Summary
Pulmonary alveolar proteinosis (PAP) is a rare syndrome characterized by accumulation of pulmonary surfactant, respiratory insufficiency, and increased infections. It occurs in various clinical settings that disrupt surfactant catabolism in alveolar macrophages, including a relatively more common autoimmune disease caused by GM-CSF autoantibodies and a rare congenital disease caused by CSF2RA mutations. Recent results demonstrate that GM-CSF is critical for alveolar macrophage terminal differentiation and immune functions, pulmonary surfactant homeostasis, and lung host defense. GM-CSF is also required for the basal functional capacity of circulating neutrophils, including adhesion, phagocytosis, and microbial killing. PAP research has illuminated the critical role of GM-CSF in innate immunity and led to novel therapy for PAP and the potential use of anti-GM-CSF therapy in other common disorders.
Introduction
Pulmonary alveolar macrophages are multifunctional tissue representatives of the bone marrow-derived mononuclear phagocyte system that serve as a first line of defense against inhaled microbial pathogens and toxins, clear inhaled debris, excess surfactant and apoptotic cells from the alveolar surface, orchestrate pulmonary inflammatory responses, and participate in wound healing and lung remodeling. A broad range of exogenous and endogenous factors interact with and modify the functions of these cells, including colony stimulating factors such as M-CSF, GM-CSF, and IL-3. GM-CSF, initially identified by its ability to stimulate the formation of neutrophil and macrophage colonies from bone marrow precursors, is now regarded as an important immunoregulatory cytokine with pleiotropic effects on myeloid cells in health and disease (reviewed in [1]) mediated through complex signaling pathways (Figure 1). The serendipitous discovery that GM-CSF deficient mice accumulate surfactant in the lungs identified the critical role of GM-CSF in alveolar macrophage function and surfactant homeostasis (reviewed in [2]). Early studies showed that this phenotype is caused by the absence of GM-CSF in the lungs where it is required to stimulate alveolar macrophages to catabolize surfactant lipids and proteins. Subsequent studies demonstrated that GM-CSF-deficient mice have increased mortality from pulmonary and systemic infections, and that myeloid cells from these mice have multiple innate immune defects (reviewed in [3]).
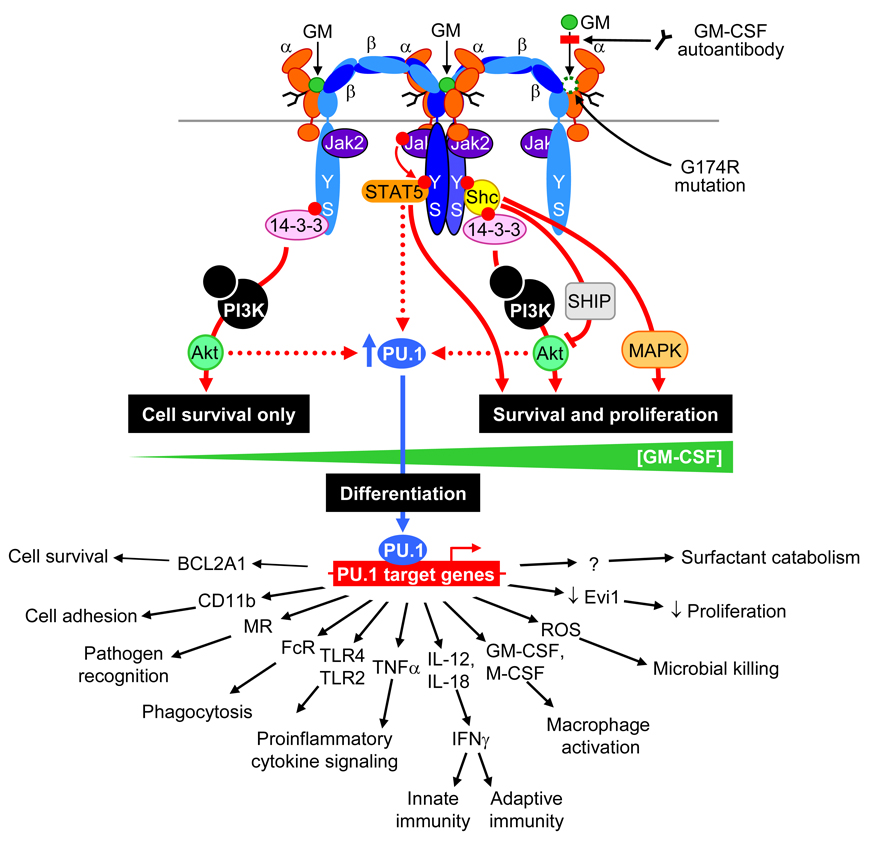
Figure 1. Mechanisms by which GM-CSF regulates the survival, differentiation, functions and activation of alveolar macrophages.
