Abstract
Using isogenic clinical bloodstream Staphylococcus aureus strains from a patient with relapsing endocarditis, we investigated transcriptional profiles of mprF and dlt genes in the context of cell surface charge and daptomycin nonsusceptibility. As in prior studies, a point mutation within mprF was observed in the daptomycin-nonsusceptible strain. However, neither the transcriptional profile of mprF, nor membrane phospholipid analyses were compatible with the anticipated mprF gain-in-function phenotype. In contrast, we demonstrated enhanced dlt expression coincident with increased positive surface charge and reduced daptomycin binding.
Keywords: Staphylococcus aureus, daptomycin, cationic antimicrobial peptide (CAP), mprF, dlt
Introduction
With the increasing use of daptomycin in clinical practice, there have been a number of recent reports of strains of Staphylococcus aureus (especially MRSA) developing daptomycin nonsusceptibility during therapy with this agent [1, 2]. Some, but not all, of these daptomycin-nonsusceptible clinical strains exhibit distinct single nucleotide polymorphisms (SNPs) within the mprF gene, in association with a “gain-in-function” phenotype [1, 3]. There are two major functions of the mprF gene product in modifying organism net surface charge: 1) lysinylate membrane phosphotidylglycerol (PG) to generate lysyl-PG (LPG); and 2) translocate this positively-charged phospholipid to the outer membrane leaflet. In addition to mprF, the dltABCD operon also contributes to the net positive surface charge by d-alanylating wall teichoic acids through distinct effector mechanisms [4]. Thus, greater net positive surface charge as mediated by mprF-and/or dltABCD mechanisms would theoretically reduce overall access of calcium-decorated daptomycin to its putative membrane target [4, 5].
In this study, we examined an isogenic pair of clinical bloodstream S. aureus strains from a patient failing daptomycin therapy in which the relapse strain exhibited daptomycin nonsusceptibility in vitro. We focused our investigations on the transcriptional profiles of mprF and dltABCD genes in the context of cell membrane surface charge and phospholipid profiles. These factors were hypothesized to impact interaction with and susceptibility to the calcium-decorated functional form of daptomycin, as well as susceptibility to innate cationic antimicrobial peptides (CAPs) involved in innate host defense [4, 6].
Methods
Strains and growth conditions
The S. aureus strains, BOY755 and BOY300, used in this study were methicillin-susceptible (MSSA) bloodstream isolates from a patient with prosthetic mitral valve endocarditis. BOY755 is the initial patient isolate, while BOY300 was subsequently isolated during daptomycin therapy and found to be nonsusceptible to daptomycin. Pulsed-field gel electrophoresis (with SmaI), agr sequencing (type II), and spa typing (type 2) confirmed that these two isolates are clonal (data not shown). Their MICs to daptomycin, as determined by standard micro-E-test, were 0.5 and 2 µg/ml for strains BOY755 and BOY300, respectively, using stationary phase cells. The MICs of vancomycin were 1 µg/ml for both strains (the patient was not treated with vancomycin due to allergies to vancomycin and penicillin, although nafcillin therapy was eventually utilized following desensitization). The population analyses were performed with both daptomycin and vancomycin by standard protocols. Both strains exhibited equivalent growth curve kinetics and ultimate CFU yields over a 24 hr period (data not shown)
S. aureus strains were grown in either tryptic soy broth (TSB; Difco Laboratories, Detroit, MI) or Mueller-Hinton broth (MH; Difco Laboratories, Detroit, MI). To determine the impact of growth phase–dependent dlt expression on daptomycin MICs, the above micro E-test assay was employed. For stationary growth phase cells, overnight cultures were utilized. For exponential growth phase cells, overnight cultures of S. aureus were diluted to an OD600 = 0.1 in fresh media and incubated for 3 hours at 37°C.
All daptomycin (Cubist Pharmaceuticals, Lexington, MA) assays were done in the presence of 50 µg/ml calcium as recommended by the manufacturer. Human neutrophil peptide 1 (hNP-1) from human PMNs was purchased from Peptide International (Louisville, KY). The predominant CAP in mammalian platelets, tPMP-1, was prepared from rabbit blood as previously described [7]. RP-1 (a synthetic congener modeled after the microbicidal domains of PMPs) was prepared and bioassayed as detailed before [8, 9]. Synthetic RP-1 has a mechanism of action that recapitulates that of tPMP-1 and was substituted for tPMP-1 in assays requiring relatively large amount of peptide (e.g., surface binding assays).
