Abstract
MBP-specific autoreactive T cells are considered pro-inflammatory T cells and thought to play an important role in the pathogenesis of multiple sclerosis (MS). Here, we report that MBP83–99-specific T cells generated from MS patients (n = 7) were comprised of pro-inflammatory and regulatory subsets of distinct phenotypes. The pro-inflammatory phenotype was characterized by high production of IFN-γ, IL-6, IL-21 and IL-17 and low expression of FOXP3, whereas the regulatory subset expressed high levels of FOXP3 and exhibited potent regulatory functions. The regulatory subset of MBP-specific T cells appeared to expand from the CD4+CD25− T-cell pool. Their FOXP3 expression was stable, independent of the activation state and it correlated with suppressive function and inversely with the production of IFN-γ, IL-6, IL-21 and IL-17. In contrast, the phenotype and function of FOXP3low MBP-specific T cells were adaptive and dependent on IL-6. The higher frequency of FOXP3high MBP-specific T cells was observed when IL-6 was neutralized in the culture of PBMC with MBP. The study provides new evidence that MBP-specific T cells are susceptible to pro-inflammatory cytokine milieu and act as either pro-inflammatory or regulatory T cells.
Keywords: forkhead/winged helix transcription factor gene (FOXP3), IL-6, MS, MBP, Tregs
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating disease of the central nervous system (CNS). Although the etiology and pathogenesis of the disease remain unknown, it is generally considered an autoimmune condition in which autoreactive T cells specific for myelin antigens, such as MBP, are thought to play an important role (1–5). MBP-specific T-cell responses, in particular, to the 83–99 immunodominant epitope of MBP in the context of DRB1*1501, are identified in patients with MS (5, 6). However, MBP83–99-specific T cells are also present in healthy individuals and their frequency does not differ significantly between MS patients and healthy individuals (7, 8). Unlike their pathogenic role in experimental autoimmune encephalomyelitis (EAE), an animal model for MS, there are some preliminary indications supporting the potential role of MBP-specific T cells in the disease process of MS (1, 4). MBP-specific T cells are generally considered pro-inflammatory-autoreactive T cells predominantly producing Th1 cytokines (9), making them an attractive target for MS therapeutic interventions (10, 11). Recently, MBP-specific T cells are found to produce Th17 cytokines, such as IL-17 (12, 13). It is also recognized that MBP-specific T cells derived from MS patients are not homogenous in terms of cytokine profile, epitope specificity and the TCR V gene usage (9, 14–16).
It has been a long-standing research focus to understand how MBP-specific T cells are activated and regulated in vivo in healthy individuals as well as in MS patients (3, 17, 18). There is evidence suggesting that MBP-specific T cells could be activated through various mechanisms, such as molecular mimicry (19). Indeed, early studies indicate that one of the major phenotypic or functional discrepancies in MBP-specific T cells derived from MS and healthy individuals is the in vivo activation state (5, 20). Studies further suggest that MBP-specific T cells are kept in check by a number of in vivo regulatory mechanisms and that they undergo in vivo clonal expansion when altered regulatory mechanisms fail to control MBP-specific T cells in MS (21–24). One of the critical regulatory mechanisms is related to regulatory T cells (Tregs). Naturally occurring Tregs, as a distinctive lineage of T cells, play an important role in preventing autoimmunity and maintaining homeostasis. FOXP3 is not only a most critical marker for Tregs but also functionally required for their regulatory activity. In addition to naturally occurring Tregs that undergo differentiation in thymus, adaptive Tregs can be induced and expanded from the CD4+CD25− T-cell pool through a process of peripheral conversion to acquire the expression of FOXP3 (25, 26) and regulatory function. Such conversion of adaptive Tregs critically requires a unique cytokine milieu comprised of IL-2, transforming growth factor (TGF)-β and IFN-γ (26–30). Other cytokines, such as IL-6 and IL-1β, are found to specifically antagonize the conversion (31–33). IL-6, in particular, has been shown to regulate the IL-17 pathway through STAT3, skewing susceptible T cells toward differentiation into Th17 pro-inflammatory or pathogenic T cells but not FOXP3+ Tregs (34).
This study was prompted by our initial observation that a proportion of MBP-specific T-cell clones derived from MS patients had high FOXP3 expression with regulatory activity, a Treg phenotype unexpected from MS-derived MBP-specific T cells. A large panel of independent MBP-specific T-cell clones was subsequently included in the analysis to further characterize in detail the phenotypes and functional properties based on FOXP3 expression, inhibitory function and cytokine profile. The identified regulatory subset of MBP-specific T cells was further scrutinized to determine whether FOXP3 expression was related to a transient event due to T-cell activation or whether it was stably expressed as a sustained intrinsic property. Experiments were undertaken to further investigate the role of IL-6 in the differentiation and maintenance of the two subsets, FOXP3high regulatory and FOXP3low pro-inflammatory, of MBP-specific T cells in both established T-cell clones and PBMC derived from MS patients. The study provides new evidence indicating, for the first time, that MBP-specific T cells, traditionally regarded as pro-inflammatory-autoreactive T cells, could stably express FOXP3 and acquire regulatory function in relation to IL-6. The novel findings described here have therapeutic implications in MS and provide new insights into the understanding of the role of MBP-specific T cells in MS.
Methods
Generation of MBP83–99-specific T-cell clones from patients with MS
Relapsing–remitting MS patients with recent attacks have been selected for this study. All MS patients are HLA-DR2 positive. To generate specific T-cell lines (5, 35), PBMC were isolated from heparinized venous blood by Ficoll density gradient separation and washed three times with sterile HBSS (Invitrogen). PBMC were seeded at 200 000 cells per well in a 96-well, U-bottomed plates (Costar, Cambridge, MA, USA) in the presence of synthetic peptide of MBP83–99 (10 μg ml−1, >90% purity) in 10% FCS AMV media (Invitrogen). Seven days later, all cultures were re-stimulated with peptide in the presence of 105 irradiated (6000 rad)-autologous PBMC as a source of antigen-presenting cells (APC). rIL-2 (50 IU ml−1) was added 72 h later to supplement T-cell growth. Two weeks later, each culture was examined for specific proliferation in response to the peptide in a proliferation assay. Briefly, each well was split into four aliquots (∼104 cells per aliquot) and cultured in duplicate with 105 irradiated-autologous PBMC in the presence and the absence of the peptide in 10% RPMI 1640 media. The cultures were maintained for an additional 48 h and pulsed subsequently with [3H]thymidine ([3H]TdR; Amersham, Arlington Heights, IL, USA) at 1 μCi per well during the last 16 h of culture. Cells were then harvested using an automated cell harvester (Tomtec, Orange, CT, USA) and [3H]TdR incorporation was measured in a beta-counter. A T-cell line was considered to be specific for the 83–99 peptide when the c.p.m. was >1500 (in the presence of the peptide) and exceeded the reference c.p.m. (in the absence of the peptide) by at least 3-fold (5, 11).