GM-CSF (GM) initiates signaling by first binding to the GM-CSF receptor α chain (α), which then associates with homodimers of the affinity-enhancing GM-CSF receptor β chain (β). Jak2 is bound constitutively to the β chain and signals through an intracytoplasmic β chain motif including residues tyrosine577 and serine585, which is necessary and sufficient for GM-CSF receptor signaling. At low GM-CSF concentrations (0.01 – 10 pM), phosphorylation of serine585 couples signaling via the adapter protein, 14-3-3 through PI3K and Akt, resulting in cell survival without proliferation. At high GM-CSF concentrations (10 – 10,000 pM), phosphorylation of tyrosine577 couples signaling via STAT5 or Shc-dependent pathways, stimulating cell survival, cellular activation and proliferation. Pulmonary GM-CSF stimulates expression of PU.1 in alveolar macrophages, which in turn regulates the expression of numerous genes enabling multiple immune and non-immune functions consistent with terminal differentiation of alveolar macrophages in the lungs. Interruption of GM-CSF signaling, either by neutralizing autoantibodies or function-altering amino acid changes in GM-CSF receptor α (G196R) impair GM-CSF receptor signaling and alveolar macrophage maturation. One of the functions affected is the ability to catabolize surfactant lipids internalized into endosomes, thereby reducing surfactant clearance and causing PAP.
Pulmonary alveolar proteinosis (PAP) causes lung pathology similar to that of GM-CSF-deficient mice and occurs in a heterogenous group of diseases (reviewed in [4]). While function-altering GM-CSF mutations have not been identified in humans, PAP is associated with disruption of GM-CSF signaling caused by high levels of neutralizing GM-CSF autoantibodies in autoimmune PAP or by mutations in CSF2RA, the gene encoding the GM-CSF receptor α protein in congenital PAP. Both PAP patients and GM-CSF deficient mice have increased susceptibility to opportunistic microbial pathogens and increased mortality from uncontrolled infections [5]. Here, we review recent studies that helped elucidate the pathogenesis of autoimmune and congenital PAP, the role of GM-CSF in alveolar macrophage and neutrophil function in mice and man, and studies that implicate GM-CSF in the pathogenesis of serious inflammatory and autoimmune diseases.
Autoimmune PAP: an attack of adaptive immunity on innate immunity
First described by Rosen in 1958, the pathogenesis of PAP remained enigmatic for more than 4 decades. Following the discovery of PAP in GM-CSF-deficient mice, neutralizing GM-CSF autoantibodies were detected in patients with the common clinical PAP subtype (idiopathic PAP) [6]. These were comprised of polyclonal IgG, primarily IgG1 and IgG2 with only small amounts of IgG3 and IgG4, and were highly specific for human GM-CSF recognizing multiple epitopes and binding with an affinity of 20 ± 7.5 pM [7,8]. High levels of GM-CSF autoantibodies were present only in patients with this clinical subtype, and not in other PAP subtypes, individuals with other lung diseases or healthy people [4,9,10]. Autoantibody levels in these patients were sufficient to neutralize far more GM-CSF than is present physiologically, suggesting they eliminate GM-CSF bioactivity in vivo and that autoimmune PAP is a functional equivalent of the GM-CSF-deficient mouse [7]. GM-CSF autoantibodies are consistently detected in pharmaceutical immunoglobulin preparations and were recently reported to be ubiquitous in healthy human individuals, albeit at levels far lower than in idiopathic PAP patients [8]. This apparent paradox was reconciled by the hypothesis that a GM-CSF autoantibody level exceeding a critical threshold is required to increase the risk of PAP [11]. Evaluation of healthy individuals and PAP patients permitted estimation of this critical threshold [8] and suggested that physiological levels of GM-CSF autoantibodies may rheostatically regulate myeloid cell reactivity via continuous in vivo priming (Figure 2). The ability to purify GM-CSF autoantibodies by affinity chromatography has facilitated evaluation of their biological effects on myeloid cells in vitro and in vivo [7,12]. Passive immunization studies showed that GM-CSF autoantibodies from PAP patients faithfully reproduced the histopathological, biochemical, and immunological manifestations of PAP in healthy, non-human primates [13]. Further, idiopathic PAP patients and GM-CSF-deficient mice have similar defects in neutrophil functions (adhesion, phagocytosis, oxygen radical production, microbial killing). Neutrophil dysfunction could be reproduced by incubation of normal cells with GM-CSF autoantibodies ex vivo [12]. Alveolar macrophages from PAP patients [8] and GM-CSF-deficient mice [14] have similarly impaired phagocytosis and other immune defects (Table 1) [8]. These myeloid cell defects provide an explanation for the increased infection risk and mortality in PAP patients and GM-CSF deficient mice.