Transcription analyses
Total RNA was isolated from the cell pellets by using the RNeasy kit (Qiagen, Valencia, CA) and the FASTPREP FP120 instrument (BIO 101, Vista, CA), according to the manufacturer’s recommended protocols.
Reverse transcriptase (RT)-PCR was performed as described previously [10]. The dlt cDNA products were detected using a primer pair dlt-F (5’-ATATGATTGTTGGGAT GATTGGTGCCA-3’) and dlt-R (5’-ACATATGGTCCAACTGAAGCTACG-3’). The mprF cDNA products were detected using a primer pair mprF-F (5’-GTAGTAATCACA TTGTATCGGGAGT-3’) and mprF-R (5’-GATGCATCGAAAACATGGAATAC-3’).
CAP susceptibility testing
Previous studies have shown cross resistance among daptomycin-nonsusceptible strains with both hNP-1 and tPMP-1 [1]. In vitro bactericidal assays were carried out with tPMP-1 and hNP-1 as described previously using a two-hour microdilution method [1, 6].
CAP and daptomycin binding assays
To determine peptide binding to S. aureus cells, RP-1 or daptomycin (40 µg/ml and 6 µg/ml, respectively) was added to 108 CFUs of each strain. Supernatants were then analyzed for residual unbound peptide by a radial diffusion assay and standard curve techniques as described previously [1, 7], and the amount of bound peptide calculated. For the daptomycin assay, the within-day and between-day variations were 25 % and 10 %, respectively. For the RP-1 binding assay, both the within- and between-day variations were < 5%.
Net cell surface charge
