Abstract
Objective
Elevated concentrations of adiponectin are associated with a favorable metabolic profile but also with adverse cardiovascular outcomes. This apparent discrepancy has raised questions about whether adiponectin is associated with an increased or decreased risk of coronary heart disease (CHD). We sought to determine whether higher adiponectin levels are associated with exercise-induced ischemia in patients with stable CHD.
Methods and results
We measured total serum adiponectin concentrations and evaluated exercise-induced ischemia by stress echocardiography in a cross-sectional study of 899 outpatients with documented stable CHD. Of these, 217 (24%) had inducible ischemia. Although adiponectin levels correlated negatively with diabetes prevalence, body mass index, serum insulin, fasting glucose, low-density lipoprotein cholesterol, and triglycerides and positively with high-density lipoprotein cholesterol (all P< 0.005), elevated adiponectin concentrations were also associated with a greater risk of inducible ischemia. Each standard deviation (0.08 μg/mL) increase in log adiponectin was associated with a 35% greater odds of inducible ischemia (unadjusted odds ratio 1.35; 95% confidence interval 1.15–1.57; P=0.0002). Although attenuated, this association remained present after multivariable adjustment for traditional cardiovascular risk factors and other measures of cardiac function (adjusted odds ratio 1.21; 95% confidence interval 1.02–1.43; P=0.03).
Conclusions
Elevated concentrations of adiponectin are independently associated with inducible ischemia in patients with stable CHD. These findings raise the possibility that the presence of chronic inducible ischemia may alter the cardio-protective effects afforded by adiponectin secretion in the healthy population.
Keywords: Adiponectin, Coronary heart disease (CHD), Ischemia, Adipokine, Reverse epidemiology
1. Introduction
Adipose tissue produces and secretes several signaling molecules that are important for glucose and lipid metabolism, inflammation, and other physiological functions [1]. Adiponectin, one such hormone that is secreted abundantly and exclusively by adipocytes, has generated particular interest as a potential biomarker in patients with coronary heart disease (CHD) [2]. Adiponectin has been shown to exert anti-inflammatory [3], anti-atherogenic [3–5], and insulin-sensitizing [4] effects by modulating endothelial adhesion molecules and suppressing the vascular inflammatory response [3,6]. Administered adiponectin improves insulin sensitivity and decreases atherosclerosis in mouse models [4]. Furthermore, adiponectin is involved in post-ischemic myocardial remodeling and protects against ischemia/reperfusion injuries in mice via inflammatory, oxidative, and metabolic pathways to inhibit apoptosis and reduce infarct size [7,8]. Thus, on the basis of these in vitro models and animal studies, adiponectin appears to be cardio-protective against CHD and ischemic injuries.
Clinical studies on the relationship between adiponectin and CHD in humans have produced inconsistent results. Although many studies have supported the notion that adiponectin may be protective against both incident and prevalent CHD [9–11], a sizable number of studies have failed to demonstrate an independent association [12,13], and indeed several large prospective studies have found that elevated adiponectin levels predict a paradoxically increased risk of CHD events and mortality [14–16]. The authors of one such study suggested that elevated adiponectin may represent an enhanced secretory response of adipose tissue to the metabolic environment present in the early development of macrovascular disease [14]. To further evaluate the association of adiponectin with metabolic risk factors and cardiovascular function, we measured serum adiponectin and performed stress echocardiography in a cross-sectional study of 899 outpatients with stable CHD.
2. Methods
2.1. Study participants
The Heart and Soul study is an ongoing prospective cohort study designed to investigate the effects of psychosocial factors on health outcomes of patients with stable CHD. Methods have been previously described [17,18]. Patients were eligible if they had a documented history of (1) myocardial infarction, (2) coronary revascularization, (3) angiographic evidence of ≥50% stenosis in one or more coronary vessels, or (4) exercise-induced ischemia on treadmill electrocardiogram (ECG) or nuclear perfusion imaging. Patients were excluded if they were unable to walk one block, had an acute coronary syndrome within the previous six months, or were likely to move out of the area within three years. Institutional Review Boards at each site approved this study protocol. All participants provided written informed consent.