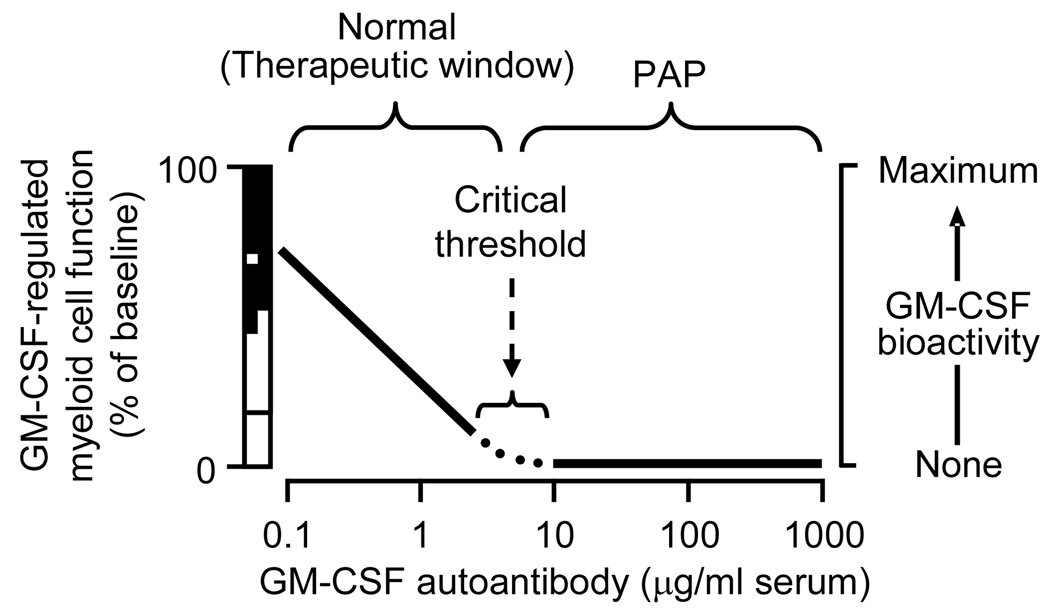
Figure 2. Relationship between GM-CSF autoantibody concentration, GM-CSF bioactivity and regulation of GM-CSF-dependent myeloid cell functions.
Over a range of low GM-CSF autoantibody levels present in healthy subjects, increasing GM-CSF autoantibody concentrations (abscissa) rheostatically lower GM-CSF bioactivity (right ordinate) thereby reducing in tandem, GM-CSF-dependent myeloid cell functions (left ordinate). Some functions have activity that is GM-CSF-independent (open bar, left ordinate), modulated by physiologic changes in GM-CSF concentration (hatched bar, left ordinate), or stimulated to supranormal levels by exogenous or pathologically increased GM-CSF levels (black bar, left ordinate). Above a concentration sufficient to block GM-CSF completely (the critical threshold), GM-CSF bioactivity is zero and GM-CSF-dependent functions are minimal. GM-CSF autoantibody concentrations between zero and the critical threshold are present in healthy individuals and may serve a physiological role by negatively regulating myeloid cell reactivity. The critical threshold also helps to define a therapeutic window for the potential use of GM-CSF autoantibodies to treat other disorders. GM-CSF antibody levels above the critical threshold are anticipated to increase the risk of iatrogenic PAP. Adapted from reference [8].
Table 1.
Functional defects in alveolar macrophages and neutrophils from in GM-CSF deficient mice and PAP patients and correction by GM-CSF or PU.1.*
| Corrected by | Corrected | |||||
|---|---|---|---|---|---|---|
| Function / attribute | GM−/− mice | GM-CSF | PU.1 | APAP | by GM-CSF | CPAP† |
| Alveolar macrophages | ||||||
| Cell diameter | ↑[14] | Yes‡ | Yes [14] | ↑ [47,48] | Yes‡ [49] | ↑‡[18] |
| Cytoskeletal organization | Abnormal [23] | Yes [23] | ||||
| Surfactant catabolism § | ↓ [14,25] | Yes [25] | Partial [14] | |||
| SP-A binding | ↑ [25] | Yes [25] | ||||
| PU.1 expression | ↓ [14] | Yes [14] | ↓ [28] | Yes [28,49] | ||
| BS-1 lectin binding | ↓ [26] | |||||
| Cell adhesion | ↑ [14,26] | Yes [26] | Yes [14] | ↓ [48] | ||
| Integrin expression | ↓ [26] | Yes [26] | Yes [14] | |||
| Mannose receptor expression | ↓ [14] | Yes [14] | Yes [14] | ↓[28] | Yes [28,49] | |
| Toll-like receptor-4, -2 expression | ↓[14] | Yes [14] | Yes [14] | ↓[28] | Yes [28] | |
| Fcγ receptor expression | ↓[27] | Yes [27] | Yes [27] | ↓[28] | Yes [28] | |
| M-CSF receptor expression | ↓[28] | Yes [28] | ||||
| Phagocytosis of | ||||||
| latex microspheres | ↓[14,23,26,27] | Yes [26,27] | Yes [14,23,27] | ↓[8] | Yes [49] | |
| opsonized latex microspheres | ↓[27] | Yes [27] | Yes [27] | |||
| transferrin-coated microspheres | ↓[23] | Yes [23] | ||||
| E. coli, S. aureus, Zymosan | ↓[14] | Yes [14] | ↓[8] | |||
| P. aeruginosa | ↓[30] | Yes [30] | ||||
| Adenovirus | ↓[23] | Yes [14] | ||||
| P. carinii | ↓[32] | |||||
| Bacterial killing of | ||||||
| E. coli | ↓[14] | Partial [14] | ||||
| Streptococcus | ↓[14] | Partial [14] | ||||
| Candida | ↓[48] | |||||
| M. tuberculosis | ↓[34] | Yes [34] | ||||
| P. aeruginosa | ↓[30] | Yes [30] | ||||
| TNFα secretion to LPS | ↓[14,26] | Yes [26] | Yes [14] | |||
| IL-6 secretion to LPS | ↓[14] | Yes [14] | ||||
| IFNγ secretion to LPS | ↓[27] | |||||
| IL-12 secretion to adenovirus | ↓[27] | |||||
| IL-18 secretion to adenovirus | ↓[27] | Yes [27] | ||||
| Leukotriene-C, -D, -E4 production | ↓[26] | Partial [26] | ||||
