant vs. pleasant pictures, possibly reflecting increased affective processing of pain aversiveness. Indeed, the role of the PHG in negative emotions, and particularly anxiety, is well documented (14). Many studies have found parahippocampal activation in response to pain (22), where it is believed to mediate the aversive drive and contribute to the negative affect inherent to pain. Interestingly, parahippocampal responses to pain are shown to be increased by anxiety in healthy volunteers (13), as well as in clinical populations suffering from anxiety-related pain disorders (23).
Our mixed blocked/event-related design also allowed us to look at the activation related to the blocks of emotional pictures separately from their effects on pain-related activation. The regions activated by the unpleasant pictures are in accordance with those associated with aversive stimuli found in previous studies on emotional processing (24). Among these regions, the PHG is of particular interest, because its activation was previously found during the anticipation of highly painful thermal stimuli (13). Also, in another study, the PHG was shown to be coactivated with the PAG and ventral tegmental area (VTA) and further predicted pain responses in the posterior insula (8). This is particularly interesting, because PAG activation was also observed here in response to unpleasant pictures, supporting the idea that the combined activation of the PHG and the PAG could be involved in the descending facilitation of pain. The present findings thus indicate that, similarly to pain-related anticipatory anxiety induced by expectation (13), negative emotions induced by unpleasant images may also affect pain-related responses through mechanisms involving the PHG and brainstem nuclei. These findings further support the Gray–McNaughton theory of anxiety (14), which posits that in situations in which environmental cues signaling danger are present, the hippocampal formation amplifies the neural representation of the aversive event to prepare the organism for the worst possible outcome. In the case of our study, unpleasant pictures would have acted as the cues signaling an increased probability of danger, thereby activating the PHG and facilitating pain-related processes.
Last, the PPI analyses revealed some of the regions that predicted the effects of emotional pictures on the pain responses observed within the PCL and right insula. The pattern of enhanced connectivity with the PCL comprised several brain regions, suggesting the implication of more than one mechanism. One of these mechanisms appears to involve the left PHG, which predicted the effects of emotions on PCL responses (Fig. 3). This finding is highly consistent with the role of the PHG in anxiety-induced descending pain facilitation. Indeed, the PPI analysis also revealed a peak of activation in the RM located in the vicinity of brainstem structures involved in the descending facilitation of spinal nociception, such as the DLPT and the RVM (9, 10), suggesting that mechanisms of descending facilitation involving the PHG and the brainstem are implicated in the effects of emotions on pain through descending modulatory pathways affecting spinal nociceptive transmission.
Other regions predicting the effects of emotional pictures on PCL activation included the supplementary motor area (SMA) and left DLPFC. Both these structures exhibit efferent connections with somatosensory cortices (25, 26), by which they could have directly influenced PCL activation. As for the PHG, these regions have been linked to pain anticipation (27, 28) and pain expectations (29), but lack the predominantly emotional function generally attributed to the PHG. Rather, they are thought to influence nociceptive processing by priming the somatosensory representation of pain through the formation of a perceptual set (28), which might be linked, in the present experiment, to the specific content of the unpleasant pictures depicting attack or body mutilation scenes.
The PPI analyses also revealed that the effects of emotions on right insula activation were predicted by the OFC, suggesting that the influence of emotions on the integrative processes of the right insula could reflect direct or indirect interactions between these structures. It has been shown that the OFC has an important role in emotional evaluation (30), and particularly, in the evaluation of punishers (31). Moreover, risk assessment and harm avoidance also produce activation in the anterior insular/OFC, rostral to those implicated in the processing of the aversive stimulus itself (32), suggesting a predictive role for this structure on insula activation during the processing of aversive stimuli. This result is thus consistent with the proposed role of the right insula in the integration of pain signals with ongoing emotional states, which are represented in many cerebral structures, including the OFC (30). The fact that the integration of pain with negative emotions occurred in the right hemisphere, ipsilateral to the pain stimulus, also supports current theories of forebrain emotional asymmetry which propose a preferential engagement of the right forebrain in negative emotions (33).