Between September 2000 and December 2002, a total of 1024 participants were recruited from 12 outpatient clinics in the San Francisco Bay Area, including 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with a diagnosis of coronary disease that was documented by their physician, based on a positive angiogram or treadmill test in over 98% of the cases. All participants completed a full-day study that included a comprehensive medical history and physical examination, health status questionnaires, and an exercise treadmill test with baseline and stress echocardiograms. 12-h fasting serum samples were obtained in the morning prior to the stress test and frozen at −70 °C. We excluded 39 subjects for whom frozen serum was not available and an additional 86 subjects who could not complete the stress test, resulting in a final sample size of 899 participants for this analysis.
2.2. Serum adiponectin
The primary predictor variable was serum adiponectin level as determined by immunoassay of thawed fasting serum samples (Linco, Millipore, St. Charles, MO). Each sample was assayed in duplicate, and adiponectin level was calculated as the average of the two measurements. The lowest detectable measurement for adiponectin was 145.4 pg/mL. The inter-assay coefficient of variation for this multiplexed immunoassay was 14.2–21.8%, and the intra-assay coefficient of variation was 1.4–7.9%. No significant antibody cross-reactivity was observed within the panel. The laboratory technicians who performed the assays were blinded to the patient characteristics and echocardiographic results.
2.3. Stress echocardiography
The outcome variable was exercise-induced cardiac ischemia as measured by stress echocardiography. After the blood draw, participants underwent a symptom-limited, graded exercise treadmill test based on a standard Bruce protocol with continuous 12-lead ECG monitoring until the patient experienced dyspnea, symptom-limited fatigue, chest discomfort, or ECG changes suggestive of ischemia [19]. Exercise capacity was calculated as the total number of metabolic equivalents (METs) achieved at peak exercise.
Complete resting two-dimensional echocardiograms with all standard views and subcostal imaging of the inferior vena cava were performed immediately before and after the exercise using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, CA) with a 3.5-MHz transducer and Doppler ultrasound examination. Prior to exercise, standard two-dimensional parasternal short-axis and apical two- and four-chamber views were obtained during held inspiration and were used to calculate the left ventricular ejection fraction. At peak exercise, precordial long- and short-axis and apical two- and four-chamber views were obtained to assess for wall motion abnormalities.
Based on prior work, we defined exercise-induced ischemia as the presence of one or more new wall motion abnormality at peak exercise that was not present at rest [17,20,21]. A single experienced cardiologist (NBS), who was blinded to the results of the adiponectin assays and clinical histories, interpreted all echocardiograms. In 20 randomly selected echocardiograms that were presented to the reviewer in blinded fashion (the reviewer did not know they were repeats of prior studies), the reviewer’s assessment of inducible ischemia was 85% reproducible. This assessment of inducible ischemia has been associated with other cardiac biomarkers [17] and validated as a predictor of cardiovascular events [22].
2.4. Other patient characteristics
Demographic characteristics, medical history, alcohol consumption, and smoking status were assessed by patient self-report questionnaires. We measured weight and height and calculated the body mass index (BMI) (kg/m2). Participants were asked to bring their medication bottles to the study appointment, and research personnel recorded all current medications. Medications were categorized using Epocrates Rx (San Mateo, CA). We considered participants users of β-blockers, statins, diuretics, and angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers if they reported taking these medications daily. We considered participants users of aspirin if they reported taking it weekly or more. High-density lipoprotein (HDL) cholesterol, triglycerides, glucose, glycosylated hemoglobin, insulin, and C-reactive protein were determined from 12-h fasting serum samples. We calculated low-density lipoprotein (LDL) cholesterol using the Friedewald equation: total cholesterol − HDL cholesterol − (triglycerides/5). Insulin levels were measured with a Linco Multiplex immunoassay (Millipore, St. Charles, MO), and C-reactive protein was measured using the Beckman Extended Range high-sensitivity assay (Beckman Coulter Inc, Fullerton, CA). Creatinine clearance was calculated from 24-h urine collections.
2.5. Statistical analysis
Because normal ranges for serum adiponectin level have not yet been established, we divided participants into quartiles on the basis of their plasma adiponectin level. Baseline participant characteristics across quartiles were compared using analysis of variance (ANOVA) for continuous variables and χ2 test for dichotomous variables.