| PGE2 production | ↓[36] | |||||
| Blood neutrophils | ||||||
| Adhesion | ↓[12] | ↓[12] | ||||
| Phagocytosis of latex beads | ↓[12] | ↓[12] | ||||
| Reactive oxygen species production | ↓[12] | ↓[12] | ||||
| Bacterial killing | ↓[12] | ↓[12] | ||||
| STAT5 phosphorylation | ↓¶ | ↓[18] | ||||
| CD11b stimulation index | No Δ [12] | ↓[12] | ↓[18] | |||
| Bronchoalveolar lavage | ||||||
| MCP-1 | ↑[26] | ↑[9,47] | ↑[18] | |||
| M-CSF | ↑[14] | ↑[9] | ↑[18] | |||
| GM-CSF | ↑[18] | |||||
| Pulmonary leukocyte number | ↑[50] | ↑[49] | Yes [49] | |||
Abbreviations: APAP, autoimmune PAP; BS-1, Bandeiraea simplicifolia clone 1; CPAP, congenital PAP; GM-CSF, granulocyte/macrophage-colony stimulating factor; IFNγ, interferon gamma; IL-Interleukin; MCP-1, monocyte chemotactic protein-1; M-CSF, macrophage-colony stimulating factor; PAP, pulmonary alveolar proteinosis; PGE2, prostaglandin E2; SP-A. surfactant protein A; STAT5, signal transducer and activator of transcription-5; TNF, tumor necrosis factor;
Supporting references are indicated in square brackets. Functions for which data are unavailable are left blank.
Caused by compound heterozygous CSF2RA mutations comprised of allelic deletion and a G196R point mutation [18].
Observed visually but not measured quantitatively.
Demonstrated for surfactant protein A, dipalmitoylphosphatidylcholine, and dipalmitoyl phosphatidylethanolamine.
Unpublished observations (T.S., B.C.T.).
In summary, GM-CSF autoantibodies appear to directly cause the common clinical form of PAP, which is now considered an autoimmune disease specifically targeting GM-CSF (i.e., autoimmune PAP). The possible physiological role(s) of GM-CSF autoantibodies in healthy individuals may be to limit the endocrine actions of GM-CSF produced at upstream sites of inflammation (Figure 3). This is consistent with the observation that more than 99.9 percent of serum GM-CSF in healthy individuals is bound to GM-CSF autoantibodies [8].
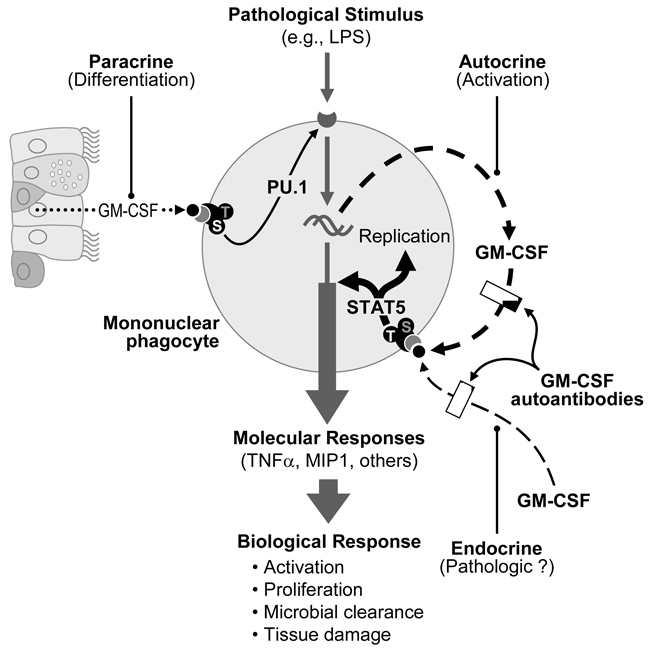
Figure 3. Proposed modes by which GM-CSF regulates alveolar macrophage functions and modulation GM-CSF autoantibodies.
GM-CSF produced locally in the lung interacts with receptors on nearby alveolar macrophages stimulating terminal differentiation (paracrine mode) thereby enabling the numerous functions and signaling pathways, e.g., TLR4 pathway. Pathological stimuli activate signaling pathways with biologic responses important to host defense of that cell. GM-CSF released by and binding to the cell’s own GM-CSF receptors (autocrine mode) switches them into the tyrosine577-mediated, high activity state [15], activating the macrophage, which enhances immune functions and stimulates proliferation. This autocrine mode of action provides a fine control for GM-CSF to modulate host defenses on a microscopic scale in the local microenvironment of the cell (i.e., after encountering a pathogen) independent of other components of regional or systemic immunity. GM-CSF originating from a ‘upstream’ site of inflammation can stimulate macrophages at distal sites (endocrine mode), which may result in unnecessary (pathologic) activation. Low levels of GM-CSF autoantibodies in healthy individuals appear to block endocrine signaling and may modulate autocrine modes of GM-CSF signaling, whereas high levels in PAP patients also block paracrine signaling resulting in maturational arrest of macrophages.