Overall, the results of the present study indicate that various brain mechanisms are implicated in the effects of emotions on pain (summarized in Fig. S2). One of these mechanisms involves descending modulatory controls affecting the transmission of spinal nociceptive signals to many brain regions, including the thalamus, amygdala, and the PCL. Cerebral sources of such descending modulation include the PHG and brainstem nuclei activated by unpleasant emotional pictures and predicting the effects of emotional pictures on PCL activation. Another mechanism involves the integration of pain- and emotion-related signals in the right anterior insula into a higher-order subjective representation of the emotional self. Notably, an additional potential modulatory mechanism, left unexplored by the present study, involves the autonomic nervous system. Because autonomic responses are central to both pain and emotions and that the anterior insular cortex is critically involved in the higher-order regulation of this system (34), future studies should provide a more complete portrait of brain–autonomic system interactions during the emotional modulation of pain. Altogether, the multiplicity of mechanisms underlying the emotional modulation of pain is reflective of the strong interrelations between pain and emotion, and emphasizes the powerful effects that emotions can have on pain.
Methods
Participants.
Thirteen healthy volunteers participated in the study (6 male and 7 female; mean age, 23.4 years; SD, 5.1). Data from one male participant was excluded because of equipment failure during scanning. Participants were selected on the basis of their ability to tolerate the electrical stimulations during a prescanning session. The study was approved by the Research Ethics Board of the Centre de Recherche de l'Institut de Gériatrie de Montréal, and all participants gave written informed consent.
Electrical Stimulation and RIII Reflex Recording.
Transcutaneous electrical stimulation was delivered with a Grass-S48 stimulator (Astro-Med Inc.) isolated with a custom-made constant-current stimulus-isolation unit. The stimulation consisted in a 30-ms train of 10 × 1 ms pulse, delivered on degreased skin over the retro-maleolar path of the right sural nerve by means of a pair of custom made 1-cm2 fMRI-compatible surface electrodes. The intensity of the electrical stimulation was recorded (Biopac Systems), and the intensity of the stimulation was adjusted individually at 120% of the reflex threshold using the staircase method (35). This intensity induces a stable and moderately painful pin-prick sensation. Electromyographic (EMG) activity of the biceps femoris was recorded with MRI-compatible Ag-AgCl surface electrodes with an interelectrode distance of 2 cm. EMG activity was amplified, band pass filtered (100–500 Hz), digitized, and sampled at 1,000 Hz. The raw EMG data were filtered off-line (120–130 Hz) and transformed using the rms. The resulting signal was integrated between 90 and 180 ms after stimulus onset to quantify the RIII-reflex to single shocks.
Affect Induction.
Ninety pictures that evoked pleasant, unpleasant, or neutral emotions were selected from the International Affective Picture System (4). Based on their normative ratings, 30 pictures were chosen within each category to maximize valence differences between pleasant and unpleasant pictures, while equating their arousal levels (pleasant pictures, valence: M = 6.81, arousal: M = 6.58; unpleasant pictures, valence: M = 1.64, arousal: M = 6.75). Neutral pictures were of intermediate valence and of lower arousal. (neutral pictures, valence: M = 4.99, arousal: M = 2.54). Pleasant pictures mainly consisted of erotic couples and outdoor sports, whereas unpleasant pictures represented images of threats or mutilations, and neutral pictures consisted of household objects and outdoor scenes. For each category, pictures were grouped in six blocks of five 6-s pictures, for a total of 30 s per block. A fourth condition consisting of only a white fixation cross displayed in the middle of a black screen for 30 s served as an additional control with no pictures, but the results for that condition were not reported in the present article. In a prescan session (see SI Materials and Methods), all subjects rated the valence and arousal dimensions of the pictures blocks presented in the scanning session. The mean valence and arousal ratings are displayed in Table S6 and were comparable with the normative ratings.
Procedure.
The scanning session was divided into two functional scans separated by an anatomical scan. Each functional scan consisted of 26 experimental trials. The time course of a trial is depicted in Fig. 1. Each trial consisted in a 30-s block of emotional pictures or fixation point. Two electrical stimuli were delivered during each block of visual stimulation, 2,700 ms after the onsets of the second and fourth picture (or equivalent timing for the fixation point condition). This particular timing was necessary for the shock and reflex to occur during the 500-ms intervolume (see “fMRI acquisition”) delay at the end of the pictures. The stimuli were thus always delivered at the 200th millisecond of the 500-ms intervolume delay with no jittering (i.e., 2,700 ms after the onset of the fMRI volume) (Fig. 1A).