The binding of fluorescein isothiocyanate (FITC)-labeled cationic poly-L-lysine (PLL) to the staphylococcal surface was used to quantify relative surface charge using a flow cytometric assay as detailed previously [1, 11]. A total of 10,000 events were counted and four separate experiments were analysed using a FACSCalibur® system (Becton-Dickinson Labware, San Jose, CA).
Membrane phospholipid content and asymmetry
Membrane phospholipids (PLs) were extracted from S. aureus cell pellets by standard methods [11]. The three major PLs, phosphatidylglycerol (PG), lysyl-PG (LPG), and cardiolipin (CL), were separated by two-dimensional thin-layer chromatography (TLC), removed from the plates and then quantified spectrophotometrically by a previously described chemical assay [1, 11].
Statistics
Data were analyzed by the Kruskal-Wallis ANOVA, with a P value of <0.05 considered significant.
Results
Population analyses
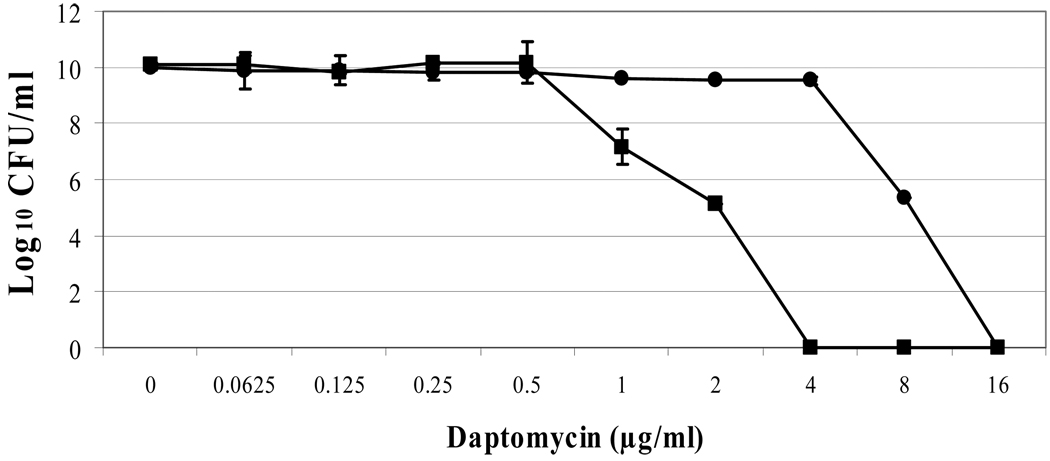
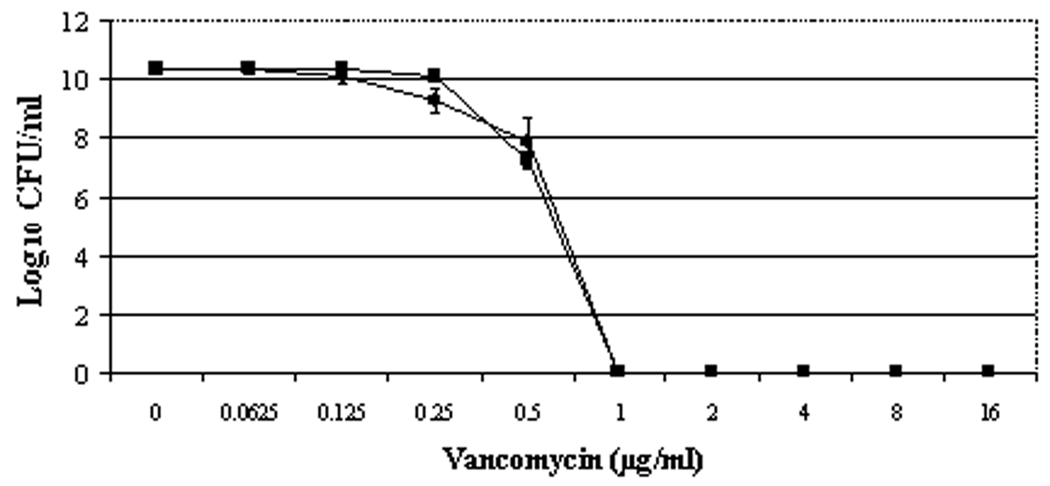
For daptomycin, the BOY300 population curve was shifted substantially to the right, with heterogenous subpopulations surviving exposures of between 0.5 and 16 µg/ml daptomycin (figure 1A). The area under the curve (AUC) values for daptomycin population analyses were ~ four-fold greater in the nonsusceptible vs. susceptible strains (89.45 ± 1.04 for BOY755 and 20.67 ± 1.52 for BOY300, respectively). Vancomycin population analyses of strains BOY755 and BOY300 were virtually identical (AUCs: 6.53 ± 0.12 vs. 6.75 ± 0.34), revealing heterogenous subpopulations surviving exposures of between 0.25 and 1 µg/ml vancomycin (figure 1B).