Congenital PAP caused by disruption of GM-CSF receptor function
GM-CSF signaling is mediated by cell surface receptors comprised of a low-affinity GM-CSF-binding α chain and an affinity-enhancing β chain common to the receptors for GM-CSF, IL-3 and IL-5 [1]. Neither the α nor β chains possess intrinsic signaling capacity but the β chain constitutively associates with Jak2, which is critical for signaling. The pleiotropic effects of GM-CSF on myeloid cell survival, proliferation, differentiation and activation appear to be mediated in part via a binary switch-like mechanism [15] initiated by assembly of a dodecameric receptor complex [16] that forms after binding GM-CSF (Figure 1). At low GM-CSF concentrations, signaling occurs via phosphorylation of serine585 of the β chain resulting only in cell survival mediated via activation of NFκB and induction of bcl-2 [17]. At higher concentrations, signaling via serine585 is extinguished and signaling via phosphorylation of tyrosine577 of the β chain results in survival as well as stimulation of STAT5-regulated pathways, including cellular activation and proliferation (Figure 1). GM-CSF, via the transcription factor PU.1, also stimulates surfactant catabolism and numerous other functions in alveolar macrophages (see below).
A six year old child with PAP in whom GM-CSF autoantibodies were undetectable recently led to identification of congenital PAP caused by recessive CSF2RA abnormalities that disrupted GM-CSF signaling [18]. Progressive dyspnea of insidious onset had been present for several years. She had a 1.6 megabase deletion in the pseudoautosomal region of her maternal X chromosome encompassing CSF2RA and a point mutation in the paternal X chromosome causing a single amino acid change (G196R) in the extracellular, cytokine binding domain of the α chain. This point mutation altered α chain glycosylation, reduced GM-CSF binding, and disrupted signaling as demonstrated by the absence of STAT5 phosphorylation and unaltered cell-surface CD11b levels after GM-CSF stimulation. Serum surfactant protein D was increased similar to results in patients with autoimmune PAP [18]. GM-CSF levels were also increased in the lungs of this patient and in the serum of her eight year old sister who was considered to be healthy but subsequently found to have identical molecular defects and radiographic findings of PAP. The parents were heterozygous for the CSF2RA abnormalities and had normal serum levels of surfactant protein D and GM-CSF. Congenital PAP was also identified in a 4 year old female with Turner’s syndrome caused by compound X chromosome deletions resulting in the disruption of both CSF2RA alleles [19]. Her serum GM-CSF level was elevated. Although, bone marrow transplantation appeared to be successful in treating PAP, the patient succumbed to fungal infection four weeks after transplantation.
Based on the hypothesis that an elevated serum GM-CSF level may be a useful biomarker of PAP due to receptor dysfunction, screening of sera from children with unexplained PAP identified 4 individuals with PAP caused by various function-altering CSF2RA mutations [20]. To date, seven children, all female and ranging in age from two to eleven years, have been identified with congenital PAP caused by CSF2RA mutations; all have elevated GM-CSF levels. Only one had a serious infection, which occurred during immunosuppression after bone marrow transplantation (described above).
PAP was been reported in four infants in whom expression of the GM-CSF β chain was not detected on blood leukocytes, implying β chain dysfunction in the pathogenesis of PAP [21]. However, these patients were poorly characterized and mutations were not excluded in other genes that cause PAP, i.e., those encoding SP-C or the lipid transporter ABCA3 [22]).
GM-CSF is critical for the terminal differentiation of alveolar macrophages
Evaluation of mice in which GM-CSF expression was normal, absent, or occurred only in the lungs revealed that pulmonary GM-CSF regulated alveolar macrophage expression of the myeloid master transcription factor, PU.1, suggesting GM-CSF was required for alveolar macrophage maturation [14]. An alveolar macrophage cell line (mAM) derived from GM-CSF deficient mice also failed to express PU.1 and had a phenotype similar to that of primary cells from these mice (Table 1). This phenotype included altered cellular morphology and expression of macrophage differentiation antigens (ERMP12, ERMP20, BM8), and impaired cell adhesion, phagocytosis, expression of cell surface receptors (TLR4, TLR2, CD14, mannose receptor, Fc receptors, integrins (α4, α5, αL αM, αv, β2, and β5)), LPS-mediated TNFα and IL-6 secretion, surfactant protein and lipid catabolism, and antimicrobial activity [14,23–27]. Importantly, reconstitution of GM-CSF in the lungs of GM-CSF deficient mice or retrovirus-mediated expression of PU.1 in mAM cells restored a normal macrophage-like appearance and corrected all the abnormalities evaluated (Table 1). Alveolar macrophages from patients with autoimmune PAP have numerous abnormalities similar to those of GM-CSF deficient mice (Table 1) and GM-CSF has been shown to be required to stimulate PU.1 expression in alveolar macrophages from these patients [28]. That GM-CSF coordinately regulates such a wide range of immune and non-immune macrophage functions strongly supports the concept that pulmonary GM-CSF is required to stimulate the terminal differentiation of alveolar macrophages in the lungs. Notwithstanding, the precise mechanism(s) by which GM-CSF stimulates PU.1 levels and stimulates macrophage terminal differentiation remain poorly understood. While GM-CSF also determines the basal functions of circulating neutrophils, neither PU.1 levels, nor expression of differentiation markers on neutrophils were affected in GM-CSF deficient mice or PAP patients, suggesting that GM-CSF is not critical for neutrophil differentiation [12].