We used logistic regression analysis to evaluate the association between adiponectin and inducible ischemia. We estimated the odds of ischemia associated with each standard deviation (SD) increase in log adiponectin (entered as a continuous variable), with adiponectin log-transformed to normalize the skewed distribution. We also evaluated the risk of ischemia across quartiles of adiponectin. Comparing participants in the bottom versus top quartiles of serum adiponectin, we had >80% power (2-tailed α = 0.05) to detect a 12% difference (20% versus 32%) in the proportion of participants who had exercise-induced ischemia.
To evaluate the independent association of adiponectin with inducible ischemia, we sequentially adjusted for demographic characteristics, comorbidities, medication use, metabolic variables, and cardiac function. At each step, any variables that were independently associated with ischemia (at P< 0.1) from the previous step were entered as covariates. All variables that were independently associated with ischemia (at P<0.1) were retained in the final logistic regression model. We report odds ratios (ORs) with 95% confidence intervals (CIs) for all analyses. To explore potential modifying effects of known clinical prognostic factors, we tested for statistical interactions between adiponectin and these clinical risk factors. Analyses were performed using Statistical Analysis Software (version 9; SAS Institute Inc, Cary, NC).
3. Results
Our 899 study participants had a median adiponectin level of 20.7 μg/mL (interquartile range, 12.2–35.5 μg/ml). Compared with those in the lowest quartile, participants in the highest quartile of adiponectin were older, less likely to be male, and more likely to be white. They were less likely to have a history of diabetes and to be taking β-blockers and aspirin. Participants with higher concentrations of adiponectin had lower BMI, fasting insulin, fasting glucose, LDL cholesterol, triglycerides, and creatinine clearance, and higher levels of HDL cholesterol. Despite this favorable metabolic risk profile, participants in the highest quartile had lower left ventricular ejection fraction and lower exercise capacity (Table 1). Notably, adiponectin levels were not correlated with a history of myocardial infarction or stroke.
Table 1.
Characteristics of 899 participants by quartile of adiponectin.
| Quartile of adiponectin (μg/mL) |
P value | ||||
|---|---|---|---|---|---|
| I, <12.2 (N =225) | II, 12.2–20.7 (N=225) | III, 20.7–35.5 (N =225) | IV, >35.5 (N=224) | ||
| Demographic characteristics | |||||
| Age (yr) | 64 ± 10 | 65 ± 10 | 67 ± 11 | 70 ± 11 | <0.0001 |
| Male sex | 204 (91) | 190 (84) | 174 (77) | 176 (79) | 0.0005 |
| White race | 114 (51) | 126 (56) | 148 (66) | 161 (72) | <0.0001 |
| Regular alcohol use | 54 (24) | 70 (31) | 67 (30) | 75 (34) | 0.14 |
| Current smoking | 46 (20) | 50 (22) | 44 (20) | 38 (17) | 0.54 |
| Medical history | |||||
| Hypertension | 166 (74) | 149 (67) | 158 (71) | 153 (68) | 0.37 |
| Myocardial infarction | 128 (57) | 119 (54) | 112 (50) | 118 (53) | 0.47 |
| Congestive heart failure | 32 (14) | 43 (19) | 36 (16) | 39 (18) | 0.53 |
| Stroke | 25 (11) | 28 (13) | 32 (14) | 32 (14) | 0.71 |
| Diabetes mellitus | 76 (34) | 66 (29) | 42 (19) | 43 (19) | 0.0001 |
| Revascularization | 138 (61) | 129 (58) | 123 (55) | 147 (66) | 0.09 |
| Current medication use | |||||