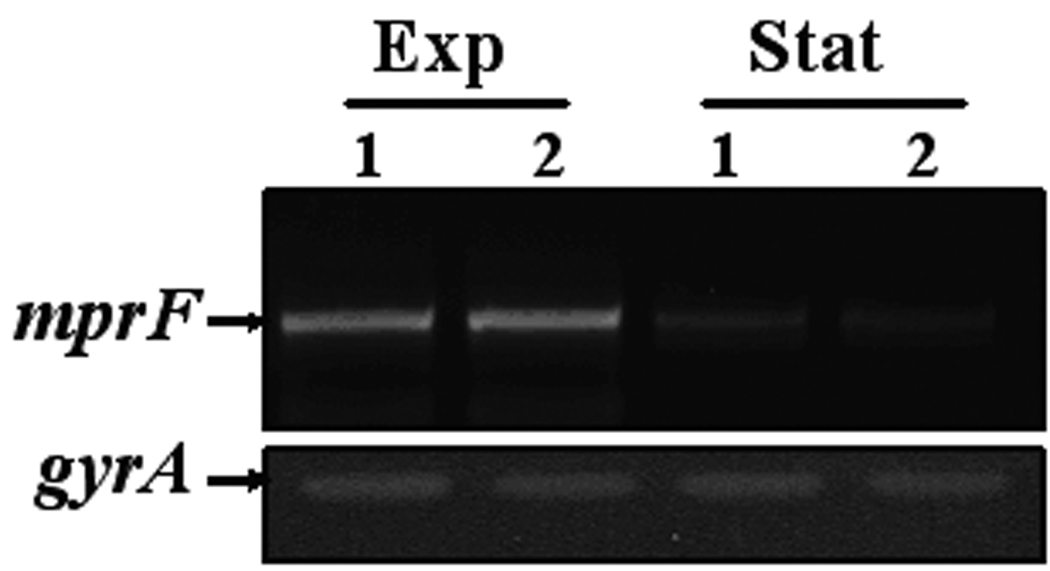
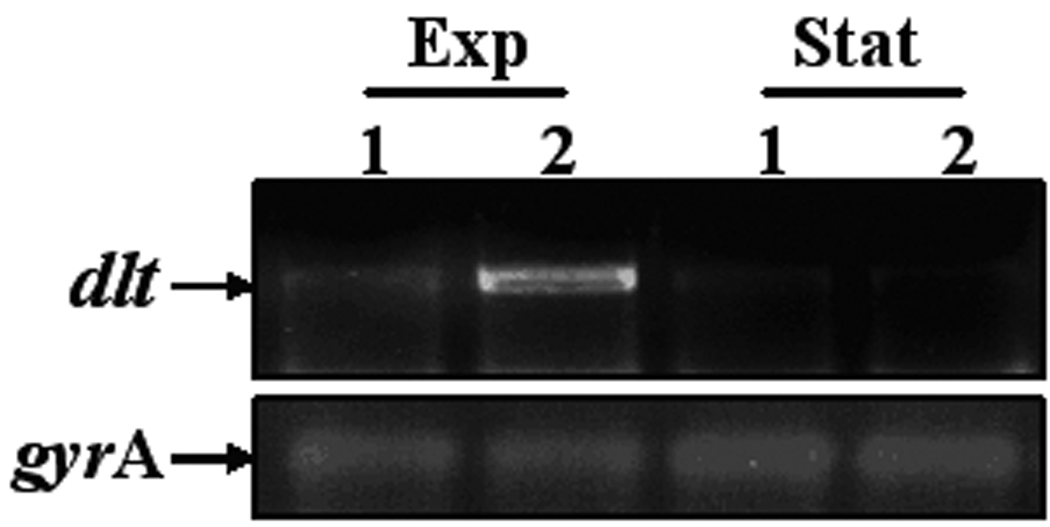
Figure 1.
Population analyses of study strains to vancomycin (A) or daptomycin (B); expression of mprF (C) and dlt operon (D). Population analyses data represent the means (±SD) for at least two separate assays. Total cellular RNA samples were isolated from exponential- and stationary-phase cultures of BOY755 (lane 1) and BOY300 (lane 2) and were subjected to RT-PCR to detect expression of mprF, dltA, and gyrA.
CAP cross-resistance in the daptomycin-nonsusceptible strain BOY300
In agreement with our previous findings [1], the parental strain, BOY755, was highly susceptible to both tPMP-1 and hNP-1, while the BOY300 strain was ~2-fold and >40-fold more resistant to tPMP-1 and hNP-1, respectively (table 1).
Table 1.
Phospholipid profiles, peptide binding, and in vitro susceptibilities of the strain set.
| Strain | % Survival (mean ± SD) after 2h exposure to** |
Concentration of drug bound (µg/ml[mean ± SD])** |
% of total phospholipid content (mean ± SD)** |
|||||
|---|---|---|---|---|---|---|---|---|
| 2 µg/ml tPMP-1 |
20 µg/ml hNP-1 |
RP-1 (40 µg) |
Daptomycin (6 µg) |
Inner LPG |
Outer LPG |
PG | CL | |
| BOY755 (parental) | 28.5 ± 12 | 1 ± 1 | 3.2 ± 0.8 | 2.32 ± 0.62 | 11.68 ± 5 | 14.7 ± 6 | 64.7 ± 9 | 7.1 ± 3 |
| BOY300 (DAP nonsusceptible) | 47.5 ± 14.8* | 46 ± 3.3* | 0.64 ± 0.32* | 0.9 ± 0.34* | 16.22 ± 6 | 16.1 ± 7 | 60.1 ± 7 | 9.4 ± 7 |
P < 0.05 versus the parental strain.
Experiments were repeated a minimum of four times.