GM-CSF is a critical regulator of myeloid cell host defense functions
Uncontrolled infection, frequently by opportunistic pathogens, account for 18% of attributable mortality in PAP patients [5]. Similarly, GM-CSF-deficient mice have increased mortality from infection and increased susceptibility to a wide range of microbial pathogens, including bacteria (Streptococcus [29], Pseudomonas a. [30], Listeria monocytogenes [31]), fungi (Pneumocystis carinii [32]), malaria (Plasmodium chabaudi [33]), virus (adenovirus [24]) and Mycobacteria (M. tuberculosis [34]) (Table 1). In both PAP patients and GM-CSF deficient mice, infections occur at pulmonary and extrapulmonary sites indicating that the predisposition to infection is systemic.
Adenovirus exemplifies how GM-CSF regulates macrophage antimicrobial functions. Macrophages normally internalize adenovirus via endosomes that are translocated to phagolysosomes where virions are destroyed [24]. In mAM cells, which don’t express GM-CSF or PU.1, virions readily escape endosomal confinement, translocate to the nucleus and transduce the cell as occurs in epithelial cells. Retroviral expression of PU.1 corrects this phenotype, restoring lysosomal translocation and virion destruction. Importantly, ectopic, retroviral-mediated PU.1 expression blocks adenoviral transduction in epithelial cells as it normally does in macrophages. Thus, GM-CSF, via PU.1, prevents infection of macrophages (i.e., transduction) and promotes viral clearance by uncoupling virion uptake from cellular transduction and by promoting virion destruction [24].
GM-CSF is important in systemic responses to infection because GM-CSF deficient mice are resistant to LPS mediated shock and the expression of PU.1 in peritoneal macrophages is critical in shock-mediated mortality from peritonitis or intraperitoneal LPS administration [35]. GM-CSF also regulates the production of oxygen radicals [29], prostaglandins (8-iso-PGF2, PGE2) and leukotrienes (LTB4) [30,32,36] by alveolar macrophages. GM-CSF-deficiency [37] or the presence of neutralizing anti-GM-CSF antibodies [38], reduces pulmonary cellular and molecular inflammation in response to LPS.
Manipulating GM-CSF bioactivity: potential new therapeutic applications
Studies evaluating GM-CSF-deficient mice in various experimental disease models and the demonstration of increased GM-CSF levels in the corresponding human disorders have implicated GM-CSF in the pathogenesis of inflammatory and autoimmune diseases (reviewed in [39,40]). For example, GM-CSF-deficient mice develop less-severe pathology in models of collagen-induced arthritis [41] and GM-CSF is increased in synovial fluid from patients with rheumatoid arthritis [42]. Similarly, GM-CSF-deficient mice are resistant to experimental allergic encephalomyelitis, a model of multiple sclerosis, and susceptibility can be restored by reconstituting GM-CSF [43]. Experimental models of antigen-driven glomerulonephritis [44], gastritis [45] and pancreatitis-associated lung injury [46] also have reduced severity in GM-CSF-deficient mice. Based on these and other studies, clinical trials are now underway evaluating the safety and efficacy of an antibody-mediated reduction in GM-CSF signaling in severe inflammatory and autoimmune disorders [40]. While this opens up exciting new potential pharmacological approaches, close monitoring for the development of pulmonary (i.e. iatrogenic PAP) and infectious (i.e., recrudescent mycobacterial infection) complications will be important.
Acknowledgments
We apologize to colleagues whose work could not be cited due to strict space limitations. Supported in part by grants from the National Heart, Lung and Blood Institute (HL0085453, to Dr. Trapnell, and the National Center for Research Resources and the National Institutes of Health Office of Rare Diseases (RR019498, to Dr. Trapnell).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
- 1. Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. The GM-CSF receptor: linking its structure to cell signaling and its role in disease. Blood. 2009 doi: 10.1182/blood-2008-12-164004. This paper presents a new model for signaling by the GM-CSF receptor, based on the recently elucidated dodecameric structure of GM-CSF bound to complexes of the GM-CSF receptor α and β chains, and Jak2. This new structure provides important new insights into the pleiotropic effects of GM-CSF derived from an understanding of the receptor’s structure.
- 2.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 3.Presneill JJ, Nakata K, Inoue Y, Seymour JF. Pulmonary alveolar proteinosis. Clin Chest Med. 2004;25:593–613. doi: 10.1016/j.ccm.2004.04.002. viii. [DOI] [PubMed] [Google Scholar]
- 4.Trapnell BC, Whitsett JA, Nakata K. Pulmonary Alveolar Proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 5. Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. This study provides a comprehensive metaanalysis summarizing most or all of the cases of PAP reported in the medical literature from the initial description of the syndrome in 1958 through 1997.
- 6. Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. This report was the first to identify that PAP in human individuals was associated with neutralizing GM-CSF autoantibodies.
- 7. Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. This report demonstrated that neutralizing GM-CSF autoantibodies were highly specific for human GM-CSF, bound it with high avidity, and eliminated GM-CSF bioactivity in the lungs of PAP patients.