| β-Blocker | 156 (69) | 124 (55) | 134 (60) | 103 (46) | <0.0001 |
| Statin | 163 (72) | 144 (64) | 144 (64) | 137 (61) | 0.07 |
| Diuretic | 57 (25) | 56 (25) | 72 (32) | 73 (33) | 0.12 |
| ACE inhibitor or ARB | 120 (53) | 111 (49) | 113 (50) | 120 (54) | 0.74 |
| Aspirin | 193 (86) | 172 (76) | 178 (79) | 163 (73) | 0.007 |
| Metabolic variables | |||||
| Body mass index (kg/m2) | 29 ± 5 | 29 ± 5 | 28 ± 5 | 27 ± 5 | <0.0001 |
| Serum insulin (pg/mL) | 463 ± 521 | 395 ± 676 | 343 ± 288 | 287 ± 253 | 0.0007 |
| Fasting glucose (mg/dL) | 128 ± 40 | 122 ± 47 | 115 ± 32 | 115 ± 49 | 0.003 |
| Glycosylated hemoglobin (%) | 6.1 ± 1.1 | 6.0 ± 1.3 | 5.8 ± 1.0 | 5.9 ± 1.2 | 0.07 |
| LDL cholesterol (mg/dL) | 100 ± 30 | 108 ± 36 | 106 ± 36 | 99 ± 30 | 0.004 |
| HDL cholesterol (mg/dL) | 39 ± 10 | 43 ± 11 | 46 ± 12 | 54 ± 17 | <0.0001 |
| Triglycerides (mmol/L) | 180 ± 181 | 153 ± 124 | 125 ± 100 | 104 ± 78 | <0.0001 |
| Creatinine clearance (mL/min) | 89 ± 29 | 89 ± 27 | 80 ± 26 | 71 ± 26 | <0.0001 |
| Cardiac function | |||||
| LV ejection fraction (%) | 62 ± 9 | 63 ± 8 | 62 ± 9 | 61 ± 10 | 0.05 |
| Exercise capacity (METs) | 7 ± 3 | 8 ± 3 | 7 ± 3 | 7 ± 3 | 0.05 |
| C-reactive protein (mg/dL) | 4.4 ± 6.4 | 3.8 ± 5.6 | 4.4 ± 6.4 | 5.0 ± 12.4 | 0.48 |
Values are expressed as N (%) or mean ± SD.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LDL, low-density lipoprotein; HDL, high-density lipoprotein; LV, left ventricular; METs, metabolic equivalent tasks.
Of the 899 patients, 217 (24%) had inducible ischemia. Each SD (0.08 μg/ml) increase in log adiponectin was associated with a 35% increased odds of inducible ischemia prior to multivariate adjustment (unadjusted OR, 1.35; 95% CI, 1.15–1.57; P = 0.0002). Although attenuated, this association remained present after multivariable adjustment for demographic variables, comorbid illnesses, medication use, metabolic factors, and cardiac function (adjusted OR, 1.21; 95% CI, 1.02–1.43; P = 0.03) (Table 2). Of the 27 covariates evaluated, only 6 (age, white race, history of myocardial infarction, glycosylated hemoglobin, left ventricular ejection fraction, and exercise capacity) were independently predictive of inducible ischemia (at P< 0.1) in this final model (Table 3).
Table 2.
Association of adiponectin with inducible ischemia, adjusted for potential confounding variables.
| Odds ratio (95% CI) per 1-SD increase | P-value | Odds ratio (95% CI) quartile IV vs. I | P-value | |
|---|---|---|---|---|
| Unadjusted | 1.35 (1.15–1.57) | 0.0002 | 2.15 (1.39–3.30) | 0.0006 |
| Adjusted models | ||||
| 1a | 1.25 (1.06–1.48) | 0.0008 | 1.78 (1.10–2.88) | 0.02 |
| 2b | 1.29 (1.09–1.53) | 0.003 | 1.97 (1.20–3.25) | 0.008 |
| 3c | 1.24 (1.05–1.48) | 0.01 | 1.91 (1.15–3.17) | 0.01 |
| 4d | 1.22 (1.02–1.46) | 0.03 | 1.74 (1.01–3.01) | 0.05 |
| 5e | 1.21 (1.02–1.43) | 0.03 | 1.63 (0.99–2.70) | 0.05 |
Model 1: Adjusted for demographic characteristics (age, sex, white race, alcohol consumption, current smoking).
Model 2: Adjusted for variables associated with ischemia (at P<0.1) in model 1 plus comorbidities and medical history (hypertension, myocardial infarction, congestive heart failure, stroke, diabetes mellitus, revascularization).
Model 3: Adjusted for variables associated with ischemia (at P<0.1)in models 1 and 2 plus current medication use (β-blocker, statin, diuretic, ACE inhibitor or ARB, aspirin).