To establish stable MBP83–99-reactive T-cell clones, the resulting T-cell lines were cloned by PHA (Sigma, St Louis, MO, USA) in the presence of autologous PBMC as accessory cells (11). Briefly, T cells were plated out at 0.3 cells per well under limiting dilution condition and cultured with 105 irradiated-autologous PBMC and 2 μg ml−1 of PHA. Cultures were fed with fresh medium containing 50 IU ml−1 of rIL-2 every 3–4 days. After ∼10–12 days, growth-positive wells became visible and were tested in proliferation assays for specific responses to the MBP83–99 peptide. Cells were then kept in frozen in liquid nitrogen until use in proposed experiments. Before each use, cells were thawed up and stimulated with anti-CD3 and anti-CD28 antibodies to obtain sufficient number of cells for the experiments. The protocol was approved by Institutional Review Board of Baylor College of Medicine.
Measurement of cytokines
Cell-free supernatant was collected from cultured cell clones 3 days after re-stimulation and was subjected to cytokine production measurement by ELISA. Antibody pairs and standards for IL-10, IL-6 and IFN-γ were purchased from BD Biosciences. ELISA kits for IL-17, TGF-β and IL-21 were purchased from eBioscience. Assays were performed according to manufacturer’s instructions. A standard curve was performed for each plate and used to calculate the absolute concentrations of cytokines.
Flow cytometric analysis
Cell surface markers were stained with fluorescent dye-labeled antibodies purchased from BD Bioscience and eBioscience. For FOXP3 intracellular staining, cells were fixed and permeabilized before addition of anti-FOXP3 antibody (PCH101, eBioscience). For intracellular cytokine assay, cells were treated with 50 ng ml−1 phorbol myristate acetate (Sigma) and 1 μg ml−1 ionomycin (Sigma) for 5 h at 37°C in the presence of monensin (GolgiStop, 1 μl ml−1 culture, BD Bioscience) and subsequently permeabilized before staining with fluorescent dye-labeled anti-cytokine antibodies (eBioscience). For cell cycle analysis, 1 × 106 cells were washed with cold PBS and fixed with cold ethanol for overnight at −20°C. Fixed cells were then re-suspended in 500 μl of propidium iodide (PI) staining solution (50 μg ml−1 PI, 0.1 mg ml−1 RNase A and 0.05% Triton X-100, BD Bioscience) and incubated for 40 min at 37°C. Stained cells were analyzed with a flow cytometer (FACSCalibur, BD Bioscience).
Inhibition assay
A total of 1 × 104 responder cells (CD4+CD25− T cells or FOXP3low effector T cells) were plated into 96-well plates. A total of 1 × 105 irradiated (4000 rads)-autologous PBMC depleted of CD4+ T cells (as APC) and 1 × 104 inhibitor cells (clones tested) were added into culture wells with 5 μg ml−1 anti-CD3 antibody and 1 μg ml−1 anti-CD28 antibody. Inhibitor cells were lightly irradiated (1000 rads) to prevent their own proliferation in response to anti-CD3 stimulation. Cells were cultured for 3 days at 37°C and 1 μCi of 3H-TdR per well was added for the last 7 h. Cells were harvested for measurement of incorporated c.p.m. counts by a beta-counter. Percent inhibition was calculated as % of 1-experimental c.p.m./(c.p.m. of responder only + c.p.m. of inhibitor only).
Analysis of cell division using CFSE
CD4+CD25− or CD4+CD25+ T cells were isolated from PBMC (human regulatory T cell isolation kit, Miltenyi Biotec) to determine from which pool of cells FOXP3high antigen-specific Tregs were derived. Cells (1 × 105) were pre-treated with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma) and subsequently stimulated with 10 μg ml−1 MBP83–99 peptide plus irradiated APC (1 × 105) for 7 days at 37°C in a round bottom 96-well plate. Cultured cells were then permeabilized and stained with allophycocyanin-labeled anti-FOXP3 antibody (eBioscience) followed by flow cytometric analysis of cell division (FACSAria, BD Bioscience). For CFSE-labeled suppression assay, CD4+CD25− responder T cells were labeled under the same condition and cultured with FOXP3high T cells (regulator) at different ratios in the presence of 5 μg ml−1 anti-CD3 and 1 μg ml−1 anti-CD28 antibodies.
Transfection of siRNA
Small interfering RNAs (siRNAs) to FOXP3 were purchased from Ambion. FOXP3high MBP-specific T cells (clone 2C6 and 1F5#9) were harvested and washed with serum-free medium. Cells (3 × 105) were re-suspended in 75 μl siPORT transfection buffer (Ambion), to which 1.5 μg of annealed siRNA was added. Cell suspension was then electrically pulsed (90 V, 2 mm gap) by an electroporator (BTX) and incubated for 10 min at 37°C. Transfected cells were transferred to pre-warmed fresh medium for culture at 37°C. After 48 h, MBP-specific T cells were collected for verification of attenuation of FOXP3 expression by western blot and subjected to inhibition assay as described above.
Western blot analysis
Cell pellets were directly lysed in Laemmli sample buffer (Bio-Rad) and separated by 10% SDS–PAGE. Western blot analysis was performed by initial transfer of proteins onto nitrocellulose filters using Mini Trans-Blot® (Bio-Rad) and followed by a blocking step using Tris-buffered saline with 0.1% Tween 20 plus 5% freeze-dried milk for 4 h. After washing, the filters were incubated with a monoclonal anti-human FOXP3 antibody (1:500 dilution, eBioscience) or anti-phosphorylated STAT3 antibody (1:1000 dilution, Cell signaling) overnight at 4°C. Actin was blotted using HRP-labeled goat anti-human actin antibody (1:1000 dilution, Santa Cruz Biotechnology). After washing and subsequent incubation with a goat anti-mouse antibody conjugated with HRP (Santa Cruz Biotechnology) for 1 h at room temperature, filters were developed with enhanced chemiluminescence technique (Amersham Biosciences) according to the manufacturer’s instructions. Protein bands were digitally captured and their intensities were analyzed by a densitometer (Bio-Rad).
Real-time PCR
Quantitative real-time reverse transcription–PCR was performed on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Hypoxanthine-guanine phosphoribosyltransferase was used as a reference for sample normalization. Total RNA isolated from FOXP3high and FOXP3low MBP-specific T cells was reverse-transcribed into complementary DNA (cDNA) using random hexamer. The amplification protocol used was described as follows: 1 μl of synthesized cDNA product was subsequently added into PCR reaction mix containing 25 μl of 2× SybrGreen master mix (Applied Biosystems), 23 μl of H2O, 1 μl of each 10 μM human RORc primers (forward: 5′-CGGGCCTACAATGCTGACA-3′; reverse: 5′-GCCACCGTATTTGCCTTCAA-3′). PCR reaction was programmed as 10 min at 94°C for denaturing and TaqGold polymerase activation followed by 40 thermal cycles of 20 s at 94°C, 20 s at 55°C and 40 s at 72°C. Relative quantification of gene expression was calculated using a delta CT method based on signal intensity of the PCR reactions according to the following formula: 2−ΔCT = [2−(sample Ct−normalizer Ct)] (Ct = threshold cycle of real-time PCR). All reactions were performed in triplicate and results were confirmed by at least one additional independent run.