Cell surface charge and binding to APs
The binding of positively-charged fluorescein-PLL to the parental strain BOY755 was significantly greater than that of the daptomycin-nonsusceptible strain BOY300 (668 ± 79 versus 506 ± 29 fluorescence units; P < 0.05). This finding suggests increased repulsion of daptomycin, putatively due to increased net surface positive charge in BOY 300. Paralleling the PLL binding data, binding of both RP-1 (the synthetic surrogate for tPMP-1) and daptomycin was reduced in the daptomycin-nonsusceptible strain, BOY300 (P < 0.05; table 1).
Membrane phospholipid (PL) content and asymmetry
The proportions of the negatively-charged species, PG and CL, were very similar between the two strains (table 1). There was only a moderate increase in the total amounts of the positively charged PL, lysyl-PG, in the nonsusceptible strain (~ 26 versus 32%), although this difference was not significant. Of note, the inner-to-outer membrane leaflet LPG ratio (as a measure of mprF translocase activity) was ~1:1 for both strains.
Genetic factors involved in the regulation of net surface charge in S. aureus
A single point mutation (C-to-A) was observed at position 884 in the mprF gene in the daptomycin-nonsusceptible BOY300 strain. This mutation yields a serine to leucine (S295L) amino acid substitution at position 295 (data not shown). In contrast, sequencing analyses of mprF and dlt promoter regions (537-bp and 721-bp regions upstream of the ATG start codon, respectively) revealed no differences between the two strains (data not shown). Sequencing studies were kindly performed at the City of Hope Medical Center (Duarte, CA)
As shown in figure 1C, RT-PCR analysis revealed that mprF is transcribed maximally and to an equivalent extent during early-exponential growth in both strains. In contrast to mprF expression, transcription of the dltABCD operon was notably enhanced in the daptomycin-nonsusceptible strain as compared with the parental strain during exponential growth (figure 1D).
Correlation of daptomycin MICs with growth phase-dependent dlt expression
For the daptomycin-nonsuceptible strain, BOY 300, the MICs at both exponential and stationary growth phases were 2 µg/ml as compared to 0.25 and 0.5 µg/ml, respectively, for the daptomycin-susceptible parental strain, BOY 755. This increased MIC profile in strain BOY 300 paralleled its enhanced expression profile of dlt, but not mprF.
Discussion
The current study focused on phenotypic and genotypic analyses of a daptomycin-susceptible and daptomycin-nonsusceptible strain pair from the bloodstream of a patient with endocarditis who failed daptomycin treatment. Of note, the current isogenic strain pair was isolated from a different hospital in a distinct geographic area than a similar previously reported strain pair [1;3]. As opposed to our prior published study, the current patient received no vancomycin therapy before daptomycin was utilized. Moreover, the genotypic profiles of the current strain pair were also distinct from the previously reported strain set in terms of PFGE pattern and spa typing. Despite these distinct genotypic profiles, the daptomycin nonsusceptible strain (BOY300) demonstrated increased positive surface charge, reduced daptomycin and CAP binding, and cross-resistance to both platelet (tPMP-1) and neutrophil (hNP-1) CAPs, paralleling our prior report [1]. This finding led us to postulate that as before [3], the current daptomycin-nonsusceptible strain would demonstrate point mutations in mprF, enhanced expression of this gene, as well as evidence of an mprF gain-in-function phenotype utilizing membrane PL profiling as the readout.
Interestingly, in the current study, a specific SNP within the mprF ORF was identified in the daptomycin-nonsusceptible strain that resulted in an identical amino acid substitution (S295L) in the putative mprF translocase domain as was described in our previously reported daptomycin-nonsusceptible S. aureus strain series [1;3]. However, despite this similarity, the current daptomycin-nonsusceptible strain (BOY300) did not exhibit an mprF gain-in-function phenotype. Thus, this latter strain exhibited only a modest increase in total LPG production (table 1), and similar inner- versus outer membrane LPG distribution ratios, relative to its daptomycin-susceptible parental strain. Furthermore, unlike the previously characterized daptomycin-nonsusceptible S. aureus strains [3, 12], BOY300 failed to show increased mprF transcription as compared to its susceptible counterpart strain BOY755. Based on these data, it is suggested that the mere presence of a point mutation within the putative translocase domain of mprF is not sufficient to yield a gain-in-function phenotype in LPG translocation. This concept would suggest the requirement for the coincident enhanced expression of the mutated form of mprF to provide a substantive contribution to the daptomycin nonsusceptible phenotype.