Model 4: Adjusted for variables associated with ischemia (at P<0.1) in models 1, 2, and 3 plus metabolic variables (BMI, serum insulin, fasting glucose, glycosylated hemoglobin, HDL and non-HDL cholesterol, triglycerides, creatinine clearance).
Model 5: Adjusted for variables associated with ischemia (at P<0.1) in models 1, 2, 3, and 4 plus cardiac function (LV ejection fraction, exercise capacity, C-reactive protein).
Table 3.
Independent predictors of inducible ischemia in final multivariable model (entered as continuous variables per standard deviation change).
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Log adiponectin (per 0.08 μg/mL increase) | 1.21 (1.02–1.43) | 0.03 |
| Age (per 11-year increase) | 1.41 (1.17–1.70) | 0.0004 |
| White race | 1.43 (0.99–2.04) | 0.05 |
| History of myocardial infarction | 1.80 (1.28–2.53) | 0.008 |
| Glycosylated hemoglobin (per 1.16% increase) | 1.30 (1.11–1.52) | 0.001 |
| LV ejection fraction (per 9% increase) | 0.72 (0.62–0.83) | <0.0001 |
| Exercise capacity (per 3.4 MET increase) | 0.80 (0.67–0.97) | 0.02 |
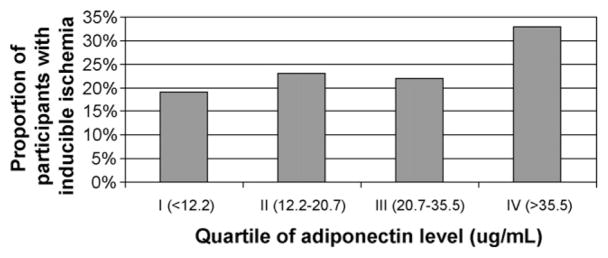
Similarly, the proportion of participants with inducible ischemia increased by serum adiponectin level (Fig. 1). A total of 33% of participants in the highest quartile of adiponectin (>35.5 μg/ml) had inducible ischemia compared with 19% of participants in the lowest quartile (<12.2 μg/ml) (P< 0.001). We found no evidence of interaction between adiponectin and age, sex, race, or BMI; history of diabetes, myocardial infarction, or revascularization; or use of statins or β-blockers (all P-values for interaction >0.1).
Fig. 1.
Proportion of participants with inducible ischemia by quartile of adiponectin (P =0.003 for trend).
4. Discussion
We found that higher total adiponectin concentrations were associated with a lower prevalence of diabetes, lower BMI, fasting insulin, fasting glucose, LDL cholesterol, and triglycerides, and higher HDL cholesterol levels in 899 outpatients with stable CHD. Despite this favorable metabolic profile, higher adiponectin concentrations were also associated with a higher prevalence of exercise-induced ischemia. Each SD increase in adiponectin was independently associated with a 21% greater odds of inducible ischemia, after adjustment for traditional clinical risk factors and measures of cardiac function. Although our cross-sectional study cannot be used to infer causal mechanisms, these findings may provide insight into the pathophysiology of adiponectin in patients with established CHD.
Some prior studies have reported that adiponectin is protective against incident CHD [9–11], while others have found no association with CHD [12,13], and still others have found that higher adiponectin levels predict an increased risk of cardiovascular events and mortality [14–16]. This apparent discrepancy has raised questions about the role of adiponectin in the pathogenesis of CHD. Although increased levels of a cardio-protective protein in patients with more severe CHD may initially seem contradictory, one possible explanation is that while elevated adiponectin may delay or prevent incident disease, the presence of chronic inducible ischemia may trigger a compensatory response that includes secretion of adiponectin. Ischemia may cause both increased production of adiponectin in the short-term and adverse cardiovascular events in the long-term.
These findings are consistent with Rathmann and Herder’s “reverse epidemiology” hypothesis that adiponectin levels may increase as an attempt to counter-regulate or compensate for systemic inflammation, and that adiponectin’s vasoprotective and anti-inflammatory effects may be superseded by the underlying disease [23]. Thus, the favorable association between adiponectin and incident CHD in the general population may be “reversed” in populations with existing CHD. This theory is supported by the fact that adiponectin appears to be more protective in higher risk individuals, such as men, those with severe stenosis, and those with elevations in the inflammatory marker C-reactive protein [11]. Furthermore, in a study of patients with stable angina and angiographic evidence of total occlusion of one coronary vessel, elevated adiponectin was associated with greater collateral development of coronary vessels [24]. Lastly, elevated adiponectin increases insulin sensitivity [4] and improves dyslipidemia [1,6], which maybe indirect mechanisms to reduce coronary risk.