Results
Polarized subsets of distinct phenotypes and functional properties in MBP-specific T cells derived from MS patients
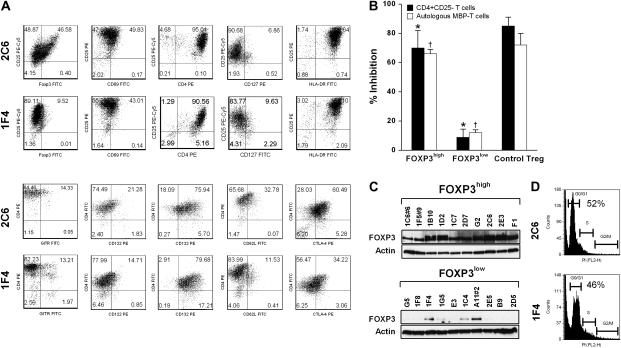
A panel of MBP-specific T-cell clones was generated from peripheral blood of seven untreated patients with relapsing–remitting MS. These T-cell clones were raised independently against the immunodominant peptide of human MBP (residues 83–99) by T-cell cloning procedure (stimulation index ≥10) (5, 11). T-cell clones were then expanded by anti-CD3 and anti-CD28 stimulations to obtain sufficient numbers to be used in the assays described here. The specificity for MBP83–99 peptide was confirmed after expansion in the proliferation assays. We first addressed whether MBP-specific T cells had differential expression levels of FOXP3 by flow cytometry and whether the expression of FOXP3 conferred inhibition on the proliferation of CD4+CD25− T cells induced by anti-CD3 antibody as well as autologous MBP-specific T effector cells in response to the MBP83–99 peptide. T-cell clones exhibiting at least 50% inhibition to the proliferation of activated T cells were considered to have regulatory function. Two polarized populations of distinct phenotypes and functions emerged. The corresponding cut-off level of FOXP3 is ∼35%. As exemplified in clone 2C6, one phenotype that represented 12 of 42 (28%) clones examined was characterized by high expression of FOXP3 with at least 46% cells found to express high level of FOXP3 (Fig. 1A) and a potent suppressive effect on the proliferation of autologous CD4+CD25− T cells and MBP-specific effector T cells (Fig. 1B). The other phenotype represented by clone 1F4 (Fig. 1A) accounted for more than two-thirds of MBP-specific T-cell clones and exhibited low expression of FOXP3 with no inhibitory activity. The differential expression of FOXP3 was confirmed by immunoblot analysis in 20 selected MBP-specific T-cell clones representing half of the T-cell panel examined (Fig. 1C). In contrast, T-cell clones of the two phenotypes had a similar expression level of other surface markers including T-cell activation-associated markers, except for CD62L and intracellular CTLA-4 (Fig. 1A). They both have high expression of HLA-DR, CD25 and CD132. CD69 expression is medium as these cells have been activated for at least 5 days prior to phenotypic analysis. In correlation with high CD25 expression, their CD127 remain low. Unlike naturally occurring Treg cells, their glucocorticoid-induced TNF receptor family-regulated gene (GITR) expression appears not so prominent, even on FOXP3high T cells. CD122 is slightly higher on FOXP3high T cells. Together with the higher levels of CD62L and CTLA-4, these markers might be related to the acquisition of regulatory function by FOXP3high T cells. A similar cell cycle profile as evidenced by PI staining (Fig. 1D), which excluded the possibility of differential FOXP3 expression due to T-cell activation state or cell cycle asynchrony. A summary of mean florescence intensity from flow cytometric analysis is presented in Table 1.
Fig. 1.
Characterization of MBP83–99-specific pro-inflammatory and Tregs. MBP83–99-specific T-cell clones were established from the peripheral blood of patients with MS. Before the experiments, cells were cultured for at least 5 days after the last stimulation with soluble anti-CD3 (2 μg ml−1) and anti-CD28 (1 μg ml−1) antibodies. (A) Two representative MBP83–99-specific T-cell clones (clone 2C6 and clone 1F4) were characterized by flow cytometric analysis for the indicated cell surface markers and FOXP3 expression. (B) Suppressive functions of FOXP3high MBP83–99-specific T-cell clones. Ten clones per group were selected from all FOXP3high and FOXP3low clones. They were analyzed for anti-proliferation properties using CD4+CD25− T cells and a selected autologous FOXP3low MBP-specific T-cell clone as responder cells. The ratio of responder to inhibitor was 1:1. Purified CD4+CD25+ natural Treg cells were used as a control. Data are presented as mean ± SD from all the clones tested. A Student's t-test was used to statistically analyze the difference between the comparable inhibition groups (asterisks indicate comparable groups in the inhibition of CD4+CD25− responder T cells; daggers indicate comparable groups in the inhibition of autologous MBP-reactive responder T cells). The P value of t-test is <0.05. The experiments for control Treg were performed independently three times. The error bar for control Treg indicates the SD of inter-experimental mean. (C) Western blot analysis of FOXP3 expression was performed for 20 selected T-cell clones representing FOXP3high and FOXP3low subsets of MBP83–99-specific T cells. (D) Cell cycle was analyzed using PI staining for clone 2C6 and clone 1F4 at the same the time point of culture as assayed in (A). The percent of cells in G0/G1 phase is indicated.
Table 1.