- 8. Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, Stevens CA, Beck DD, Denson LA, Carey BC, et al. Granulocyte/Macrophage Colony-Stimulating Factor Autoantibodies and Myeloid Cell in Healthy Individuals. Blood. 2009;113:2547–2556. doi: 10.1182/blood-2009-05-155689. This report describes the critical threshold of GM-CSF autoantibody associated with an increased risk of developing PAP.
- 9.Bonfield TL, Russell D, Burgess S, Malur A, Kavuru MS, Thomassen MJ. Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. Am J Respir Cell Mol Biol. 2002;27:481–486. doi: 10.1165/rcmb.2002-0023OC. [DOI] [PubMed] [Google Scholar]
- 10. Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, et al. Characteristics of a Large Cohort of Autoimmune Pulmonary Alveolar Proteinosis Patients in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. This report describes the largest contemporaneous cohort of PAP patients (223 patients with autoimmune PAP) ever studied and reports on the epidemiology, clinical features, and biomarkers of autoimmune PAP.
- 11.Bendtzen K, Svenson M, Hansen MB, Busch T, Bercker S, Kaisers U, Uchida K, Beck DC, Trapnell BC. GM-CSF Autoantibodies in Pulmonary Alveolar Proteinosis. N Engl J Med. 2007;356:2001–2002. [PubMed] [Google Scholar]
- 12. Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–579. doi: 10.1056/NEJMoa062505. This report describes the effects of highly purified GM-CSF autoantibodies on the functions of normal human neutrophils. The differential reduction in various functions of neutrophils from PAP patients and GM-CSF deficient mice was striking and suggests that GM-CSF may regulate neutrophil functions by similar mechanisms in man and mice.
- 13.Sakagami T, Beck D, Suzuki T, Uchida K, Wood RE, Carey BC, Wert SE, Ikegami M, Whitsett JA, Keller G, et al. Pulmonary Alveolar Proteinosis (PAP) Reproduced in Non-Human Primates. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.201001-0008OC. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. This report demonstrated that GM-CSF must be present in the lungs to stimulate the terminal differentiation of alveolar macrophages and functions via stimulating expression of the master macrophage transcription factor, PU.1.
- 15. Guthridge MA, Powell JA, Barry EF, Stomski FC, McClure BJ, Ramshaw H, Felquer FA, Dottore M, Thomas DT, To B, et al. Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch. Embo J. 2006;25:479–489. doi: 10.1038/sj.emboj.7600948. The authors identified a novel motif within the β chain that is both necessary and sufficient for GM-CSF signaling, and explained how low and high GM-CSF concentrations regulate cell survival only or cell survival and proliferation.
- 16. Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. This groundbreaking paper that describes the crystal structure of the GM-CSF receptor bound to its ligand revealed an unexpected, novel mechanism of signaling that is likely to be the paradigm used by other cytokine receptors.
- 17.Guthridge MA, Barry EF, Felquer FA, McClure BJ, Stomski FC, Ramshaw H, Lopez AF. The phosphoserine-585-dependent pathway of the GM-CSF/IL-3/IL-5 receptors mediates hematopoietic cell survival through activation of NF-kappaB and induction of bcl-2. Blood. 2004;103:820–827. doi: 10.1182/blood-2003-06-1999. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, et al. Familial Pulmonary Alveolar Proteinosis Caused by Mutations in CSF2RA. J. Exp. Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. This is the first report of familial PAP caused by mutations in the CSF2RA that encodes the GM-CSF receptor α chain. It demonstrated that a high serum level of GM-CSF is a biomarker of congenital PAP caused by GM-CSF receptor dysfunction.
- 19.Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT, Moore JP, Tavana G, Lewis LR, Zhu Y, et al. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey BC, Suzuki T, Uchida K, Sakagami T, Wood RE, Luisetti M, Rubin B, Kevill K, Trapnell BC. An Algorithm for Diagnosis of Familial Pulmonary Alveolar Proteinosis (PAP) Am J Respir Crit Care Med. 2009 (in press) [Google Scholar]
- 21.Dirksen U, Nishinakamura R, Groneck P, Hattenhorst U, Nogee L, Murray R, Burdach S. Human pulmonary alveolar proteinosis associated with a defect in GM- CSF/IL-3/IL-5 receptor common beta chain expression. J Clin Invest. 1997;100:2211–2217. doi: 10.1172/JCI119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet. 2004;13(Spec No 2):R207–R215. doi: 10.1093/hmg/ddh252. [DOI] [PubMed] [Google Scholar]
- 23.Berclaz PY, Zsengeller Z, Shibata Y, Otake K, Strasbaugh S, Whitsett JA, Trapnell BC. Endocytic Internalization of Adenovirus, Nonspecific Phagocytosis, and Cytoskeletal Organization Are Coordinately Regulated in Alveolar Macrophages by GM-CSF and PU.1. J Immunol. 2002;169:6332–6342. doi: 10.4049/jimmunol.169.11.6332. [DOI] [PubMed] [Google Scholar]
- 24.Carey B, Staudt MK, Bonaminio D, van der Loo JC, Trapnell BC. PU.1 Redirects Adenovirus to Lysosomes in Alveolar Macrophages, Uncoupling Internalization from Infection. J Immunol. 2007;178:2440–2447. doi: 10.4049/jimmunol.178.4.2440. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Ikegami M, Reed JA, Chroneos ZC, Whitsett JA. GM-CSF regulates surfacant Protein-A and lipid catabolism by alveolar macrohpages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L379–L386. doi: 10.1152/ajplung.2001.280.3.L379. [DOI] [PubMed] [Google Scholar]