Alternatively, coronary disease may reflect a state of adiponectin resistance, similar to insulin resistance in diabetes mellitus. Studies have demonstrated reduced adiponectin receptor expression and impaired response to adiponectin binding in obese and insulin-resistant mice, creating what has been termed the “vicious cycle” of hyperglycemia, hyperinsulinemia, and adiponectin resistance [25,26]. However, this process is unlikely to be the primary explanation for the findings in our study, given the favorable lipid and metabolic status of our patients. Instead, adiponectin resistance may simply attest to the limited capacity for the hormone to fully compensate for a diseased state, even when maximally secreted.
Elevated adiponectin may also be a consequence of impaired renal function. High adiponectin levels have been shown to be correlated with end-stage renal disease [27], low creatinine clearance [28], and reduced glomerular filtration rate [29]. Our findings are consistent with these previous reports; participants in the highest quartile of adiponectin had the lowest creatinine clearance and may have elevated adiponectin due to reduced renal clearance of the hormone. However, we found that adiponectin remained associated with inducible ischemia even after adjustment for creatinine clearance.
A final although less likely explanation for the association of higher adiponectin with inducible ischemia is that adiponectin may be on the causative pathway to CHD. Adiponectin has been described as a marker for catabolic wasting in congestive heart failure and is elevated in heart failure and predictive of mortality [30]. Adiponectin may be similarly detrimental in CHD and produce a downward spiral of disease progression via a currently unknown mechanism. The widely varying results from this and previous works suggest that the link between obesity, adiponectin, and coronary disease is likely complex and multifactorial, involving several if not all of the proposed mechanisms above. As such, the use of adiponectin as either a biomarker of coronary disease status or a cardio-protective therapeutic candidate for CHD would be premature. Although studies of adiponectin may eventually lead to clinically relevant therapies or interventions, our current findings by themselves have no direct clinical impact. Certainly, adiponectin is far from being a replacement for stress echocardiograms in risk stratifying CHD patients. Further studies are needed to characterize the clinical significance of the serum adiponectin level and to understand the potential role of adiponectin in patients with CHD.
Several limitations should be considered when interpreting our results. First, participants were predominantly older men, and all with documented CHD. Therefore, we are unable to generalize the results of the study to women, younger populations, or those without prevalent coronary disease. In fact, studies have found that although adiponectin is similarly associated with favorable metabolic factors in women, absolute serum adiponectin levels are elevated relative to men, and there is no association, or even a negative association, between adiponectin and CHD risk in women, suggesting that gender-specific effects may exist and warrant further investigation [31–33]. Second, the immunoassay used in this study measures total adiponectin and does not distinguish among the molecular isoforms that may be functionally distinct, with the high molecular weight variety being potentially the most biologically active [34].
Our adipokine immunoassay panel also differed from the assays used in previous studies, a possible explanation for the higher range of adiponectin levels we found compared to those in other populations. However, this would not explain the association between adiponectin and inducible ischemia, and higher adiponectin levels may represent greater underlying disease severity in this sample of patients with known CHD. Third, there was moderate variability in the adiponectin assay measurements. However, we assayed all samples in duplicate and used the average of the two samples as our measurement of serum adiponectin. Furthermore, any measurement error or short-term variation that were to occur would bias the results toward null. Lastly, we cannot establish causality in our observations of adiponectin and ischemia due to the cross-sectional design of this study.
In summary, we found that higher levels of circulating adiponectin are associated with inducible ischemia among patients with stable CHD. While secretion of this potentially protective hormone may be responsible for the favorable lipid and metabolic profile of those with increased adiponectin, its role in patients with established CHD appears more complex. Whether elevated adiponectin is a compensatory anti-atherosclerotic substance increased in an at-risk population, evidence of adiponectin resistance, or a causative risk factor in CHD, the effects of adiponectin in CHD pathogenesis and progression warrant further study.