Phenotypes of MBP83–99-specific T cells established from MS patients
| T cells | FOXP3 | CD69 | CD25 | CD127 | HLA-DR | GITR | CD122 | CD132 | CD62L | CTLA-4 |
| 1D2 | 49.3 | 32.1 | 512.1 | 3.2 | 744.8 | 6.5 | 18.5 | 186.3 | 19.2 | 101.7 |
| 1C6#6 | 21.6 | 35.6 | 544.8 | 1.9 | 703.4 | 9.1 | 6.8 | 121.5 | 19.7 | 133.5 |
| 1F5#9 | 20.3 | 11.4 | 512.9 | 4.1 | 706.3 | 5.2 | 20.3 | 100.3 | 18.3 | 201.1 |
| 1B10 | 21.7 | 20.1 | 532.5 | 3.7 | 711.1 | 2.1 | 15.2 | 89.7 | 12.1 | 127.6 |
| 1C7 | 33.1 | 21.8 | 576.6 | 2.4 | 678.5 | 3.4 | 19.1 | 179.3 | 23.4 | 196.4 |
| 2D7 | 35.5 | 33.6 | 531.1 | 3.6 | 716.8 | 5.8 | 13.6 | 111.5 | 17.3 | 157.1 |
| G2 | 37.8 | 39.5 | 517.4 | 5.3 | 732.2 | 3.6 | 11.2 | 99.3 | 21.2 | 121.3 |
| 2C6 | 24.4 | 25.8 | 542.1 | 4.5 | 736.5 | 4.3 | 11.9 | 102.1 | 18.3 | 172.9 |
| 2E3 | 41.2 | 29.1 | 432.7 | 5.7 | 721.6 | 9.3 | 21.3 | 109.8 | 24.8 | 106.5 |
| F1 | 44.5 | 34.2 | 444.7 | 5.1 | 689.9 | 6.9 | 9.7 | 97.2 | 22.3 | 132.1 |
| Meana | 32.9 ± 10.4 | 28.3 ± 8.5 | 514.7 ± 44.3 | 4.0 ± 1.2 | 714.1 ± 20.7 | 5.6 ± 2.4 | 14.8 ± 4.9 | 120.0 ± 34.4 | 19.7 ± 3.6 | 145.2 ± 35.3 |
| G5 | 1.3 | 10.7 | 501.2 | 4.7 | 735.1 | 6.3 | 16.5 | 101.6 | 8.2 | 38.3 |
| E3 | 2.1 | 15.6 | 512.9 | 3.3 | 763.5 | 6.9 | 7.2 | 167.3 | 10.6 | 71.2 |
| 1C4 | 5.8 | 31.2 | 397.9 | 5.8 | 699.1 | 7.6 | 9.1 | 97.7 | 3.5 | 33.7 |
| A11#2 | 15.1 | 14.3 | 432.2 | 4.9 | 756.3 | 3.9 | 10.7 | 94.2 | 8.9 | 89.5 |
| 2E5 | 3.1 | 19.5 | 512.7 | 3.7 | 712.2 | 5.1 | 9.3 | 171.4 | 4.7 | 73.3 |
| B9 | 2.7 | 33.7 | 532.2 | 2.9 | 756.4 | 2.3 | 15.3 | 103.5 | 7.6 | 46.8 |
| 2D5 | 3.1 | 25.6 | 576.6 | 2.0 | 788.2 | 7.5 | 7.6 | 98.2 | 5.2 | 47.6 |
| 1F8 | 3.2 | 37.5 | 478.2 | 5.5 | 725.3 | 3.9 | 11.3 | 168.3 | 6.7 | 64.8 |
| 1F4 | 10.8 | 22.3 | 279.7 | 6.7 | 798.6 | 4.2 | 8.8 | 189.1 | 5.6 | 42.6 |
| 1G5 | 7.5 | 31.4 | 532.1 | 5.4 | 709.8 | 4.6 | 7.5 | 135.2 | 11.2 | 34.1 |
| Meanb | 5.5 ± 4.4 | 24.2 ± 9.1 | 475.6 ± 85.8 | 4.5 ± 1.5 | 744.5 ± 33.7 | 5.2 ± 1.8 | 10.3 ± 3.2 | 132.7 ± 37.8 | 7.2 ± 2.5 | 54.2 ± 19.2 |
Mean florescence intensity (MFI) was determined by FACSCalibur (BD Bioscience) and the data analysis software (FCS Express, De Novo software). Data are presented for all clones tested for phenotypes. First, all MBP83–99-specific T-cell clones were analyzed for FOXP3 expression. Subsequently, 10 clones were selected for each group representing polarized FOXP3 expression and undergone detailed phenotypic analysis.
Mean ± SD of MFI from FOXP3high group.
Mean ± SD of MFI from FOXP3low group.
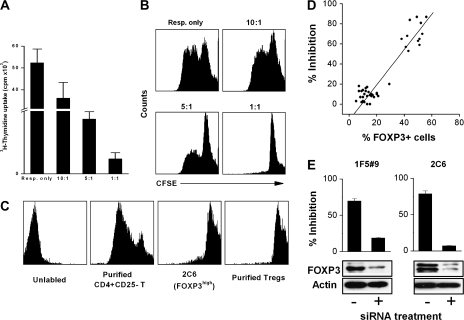
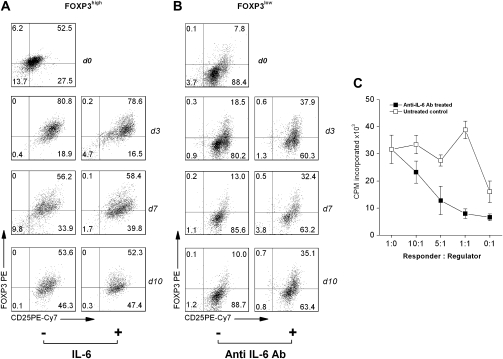
In addition, the anti-proliferation properties were confirmed by incubating FOXP3high clones with CFSE-labeled responder cells. Fig. 2(A) shows that the proliferation of responder cells in the presence of different concentrations of FOXP3high MBP83–99-specific T cells. When FOXP3high MBP83–99-specific T cells were added at 1:1 ratio, they exerted a striking inhibition against the proliferation of responder cells. In Fig. 2(B), strong CFSE signal was observed when CFSE-labeled responder cells were cultured with FOXP3high MBP83–99-specific T cells at 1:1 ratio. It indicates that there is a remarkable inhibition of the proliferation of CFSE-labeled responder cells by FOXP3high MBP83–99-specific T cells. The proliferative capability of FOXP3high MBP83–99-specific T cell itself was determined by CFSE dilution assay (Fig. 2C). As compared with purified CD4+CD25− T cells, these cells have inferior proliferative ability when they are stimulated. As shown in Fig. 2(D), there was striking phenotypic and functional polarization as well as correlation between FOXP3 expression levels and the degree of inhibition among all 42 MBP-specific T-cell clones examined. We further examined whether high expression of FOXP3 was responsible for the inhibitory activity seen in FOXP3high MBP-specific T-cell clones. To this end, a specific siRNA was transfected into FOXP3high MBP-specific T-cell clones to block the expression of FOXP3. As shown in Fig. 2(E) with representative FOXP3high MBP-specific T-cell clones (1F5#9 and 2C6), reduced FOXP3 expression resulted in significantly decreased inhibitory activity. Furthermore, our parallel experiments revealed that activation of MBP-specific T-cell clones in both subsets led to a transient increase of FOXP3 expression that returned to baseline in FOXP3low clones, whereas the heightened expression was sustained in FOXP3high MBP-specific T cells (Fig. 3).
Fig. 2.
The inhibitory function of MBP83–99-specific T cells and their correlation with FOXP3. (A) CD4+CD25− T cells (responder cell or Resp.) were stimulated with anti-CD3 and anti-CD28 antibodies. FOXP3high T cells (regulator cell, clone 2C6) were added to the culture at different responder to regulator ratio. After 72 h, cells were pulsed with [3H]TdR and harvested for c.p.m. determination. (B) In another parallel experiment, responder cells were labeled with CFSE and the proliferation was measured by CFSE signal by flow cytometry. (C) The proliferative ability of FOXP3high cells was analyzed by CFSE dilution. CFSE-labeled cells were stimulated with anti-CD3 and anti-CD28 antibodies for 72 h. CFSE dilution was determined by flow cytometry. Purified CD4+CD25− T cell and CD4+CD25+ Treg were used as controls. (D) Correlation of FOXP3 expression with suppressive function was analyzed in a total of 42 MBP83–99-specific T-cell clones. Suppressive function was assessed by the inhibition of the proliferation of autologous CD4+CD25− T cells activated by anti-CD3 and anti-CD28 antibodies. Clones with an inhibition rate >50% were considered Tregs for this study. (E) Inhibitions of clones 1F5#9 and 2C6 after the treatment with siRNA specific for FOXP3 were examined by the proliferation of activated CD4+CD25− T cells. Effectiveness of FOXP3 repression was assessed by western blot for samples transfected with negative control siRNA (Ambion) or FOXP3 siRNA.