At the end of each block of pictures, participants had 18 s to rate the pain elicited by the electrical stimulation on a visual analogue scale (VAS: 0, no pain; 100, extremely painful). Participants were asked to provide an overall rating of the pain induced by the two stimulations in the preceding block. They were also instructed that their pain ratings should reflect the perceived intensity (sensory dimension), as well as the discomfort (affective dimension) elicited by the electrical stimulations. The purpose of asking for only one compound evaluation of the sensory and affective dimension of pain was to reduce rating times to maximize the number of pain events. However, it should be noted that these pain ratings cannot distinguish the effects of emotions on the sensory and affective dimension of pain.
Each scan started with two control trials with only the fixation point. These trials controlled for potential habituation effects, allowing the RIII reflex to stabilize. After these two trials, the remaining 24 trials were presented in a pseudorandom order (see SI Materials and Methods).
Functional MRI Acquisition.
Imaging data were acquired at the Unité de Neuroimagerie Fonctionnelle of the Centre de Recherche de l'Institut de Gériatrie de Montréal on a 3T Siemens Trio scanner using a CP head coil. The anatomical scans were T1-weighted high-resolution scans [repetition time (TR), 13 ms; echo time (TE), 4.92 ms; flip angle, 25°; field of view, 256 mm; voxel size, 1 × 1 × 1.1 mm]. The functional scans were collected using a blood-oxygen-level-dependent (BOLD) protocol with a T2*-weighted gradient echo-planar imaging sequence (TR, 3.0 s with an intervolume delay of 500 ms; TE, 30 ms; flip angle, 90°; 64 × 64 matrix; 41 × 3.44 × 3.44 × 5 mm slices; 433 volume acquisitions). Electrical stimuli were always administered during the intervolume delay (Fig. 1A), thereby avoiding the potential contamination of fMRI images by shock-induced artefacts and of EMG recordings by RF-pulse artefacts.
Pain Ratings and RIII Reflex Analyses.
RIII reflex amplitudes were first standardized within participants by converting them into z-scores. Normalized RIII reflex amplitudes and raw pain ratings were then averaged for each condition and compared through ANOVA with the experimental condition as a within-subject factor. Two participants were excluded from the RIII-reflex analysis. In one participant, the stimulation intensity required to reach 120% of the RIII-reflex threshold could not be attained at the maximal level of stimulation allowed by the isolation unit. In the other participant, the reflex habituated quickly after the onset of the experiment and was undetected in ≈90% of the following trials.
Functional MRI Data Analyses.
Pain and picture activations.
After preprocessing (see SI Materials and Methods), activation related to the pictures and the electrical stimulation were identified with a mixed block/event-related design where electrical stimulations were modeled as instantaneous events and experimental conditions as 30-s blocks of images. The vectors created for the electrical stimulations and experimental conditions were then convolved with a canonical hemodynamic response function (hrf). At the second level, group analyses were conducted using random-effect models with contrast images of individual-subject effects.
Correlations with RIII reflexes and pain ratings modulation.
For the correlation analyses, individual indexes of RIII reflexes or pain ratings modulation were computed for each participant by subtracting their mean RIII reflex or pain rating during the unpleasant vs. pleasant pictures conditions. The correlation between the modulation of pain ratings and RIII reflexes was nonsignificant (r = 0.344, n.s.). However, according to Cohen's guidelines (36), the size of this correlation can be considered as “medium” (r between 0.3 and 0.5), suggesting that RIII reflex and pain ratings modulation are related, but dissociable processes. These individual indexes of RIII reflexes or pain ratings modulation were then correlated with the effects of unpleasant vs. pleasant pictures on shock-related activations to reveal the regions where the modulation of shock-evoked activation correlated with RIII reflexes or pain ratings modulation.
PPI.
For the PPI analyses, the effect of unpleasant vs. pleasant pictures on the event-related responses to the electrical stimulations was chosen as the psychological variable of interest. The seed regions used as the physiological variables were chosen among the most representative pain-related areas modulated by the unpleasant vs. pleasant contrast (i.e., insula and PCL; see Results). Time series were extracted from a sphere (6-mm radius) centered on the selected seed regions maximum of the unpleasant vs. pleasant contrast for each participants using the first eigen time series (principal component) of this area. The PPI regressor was then computed as the element-by-element product of the mean-corrected seed region activity and a vector coding for the differential effect of unpleasant compared with pleasant pictures (1 for unpleasant and −1 for pleasant). The results of these PPIs thus revealed the brain regions where activity positively covaried more strongly with the seed regions during shocks delivered in the context of unpleasant vs. pleasant pictures.