Acknowledgments
Mary Zhang was supported by a fellowship from the University of California, San Francisco (Medical Student Research Training Program). The Heart and Soul Study was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute (R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund. None of these funding sources had any role in the collection of data, interpretation of results, or preparation of this manuscript.
References
- 1.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13:51–9. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–6. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 5.Tian L, Luo N, Klein RL, et al. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaverkova H, Karasek D, Novotny D, et al. Positive association of adiponectin with soluble vascular cell adhesion molecule sVCAM-1 levels in patients with vascular disease or dyslipidemia. Atherosclerosis. 2008;197:725–31. doi: 10.1016/j.atherosclerosis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao L, Gao E, Jiao X, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 10.Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–53. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 11.Wolk R, Berger P, Lennon RJ, et al. Association between plasma adiponectin levels and unstable coronary syndromes. Eur Heart J. 2007;28:292–8. doi: 10.1093/eurheartj/ehl361. [DOI] [PubMed] [Google Scholar]
- 12.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–9. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 13.Lawlor DA, Davey Smith G, Ebrahim S, et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–83. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 14.Steffes MW, Gross MD, Lee DH, et al. Adiponectin, visceral fat, oxidative stress, and early macrovascular disease: the Coronary Artery Risk Development in Young Adults Study. Obesity (Silver Spring) 2006;14:319–26. doi: 10.1038/oby.2006.41. [DOI] [PubMed] [Google Scholar]
- 15.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–9. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 16.Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93:1489–96. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 17.Beattie MS, Shlipak MG, Liu H, et al. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107:245–50. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibbins-Domingo K, Gupta R, Na B, et al. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–76. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–92. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 20.Bibbins-Domingo K, Ansari M, Schiller NB, et al. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108:2987–92. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Gehi AK, Ali S, Na B, et al. Inducible ischemia and the risk of recurrent cardiovascular events in outpatients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2008;168:1423–8. doi: 10.1001/archinte.168.13.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for “reverse epidemiology”. Horm Metab Res. 2007;39:1–2. doi: 10.1055/s-2007-958630. [DOI] [PubMed] [Google Scholar]
- 24.Soydinc S, Davutoglu V, Sari I. High serum levels of adiponectin improve coronary collateral development in patients with coronary artery disease. Tohoku J Exp Med. 2007;211:347–52. doi: 10.1620/tjem.211.347. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchida A, Yamauchi T, Ito Y, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–22. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 26.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 27.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–41. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 28.Mallamaci F, Zoccali C, Cuzzola F, et al. Adiponectin in essential hypertension. J Nephrol. 2002;15:507–11. [PubMed] [Google Scholar]
- 29.Risch L, Saely C, Hoefle G, et al. Relationship between glomerular filtration rate and the adipokines adiponectin, resistin and leptin in coronary patients with predominantly normal or mildly impaired renal function. Clin Chim Acta. 2007;376:108–13. doi: 10.1016/j.cca.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, Watt P, Cherry L, et al. High molecular weight adiponectin is not associated with incident coronary heart disease in older women: a nested prospective case-control study. J Clin Endocrinol Metab. 2008;93:1846–9. doi: 10.1210/jc.2007-2603. [DOI] [PubMed] [Google Scholar]
- 32.Zyriax BC, Algenstaedt P, Hess UF, et al. Factors contributing to the risk of cardiovascular disease reflected by plasma adiponectin: data from the coronary risk factors for atherosclerosis in women (CORA) study. Atherosclerosis. 2008;200:403–9. doi: 10.1016/j.atherosclerosis.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Rathmann W, Haastert B, Herder C, et al. Differential association of adiponectin with cardiovascular risk markers in men and women? The KORA survey 2000. Int J Obes (Lond) 2007;31:770–6. doi: 10.1038/sj.ijo.0803471. [DOI] [PubMed] [Google Scholar]
- 34.von Eynatten M, Humpert PM, Bluemm A, et al. High-molecular weight adiponectin is independently associated with the extent of coronary artery disease in men. Atherosclerosis. 2008;199:123–8. doi: 10.1016/j.atherosclerosis.2007.10.002. [DOI] [PubMed] [Google Scholar]