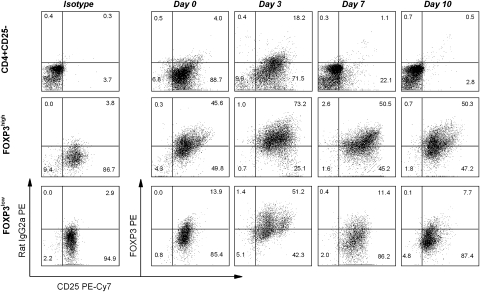
Fig. 3.
Independent relationship between FOXP3 expression and T-cell activation state in FOXP3high MBP83–99-specific T-cell clones. Clone 2C6 and clone 1F4 were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of irradiated APC. Purified CD4+CD25− T cells were used as a control. Cells were maintained by medium change and supplementation of 50 U ml−1 IL-2 every other day. Cells were collected at each time point for analysis of the expression of FOXP3 and CD25 by flow cytometry. It should be noted that cultured MBP83–99-specific T-cell clones showed sustained expression of CD25.
Polarized pro-inflammatory cytokine profile in FOXP3low MBP-specific T cells
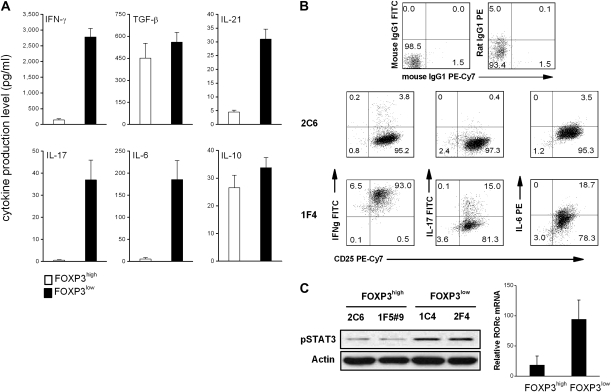
We next examined whether the two subsets of MBP-specific T cells described here could be further distinguished by their cytokine profile. As illustrated in Fig. 4(A and B), the two subsets of MBP-specific T cells exhibited a strikingly polarized cytokine profile. FOXP3low MBP-specific T cells produced large amounts of pro-inflammatory cytokines, including IFN-γ, IL-6, IL-17 and IL-21 by both ELISA and flow cytometry, whereas FOXP3high regulatory MBP-specific T cells secreted little or no such cytokines. Both subsets also produced similar levels of TGF-β and IL-10 (Fig. 4A). Furthermore, consistent with the characteristic cytokine profile were the high levels of STAT3 and RORc, a nuclear factor associated with IL-17, seen in FOXP3low MBP-specific T cells as opposed to FOXP3high T cells (Fig. 4C), providing additional intrinsic markers to FOXP3low effector cells. The results indicate that in contrast to FOXP3high Tregs, FOXP3low MBP-specific T cells derived from MS patients had an intrinsic pro-inflammatory property consistent with that of Th1 or Th17 families.
Fig. 4.
Cytokine production and level of pSTAT3 and RORc in FOXP3high and FOXP3low MBP83–99-specific T cells. (A) Culture supernatants of MBP83–99-specific T-cell clones (FOXP3high clones n = 10, FOXP3low clones n = 12) were harvested 3 days after initial antigen stimulation and measured by ELISA for the production of cytokines. (B) Clones 2C6 and 1F4 were stimulated with 50 ng ml−1 of phorbol myristate acetate and 1 μg ml−1 of calcium ionophore for 5 h in the presence of GolgiStop and subsequently harvested for intracellular cytokine analysis by flow cytometry. (C) Cells were collected from the culture of clones 2C6, 1F4, 1C4 and 1F5#9. Phosphorylated STAT3 was examined by western blot and mRNA level of RORc was measured by real-time PCR.
Original pool of FOXP3high MBP-specific Tregs
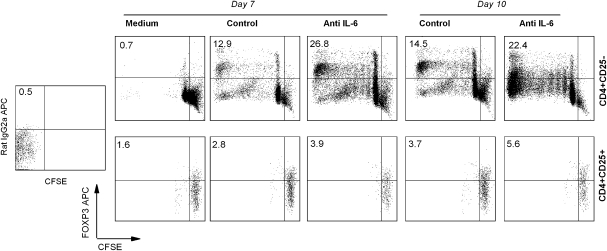
It was important to address whether FOXP3high MBP-specific T cells with regulatory property could have differentiated or expanded from the pre-existing naturally occurring Treg pool or whether they were derived from the CD4+CD25− T-cell pool. To this end, naturally occurring Treg or CD4+CD25− T cell preparations were purified from MS patients, labeled with CFSE and subjected to in vitro stimulation with MBP83–99 peptide to monitor cell proliferation and expansion. The results revealed that stimulation of CD4+CD25− T-cell preparations led to marked proliferation and expansion of FOXP3high MBP-reactive T cells, whereas purified Treg preparations did not expand under the same experimental conditions. A representative analysis is shown in Fig. 5 and a summary of the results from four MS patients is presented in Table 2. It should be noted that the expression of FOXP3 in CD4+CD25− T cells in response to MBP peptide stimulation was not all transient and a significant proportion of resulting FOXP3+ T cells maintained high level of FOXP3 expression at day 10 when transient expression of FOXP3 induced by T-cell activation receded as described in Fig. 3. In parallel experiments shown in Fig. 5, neutralization of IL-6 appeared to markedly enhance the expression of FOXP3 in CD4+CD25− T cells activated by MBP83–99 peptide, while it had no effect on purified Treg.
Fig. 5.
Expansion of antigen-induced FOXP3high Tregs from naive CD4+CD25− T-cell pool. PBMCs were obtained from MS patients that had significant response to MBP83–99 peptide stimulation as evidenced by proliferation assay. Purified CD4+CD25− T cells or CD4+CD25+ Treg cells (Miltenyi) from PBMC were pre-treated with 5 μM CFSE and cultured with 10 μg ml−1 MBP83–99 peptide plus irradiated APC in the presence of anti-IL-6 neutralizing antibody or isotype-matched control antibody. At day 7 and 10 after initial stimulation, cells were harvested and stained with anti-FOXP3 antibody. Cell division was analyzed by flow cytometry. The upper left quadrant is used to determine the frequency of FOXP3 up-regulated cells in CD4+ T cells.
Table 2.
Up-regulation of FOXP3 in CD4+ T cells stimulated with MBP83–99 peptide
| Purified T cells | Patients | After 7 days |
After 10 days |
|||
| Medium | Control | Anti-IL-6 | Control | Anti-IL-6 | ||
| CD4+CD25− | MS1 | 1.1 | 15.4 | 36.2 | 13.9 | 40.6 |
| MS2 | 0.7 | 12.9 | 26.8 | 14.5 | 22.4 | |
| MS3 | 1.0 | 17.3 | 29.2 | 21.4 | 36.1 | |
| MS4 | 0.9 | 13.1 | 30.5 | 17.2 | 33.7 | |
| CD4+CD25+ | MS1 | 1.2 | 1.6 | 2.4 | 2.1 | 3.2 |
| MS2 | 1.6 | 2.8 | 3.9 | 3.7 | 5.6 | |
| MS3 | 0.7 | 1.6 | 2.4 | 2.1 | 3.7 | |
| MS4 | 2.1 | 2.9 | 3.4 | 4.1 | 4.8 | |
CD4+CD25− and CD4+CD25+ T cell preparations were isolated from PBMCs of four MS patients. Cells were labeled with CFSE and stimulated with MBP83–99 peptide in the presence of irradiated APC. FOXP3 up-regulation and cell division were analyzed at day 7 and day 10 after stimulation. Dead cells and APC were gated out using forward scatter and side scatter in the analysis of flow cytometric data.