Thresholding.
The significance of the t values obtained for the different neuroimaging analyses described in the results were thresholded at P < 0.05, Bonferroni-corrected for multiple comparisons (one-tail), based on Random Field Theory (37). Separate directed-search volumes were examined for shock-pain and picture processing. The directed search volume for pain analyses was estimated based on the cumulative size of structures previously shown to be involved in pain (SI and SII, the cingulate cortex, INS, OFC, the PGH/amygdala, hypothalamus, thalamus, and brainstem), whereas the directed search volume for picture analyses was based on the cumulative size of structures previously shown to be involved in emotional picture processing (occipital lobe, medial temporal lobe, thalamus, brainstem, OFC, insula, and prefrontal cortices). The pain directed search volume was estimated at 109.58 resels (using the effective FWHM) and led to a T-threshold of 4.1 for peaks of pain-related activation found within this volume. The picture directed search volume was estimated at 153.12 resels (using the effective FWHM) and led to a T-threshold of 4.2 for peaks of picture-related activation found within this volume. Additional peaks are reported in these directed search areas based on a more permissive threshold of P-uncorrected < 0.005 to protect against type II error. Activation peaks falling outside of these directed-search areas were thresholded at a t value of 5.1, according to a global search volume of 626.38 resels accounting for the whole-brain gray matter.
Supplementary Material
Acknowledgments.
This work was supported by a grant from the Canadian Institutes of Health Research (to P.R.), a doctoral scholarship from the Natural Science and Engineering Research Council of Canada (to M.R.), and a research fellowship from the Canadian Institutes of Health Research (to M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.R.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904706106/DCSupplemental.
References
- 1.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 2.Villemure C, Bushnell MC. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 3.Rhudy JL, Williams AE, McCabe KM, Nguyen MATV, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 4.Lang PJ, Bradley MM, Cuthbert BN. International affective pictures system (IAPS): Digitized photographs, instruction manual and affective ratings. Gainesville: University of Florida, NIMH Center for the Study of Emotion and Attention; 1998. (Tech. Rep. A-6) [Google Scholar]
- 5.Sandrini G, et al. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YQ, Tang JS, Yuan B, Jia H. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain. 1997;72:127–135. doi: 10.1016/s0304-3959(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Zhang Y, Zhao Z-Q. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur J Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]
- 8.Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128:101–110. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva L, Fields HL. The Pain System in Normal and Pathological States: A Primer for Clinicians. Progress in Pain Research and Management. In: Villanueva L, Dickenson A, Ollat H, editors. Seattle, WA: International Association for the Study of Pain Press; 2004. pp. 223–243. [Google Scholar]
- 10.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 13.Ploghaus A, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray JA, McNaughton N. The Neuropsychology of Anxiety. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 15.Villemure C, Bushnell MC. Mood Influences Supraspinal Pain Processing Separately from Attention. J Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernat E, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43:93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 18.LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenntner-Mabiala R, Andreatta M, Wieser MJ, Muhlberger A, Pauli P. Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biol Psychol. 2008;78:114–122. doi: 10.1016/j.biopsycho.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Kenntner-Mabiala R, Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology. 2005;42:559–567. doi: 10.1111/j.1469-8986.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 22.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Gündel H, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137:413–421. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 25.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connections patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.Jurgens U. The efferent and afferent connections of the supplementary motor area. Brain Res. 1984;300:63–81. doi: 10.1016/0006-8993(84)91341-6. [DOI] [PubMed] [Google Scholar]
- 27.Ploghaus A, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 28.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci USA. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wager TD, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 30.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 31.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cog Affect Behav Ne. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 32.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 33.Davidson RJ. Well-being and affective style: Neural substrates and biobehavioural correlates. Philos Trans R Soc London Ser B. 2004;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J, editor. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed. Hillsdale, UK: Erlbaum; 1988. [Google Scholar]
- 37.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cerebr Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.