As increased net positive surface charge and, thus, reduced CAP and daptomycin binding in the daptomycin-nonsusceptible strain (BOY300) could not be explained by total LPG production or translocation differences, expression of the dltABCD operon was also determined. Interestingly, RT-PCR analysis revealed increased dltABCD expression in the daptomycin-nonsusceptible BOY300 as compared to BOY755 during exponential growth. This correlated with increases in daptomycin MICs in strain BOY300 at both exponential and stationary phases of growth as compared to the parental strain. These findings strongly support the concept that the enhanced surface positive charge in BOY300 likely results from dltABCD-mediated increases in alanylation of wall teichoic acids, with resultant charge-dependent repulsion of daptomycin. Thus, the current data are the first to our knowledge to document an mprF-independent, dltABCD-dependent pathway for daptomycin nonsusceptiblity in clinical isolates. Furthermore, analyses of the dlt promoter region showed no difference in sequence between the BOY755 and BOY300 strains (data not shown), indicating that increased expression of the dlt operon in BOY300 likely results from the influence of regulatory factor(s) other than a cis acting element.
Current studies are in-progress to further examine the detailed genetic regulatory pathway(s) that control mprF and dltABCD expression in the context of daptomycin nonsusceptibility. Of interest to the current findings, it has been recently shown that in certain strains of S. aureus, the aps (graRS) genes appear to positively regulate mprF and dltABCD expression in response to specific CAPs [13].
Acknowledgments
Financial support: National Institute of Health to A.S.B. (AI-39108) and M.R.Y (AI-39001), and an American Heart Association grant to Y.Q.X. (0465142Y). GS has received research grant support from Cubist and Pfizer Pharmaceuticals, consulting fees from Cubist, Ortho-McNeail, and Pfizer Pharmaceuticals and speaking fees from Cubist, Pfizer, and Wyeth Pharmaceuticals.
Footnotes
Potential conflicts of interest: none reported
References
- 1.Jones T, Yeaman MR, Sakoulas G, et al. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 2008;52:269–278. doi: 10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skiest DJ. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 2006;44:655–656. doi: 10.1128/JCM.44.2.655-656.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SJ, Xiong YQ, Dunman PM, et al. Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2636–2637. doi: 10.1128/AAC.01415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidenmaier C, Peschel A, Kempf VAJ, Lucindo N, Yeaman MR, Bayer AS. DltABCD-and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 2005;73:8033–8038. doi: 10.1128/IAI.73.12.8033-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman JA, Perlmutter NG, Shapiro HM. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003;47:2538–2544. doi: 10.1128/AAC.47.8.2538-2544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong YQ, Mukhopadhyay K, Yeaman MR, Adler-Moore J, Bayer AS. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005;49:3114–3121. doi: 10.1128/AAC.49.8.3114-3121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeaman MR, Bayer AS, Koo S-P, Foss W, Sullam PM. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Invest. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong YQ, Bayer AS, Elazegui L, Yeaman MR. A synthetic congener modeled on a microbicidal domain of thrombin-induced platelet microbicidal protein-1 recapitulates staphylocidal mechanisms of the native molecule. Antimicrob. Agents Chemother. 2006;50:3786–3792. doi: 10.1128/AAC.00038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeaman MR, Gank KD, Bayer AS, Brass EP. Synthetic peptides that exert antimicrobial activities in whole blood and blood-derived matrices. Antimicrob. Agents Chemother. 2002;46:3883–3891. doi: 10.1128/AAC.46.12.3883-3891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S-J, Rice KC, Brown RJ, et al. A LysR-type regulator, CidR, is required for induction of the Staphylococcus aureus cidABC operon. J. Bacteriol. 2005;187:5893–5900. doi: 10.1128/JB.187.17.5893-5900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhopadhyay K, Whitmire W, Xiong YQ, et al. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbicidal protein-1 (tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology. 2007;153:1187–1197. doi: 10.1099/mic.0.2006/003111-0. [DOI] [PubMed] [Google Scholar]
- 12.Mishra NN, Yang SJ, Sawa A, et al. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus (MRSA) Antimicrob Agents Chemother. 2009;53:2312–2318. doi: 10.1128/AAC.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]