Role of IL-6 in the differentiation and maintenance of the two subsets of MBP-specific T cells
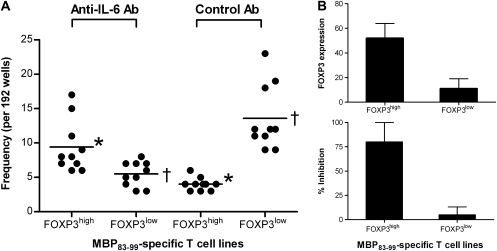
It was hypothesized that IL-6 played a critical role in driving the differentiation and maintenance of MBP-specific T cells toward the two functional subsets. To this end, we first evaluated the role of IL-6 in the expression of FOXP3 and the functional activity of the two subsets of MBP-specific T cells. As illustrated in Fig. 6(A), the addition of IL-6 at the indicated concentrations did not alter the baseline levels of FOXP3 expression or the regulatory property of FOXP3high MBP-specific T-cell clones. In contrast, neutralization of IL-6 significantly increased the expression of FOXP3 in FOXP3low T-cell clones examined (Fig. 6B). The increased FOXP3 expression appeared to sustain at day 10 and led to the acquisition of regulatory property (Fig. 6C). Given purified CD4+CD25+FOXP3+ T cells merely proliferated even in the presence of antigen stimulation, the observation suggested that the increased numbers of FOXP3+ cells in cultures might, at least in part, result from de novo induction of FOXP3 expression. The results also suggested that unlike FOXP3high MBP-specific T cells, pro-inflammatory FOXP3low T cells were dependent on IL-6. To further investigate the observed role of IL-6 in the differentiation of FOXP3high and FOXP3low MBP-specific T cells, PBMC preparations derived from 10 untreated relapsing-remitting MS patients were stimulated with MBP83–99 peptide to determine the frequency of FOXP3high and FOXP3low MBP-specific T cells, using the same protocol from which the original MBP-specific T-cell clones were generated, in the presence and absence of an IL-6-blocking antibody. The results showed that FOXP3high and FOXP3low MBP-specific T cells occurred at an average frequency of 9 ± 4 of 192 and 5 ± 2 of 192, respectively, in the presence of anti-IL-6 antibody as compared with 4 ± 1 of 192 and 14 ± 5 of 192, respectively, in the absence of the antibody (Fig. 7). When characterized for cytokine profile and regulatory functions, the resulting FOXP3high and FOXP3low T-cell isolates/lines displayed the same patterns as those of the two subsets in established MBP-specific T-cell clones (data not shown). The findings confirmed the critical role of IL-6 in influencing the expression of FOXP3 and further differentiation of FOXP3high and FOXP3low subsets of MBP-specific T cells.
Fig. 6.
Increased expression of FOXP3 in FOXP3low MBP83–99-specific T cells by IL-6 antagonism. (A and B) FOXP3high MBP83–99-specific T-cell clone 2E3 or FOXP3low MBP83–99-specific T-cell clone 1F4 (5 × 104) was cultured with anti-CD3 and anti-CD28 stimulation (5 μg ml−1 of each antibody) in the presence or absence of IL-6 (10 ng ml−1) or IL-6 neutralizing antibody (5 μg ml−1), respectively. During the culture, medium containing fresh IL-6 or anti-IL-6 antibody was supplied at the indicated concentration every other day. The resulting cells were collected at days 0, 3, 7 and 10 of culture for intracellular staining of FOXP3 by flow cytometery. (C) For inhibition assay, purified naive CD4+CD25− T cells (1 × 104, responder) were cultured with anti-IL-6 antibody-treated FOXP3low MBP83-99-specific T cells (regulator) harvested at day 3 of treatment in the presence of 1 × 105 irradiated-autologous APC for 72 h at the indicated ratios of regulator to responder. In parallel, FOXP3low MBP83–99-specific T cells cultured without anti-IL-6 antibody were harvested at the same time to serve as control regulator. The proliferation of responder cells was measured by [3H]TdR uptake and expressed as c.p.m. Results are the representative of three independent experiments.
Fig. 7.
The role of IL-6 in the generation of MBP83–99-specific T cells in MS patients. (A) PBMCs isolated from MS patients (n = 10) were stimulated with MBP83–99 peptide in the presence and absence of 5 μg ml−1 anti-IL-6 antibody in 96-well plates for 7 days. Fresh antibody was supplied every other day with medium change. Cells were then tested for their specificity to MBP83–99 peptide in a proliferation assay. MBP-specific T-cell lines were selected and assayed for the expression of FOXP3 by FACS and the inhibitory function. Frequencies of MBP-specific T-cell lines expressing high or low levels of FOXP3 were calculated as number of wells of interest per total wells tested (n = 192). The statistical difference of frequencies between cell line groups was analyzed by the Student’s t-test. Asterisks (P = 0.004) and daggers (P = 0.001) indicate the comparable cell line groups with significant differences, respectively. (B) Average levels of FOXP3 in MBP83–99-specific T-cell lines (94 for FOXP3high, 55 for FOXP3low) generated in the presence of anti-IL-6 antibody from 10 MS patients were analyzed by flow cytometry. Inhibitory functions of these resultant MBP83–99-specific T-cell lines were examined by the proliferation assay using autologous CD4+CD25− T cells as responder as described in the Methods.
Discussion
The study described here provides evidence indicating that MBP-specific T cells are not a homogenously committed pro-inflammatory T-cell population. Several investigators have described antigen-specific human Tregs in other status, especially in cancers or infections (36–39). In autoimmunity, antigen-specific Treg cells have also been observed by tetramer staining and suspected to counter autoreactive T cells (40, 41). The observation that MBP-specific T cells generated from MS patients in vitro contain a FOXP3high regulatory subset is particularly interesting. Unlike naturally occurring CD4+CD25+ Treg cells that differentiate in the thymus, these MBP-specific Tregs could emerge in accompany with inflammatory responses in the periphery. Phenotypically, these cells could share some Treg-related markers such as FOXP3, CD62L and CTLA-4, although they are not exactly the same as thymus-derived naturally occurring CD4+CD25+ Treg cells. In contrast to CD4+CD25+ Treg cells, GITR appears not prominent on these cells. They express L selectin (CD62L) indicating that they may recirculate like naive T cells from inflammatory site to other lymph organs. The expression of CTLA-4 might mediate their suppression on their target cells as do naturally occurring Treg cells. CD122 is found to be related to CD8+ Treg cells in mice (42). In humans, its counterpart is thought to be CD8+CXCR3+ T cells (43). As these MBP-specific Treg cells are CD4 phenotype, they do not seem to fit in the category of CD8+ Treg cells. It is unclear if the slightly higher expression of CD122 is characteristic of these cells. Although these Treg-related markers can be used to identify this population, however, the key phenotype and the functional activity of the two subsets are related to the expression levels of FOXP3. It has been shown that transient expression of FOXP3 can be induced during T-cell activation (44). In this study, a component of transient FOXP3 expression was also seen in both FOXP3low and FOXP3high MBP-specific T cells when activated. However, when the component of transient FOXP3 expression recedes to the resting T cell state, the expression of FOXP3 in FOXP3high MBP-specific T cells still remains stable at a high level. It is unknown why the FOXP3 expression in FOXP3high cells does not recede at ≥7 days after stimulation as seen in Fig. 3. In consideration of the biological role of FOXP3 in Treg cells, the sustained expression of FOXP3 in MBP-specific Treg cells might largely contribute to their suppressive function. FOXP3 has been found to cooperate with nuclear factor of activated T cell and nuclear factor kappa B to regulate T-cell activation (45, 46). It was reported that the transient FOXP3 induced by activation might not mediate suppressive function (44, 47). Nevertheless, it is demonstrated here that the sustained high expression of FOXP3 correlated closely with the regulatory activity in FOXP3high MBP-specific T cells as shown in Fig. 2(B). In contrast, although FOXP3low MBP-specific T cells are equally capable of responding to T-cell activation and result in FOXP3 expression, the expression is transient and returns to a low baseline level without regulatory function. In addition to FOXP3 expression and the regulatory property, the two subsets can be further differentiated by an additional set of cytokines that are closely related to their respective functions. In this regard, FOXP3low MBP-specific T cells are characteristic of pro-inflammatory T cells as they uniformly produce high levels of IFN-γ, IL-6, IL-17 and IL-21, whereas their counterparts secrete little or no such pro-inflammatory cytokines, which markedly polarize MBP-specific T cells into two subsets. The pro-inflammatory nature of FOXP3low MBP-specific T cells appears intrinsic as evidenced by elevated RORc expression and STAT3 phosphorylation in comparison with that of FOXP3high T cells. Moreover, the hypoproliferative nature of FOXP3high T cells should also be mentioned. As shown in Fig. 2(C), The FOXP3high T cells grow slowly when compared with purified CD4+CD25− T cells. While in Fig. 5, the FOXP3high cells newly propagated from CD4+CD25− T cells still possess remarkable proliferative capability. This discrepancy is due to the FOXP3high T cells used in Fig. 2(C) are in vitro cloned MBP-specific T cells, which were cultured with repetitive antigenic stimulations. Therefore, clonal exhaustion could also play a role in the hypoproliferative phenotype of FOXP3high T-cell clones.
The finding that MBP-reactive T cells could act as Tregs in a given cytokine milieu challenges the traditional view of autoreactive T cells. In addition to the known heterogeneity in TCR V gene usage and cytokine profile, MBP-reactive T cells in an autoimmune state are functionally heterogenous. The finding may also provide a possible explanation for the poor concordance between the levels of MBP-specific T cells and CNS inflammation in MS. In fact, the frequency of MBP-specific T cells, if not in vivo activated, does not seem to differ significantly between healthy individuals and MS patients (8). The finding prompts further investigation into the role of FOXP3high regulatory MBP-specific T-cell response in MBP-induced tolerance. In a recent Canadian clinical trial, intravenous administration of synthetic peptide MBP82–98 was shown to delay disease progression in some patients with progressive MS (48). The mechanism of MBP-induced tolerance is currently unknown. However, this results may provide an explanation that administration of the MBP peptide is likely to induce MBP-specific Tregs. In a recent study, neuron-mediated generation of Tregs from encephalitogenic T cells is shown to suppress EAE (49). Taken together, this raises the possibility that the fine balance of pro-inflammatory and regulatory properties of autoreactive T cells might be the key to determine whether autoreactive T cells are pathogenic. A recent study in EAE showed that a proportion of autoreactive T cells specific for proteolipid protein, another myelin autoantigen implicated in MS, is found to have a similar phenotype and regulatory function in rodents (50).
Although the FOXP3 expression sustains in FOXP3high MBP-specific T cells, the origin of these cells are still unclear. However, it seems that the differentiation and balance of the two subsets is significantly influenced by the cytokine milieu. Our study demonstrates that IL-6, in particular, plays a critical role in driving or maintaining the polarization of the two subsets. The conclusion is supported by several observations in different experimental settings with established MBP-specific T-cell clones and MS-derived PBMC preparations. First, IL-6 production correlates with the phenotype of MBP-specific T cells and is required for FOXP3low T cells but not for FOXP3high T cells. The expression of FOXP3 is stable and independent of exogenous IL-6 in FOXP3high MBP-specific T cells. One explanation is that FOXP3high MBP-specific T cells have developed into an adaptive Treg cell lineage, rendering them refractory to other exogenous modulating agents. In contrast, the FOXP3low T cell subset tends to maintain increased FOXP3 expression and regulatory function when IL-6 is absent. More importantly, when analyzed for the frequency of MBP-specific T cells in PBMC, IL-6 neutralization appears to markedly skew the differentiation of MBP-specific T cells toward the increased frequency of FOXP3high MBP-specific T cells despite only 2% (4 of 192) FOXP3high cell lines in these primary T-cell lines versus 28% (12 of 42) in the established long-term T-cell lines. Reciprocal regulation between inflammatory T cells and Tregs by IL-6 has recently been reported in mice (51). Explained in another way, while IL-2 and TGF-β induce naive T cells to become FOXP3+ regulatory cells, the combination of IL-6 and TGF-β induce pro-inflammatory IL-17-producing cells (Th17). It is unknown whether or not IL-6 lowers the activation level of FOXP3low T cells. However, one speculation is the combination of IL-2 and TGF-β enables Tregs to become resistant to IL-6 by down-regulating IL-6R. The finding described here bears important relevance to the understanding of the role of IL-6 in autoimmune processes involved in MS and its potential therapeutic implication. Other investigators described that blockade of IL-6-gp130-STAT3 pathway in CD4+ T cells could be a good target for controlling unwanted Th17-mediated immune responses including autoimmune diseases (33). Responsiveness of T cells to IL-6 determines susceptibility to EAE (52). Consistent with the observed role of IL-6 described here are previous reports indicating significant correlation between IL-6 concentration in cerebral spinal fluid and disease activity in MS (53). Furthermore, the treatment effect of IFN-β in MS is likely to involve the reduction of IL-6 (54). Further investigation is warranted to evaluate the potential role of an anti-IL-6 therapy in the treatment of MS. It is believed that MBP-specific T cells also exist in healthy individuals and they respond to MBP under the same HLA restrictions (55, 56). However, MBP-specific T cells pre-existing in healthy individuals do not cause MS. Although there are other assumptions that explain why MBP-specific T cells are not pathogenic in healthy individuals (57, 58), it is conceivable that the different composition of MBP-specific T cells might determine the virulence of MBP-specific T cells. The FOXP3high to FOXP3low ratio of MBP-specific T cells in healthy individuals might be higher than that in MS patients due to the distinctive pro-inflammatory cytokine milieu caused by MS etiologic factors. In conclusion, this study suggests that MBP-specific T cells may display either pro-inflammatory or regulatory phenotypes in the periphery under the influence of distinctive cytokine milieu. IL-6 is one of the cytokine that promotes this dichotomy.
Funding
National Institutes of Health (R01-NS48860); Richardson Foundation.
Glossary
Abbreviations
- APC
antigen-presenting cells
- cDNA
complementary DNA
- CNS
central nervous system
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- EAE
experimental autoimmune encephalomyelitis
- [3H]TdR
[3H]thymidine
- GITR
glucocorticoid-induced TNF receptor family-regulated gene
- MS
multiple sclerosis
- PI
propidium iodide
- siRNA
small interfering RNA
- TGF
transforming growth factor
- Tregs
regulatory T cells
References
- 1.Ben-Nun A, Cohen IR. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J. Immunol. 1982;129:303. [PubMed] [Google Scholar]
- 2.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 3.Zamvil S, Nelson P, Trotter J, et al. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 4.Mokhtarian F, McFarlin DE, Raine CS. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984;309:356. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J. Exp. Med. 1994;179:973. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin R, Howell MD, Jaraquemada D, et al. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J. Exp. Med. 1991;173:19. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pette M, Fujita K, Wilkinson D, et al. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc. Natl Acad. Sci. USA. 1990;87:7968. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellings N, Baree M, Verhoeven C, et al. T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J. Neurosci. Res. 2001;63:290. doi: 10.1002/1097-4547(20010201)63:3<290::AID-JNR1023>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Hermans G, Stinissen P, Hauben L, Van den Berg-Loonen E, Raus J, Zhang J. Cytokine profile of myelin basic protein-reactive T cells in multiple sclerosis and healthy individuals. Ann. Neurol. 1997;42:18. doi: 10.1002/ana.410420106. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JZ, Rivera VM, Tejada-Simon MV, et al. T cell vaccination in multiple sclerosis: results of a preliminary study. J. Neurol. 2002;249:212. doi: 10.1007/pl00007867. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 12.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008:172. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Mor F, Kantorowitz M, Cohen IR. The dominant and the cryptic T cell repertoire to myelin basic protein in the Lewis rat. J. Neurosci. Res. 1996;45:670. doi: 10.1002/(SICI)1097-4547(19960915)45:6<670::AID-JNR3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Wucherpfennig KW, Ota K, Endo N, et al. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990;248:1016. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- 16.Oksenberg JR, Panzara MA, Begovich AB, et al. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993;362:68. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- 17.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 1992;10:153. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Weiner HL, Hafler DA. Autoreactive T cells in multiple sclerosis. Int. Rev. Immunol. 1992;9:183. doi: 10.3109/08830189209061790. [DOI] [PubMed] [Google Scholar]
- 19.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann. Neurol. 2003;53:189. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 20.Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira EM, Bar-Or A, Waliszewska AI, et al. CTLA-4 dysregulation in the activation of myelin basic protein reactive T cells may distinguish patients with multiple sclerosis from healthy controls. J. Autoimmun. 2003;20:71. doi: 10.1016/s0896-8411(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 22.Kantarci OH, Hebrink DD, Achenbach SJ, et al. CTLA4 is associated with susceptibility to multiple sclerosis. J. Neuroimmunol. 2003;134:133. doi: 10.1016/s0165-5728(02)00395-8. [DOI] [PubMed] [Google Scholar]
- 23.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007;357:851. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 24.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J. Clin. Invest. 2003;112:1437. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Hong J, Sun W, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J. Clin. Invest. 2006;116:2434. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3(+)CD4(+) Treg. Eur. J. Immunol. 2008;38:912. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 29.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 2007;7:443. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 30.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan BJ, Thomas HE, Pai S, et al. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J. Immunol. 2006;176:7278. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- 32.Kryczek I, Wei S, Vatan L, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 2007;179:1423. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 33.Nishihara M, Ogura H, Ueda N, et al. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int. Immunol. 2007;19:695. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 35.Jingwu Z, Medaer R, Hashim GA, Chin Y, van den Berg-Loonen E, Raus JC. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursor frequency, fine specificity, and cytotoxicity. Ann. Neurol. 1992;32:330. doi: 10.1002/ana.410320305. [DOI] [PubMed] [Google Scholar]
- 36.Voo KS, Peng G, Guo Z, et al. Functional characterization of EBV-encoded nuclear antigen 1-specific CD4+ helper and regulatory T cells elicited by in vitro peptide stimulation. Cancer Res. 2005;65:1577. doi: 10.1158/0008-5472.CAN-04-2552. [DOI] [PubMed] [Google Scholar]
- 37.Vigouroux S, Yvon E, Biagi E, Brenner MK. Antigen-induced regulatory T cells. Blood. 2004;104:26. doi: 10.1182/blood-2004-01-0182. [DOI] [PubMed] [Google Scholar]
- 38.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji NM, Mizumachi K, Kurisaki J. Antigen-specific, CD4+CD25+ regulatory T cell clones induced in Peyer’s patches. Int. Immunol. 2003;15:525. doi: 10.1093/intimm/dxg051. [DOI] [PubMed] [Google Scholar]
- 40.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat. Rev. Immunol. 2003;3:223. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 42.Rifa'i M, Shi Z, Zhang SY, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int. Immunol. 2008;20:937. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Shi Z, Okuno Y, Isobe K. Are CD8+CD122+ cells regulatory T cells or memory T cells? Hum. Immunol. 2008;69:751. doi: 10.1016/j.humimm.2008.08.285. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 46.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl Acad. Sci. USA. 2005;102:5138. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 48.Warren KG, Catz I, Ferenczi LZ, Krantz MJ. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA Class II-defined cohort of patients with progressive multiple sclerosis: results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 2006;13:887. doi: 10.1111/j.1468-1331.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat. Med. 2006;12:518. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 50.Yu P, Gregg RK, Bell JJ, et al. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J. Immunol. 2005;174:6772. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- 51.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 52.Korn T, Mitsdoerffer M, Croxford AL, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2008;105:18460. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malmestrom C, Andersson BA, Haghighi S, Lycke J. IL-6 and CCL2 levels in CSF are associated with the clinical course of MS: implications for their possible immunopathogenic roles. J. Neuroimmunol. 2006;175:176. doi: 10.1016/j.jneuroim.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Ozenci V, Kouwenhoven M, Teleshova N, Pashenkov M, Fredrikson S, Link H. Multiple sclerosis: pro- and anti-inflammatory cytokines and metalloproteinases are affected differentially by treatment with IFN-beta. J. Neuroimmunol. 2000;108:236. doi: 10.1016/s0165-5728(00)00281-2. [DOI] [PubMed] [Google Scholar]
- 55.Pette M, Fujita K, Kitze B, et al. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- 56.Goebels N, Hofstetter H, Schmidt S, Brunner C, Wekerle H, Hohlfeld R. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain. 2000;123(Pt 3):508. doi: 10.1093/brain/123.3.508. [DOI] [PubMed] [Google Scholar]
- 57.Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J. Clin. Invest. 1998;101:725. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rohowsky-Kochan C, Molinaro D, Cook SD. Cytokine secretion profile of myelin basic protein-specific T cells in multiple sclerosis. Mult. Scler. 2000;6:69. doi: 10.1177/135245850000600203. [DOI] [PubMed] [Google Scholar]