- 26.Paine R, 3rd, Morris SB, Jin H, Wilcoxen SE, Phare SM, Moore BB, Coffey MJ, Toews GB. Impaired functional activity of alveolar macrophages from GM-CSF- deficient mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1210–L1218. doi: 10.1152/ajplung.2001.281.5.L1210. [DOI] [PubMed] [Google Scholar]
- 27.Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma -mediated molecular connection between innate and adaptive immunity in the lung. Blood. 2002;100:4193–4200. doi: 10.1182/blood-2002-04-1102. [DOI] [PubMed] [Google Scholar]
- 28.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, Thomassen MJ. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1132–L1136. doi: 10.1152/ajplung.00216.2003. [DOI] [PubMed] [Google Scholar]
- 29.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest. 1999;103:563–569. doi: 10.1172/JCI5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballinger MN, Paine R, 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, Toews GB, Moore BB. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:766–774. doi: 10.1165/rcmb.2005-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan Y, Basu S, Lieschke GJ, Grail D, Dunn AR, Cheers C. Functional deficiencies of peritoneal cells from gene-targeted mice lacking G-CSF or GM-CSF. J Leukoc Biol. 1999;65:256–264. doi: 10.1002/jlb.65.2.256. [DOI] [PubMed] [Google Scholar]
- 32.Paine R, 3rd, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol. 2000;164:2602–2609. doi: 10.4049/jimmunol.164.5.2602. [DOI] [PubMed] [Google Scholar]
- 33.Riopel J, Tam M, Mohan K, Marino MW, Stevenson MM. Granulocyte-macrophage colony-stimulating factor-deficient mice have impaired resistance to blood-stage malaria. Infect Immun. 2001;69:129–136. doi: 10.1128/IAI.69.1.129-136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, Cooper AM, Orme IM. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 35.Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP. Granulocyte-macrophage-colony-stimulating factor-dependent peritoneal macrophage responses determine survival in experimentally induced peritonitis and sepsis in mice. Shock. 2008;30:434–442. doi: 10.1097/SHK.0b013e3181673543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, 3rd, et al. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- 37.Berclaz PY, Carey B, Fillipi MD, Wernke-Dollries K, Geraci N, Cush S, Richardson T, Kitzmiller J, O'Connor M, Hermoyian C, et al. GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS. Am J Respir Cell Mol Biol. 2007;36:114–121. doi: 10.1165/rcmb.2006-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bozinovski S, Jones J, Beavitt SJ, Cook AD, Hamilton JA, Anderson GP. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L877–L885. doi: 10.1152/ajplung.00275.2003. This report demonstrated that administration of neutralizing GM-CSF autoantibodies modulates inflammatory host responses in a rheostatic fashion in mice. This provided data supporting the feasibility of using GM-CSF autoantibodies therapeutically in serious inflammatory and autoimmune diseases.
- 39.Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol. 2005;25:405–428. doi: 10.1615/critrevimmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- 40. Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. This paper provides an excellent review of the role of GM-CSF autoimmune disorders and of clinical trials aimed at evaluating the use of neutralizing GM-CSF autoantibodies to treat serious inflammatory and autoimmune diseases.
- 41.Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56:364–368. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu WD, Firestein GS, Taetle R, Kaushansky K, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83:876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. This study implicated GM-CSF in experimental allergic encephalitis, suggesting a potential therapeutic application of neutralizing GM-CSF autoantibodies to treat individuals with multiple sclerosis.
- 44.Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol. 2002;13:350–358. doi: 10.1681/ASN.V132350. [DOI] [PubMed] [Google Scholar]
- 45.Alderuccio F, Biondo M, Toh BH. Organ-specific autoimmunity in granulocyte macrophage-colony stimulating factor (GM-CSF) deficient mice. Autoimmunity. 2002;35:67–73. doi: 10.1080/08916930290005954. [DOI] [PubMed] [Google Scholar]
- 46.Frossard JL, Saluja AK, Mach N, Lee HS, Bhagat L, Hadenque A, Rubbia-Brandt L, Dranoff G, Steer ML. In vivo evidence for the role of GM-CSF as a mediator in acute pancreatitis-associated lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L541–L548. doi: 10.1152/ajplung.00413.2001. [DOI] [PubMed] [Google Scholar]
- 47.Iyonaga K, Suga M, Yamamoto T, Ichiyasu H, Miyakawa H, Ando M. Elevated bronchoalveolar concentrations of MCP-1 in patients with pulmonary alveolar proteinosis. Eur Respir J. 1999;14:383–389. doi: 10.1034/j.1399-3003.1999.14b24.x. [DOI] [PubMed] [Google Scholar]
- 48.Golde DW, Territo M, Finley TN, Cline MJ. Defective lung macrophages in pulmonary alveolar proteinosis. Ann Intern Med. 1976;85:304–309. doi: 10.7326/0003-4819-85-3-304. [DOI] [PubMed] [Google Scholar]
- 49.Tazawa R, Hamano E, Arai T, Ohta H, Ishimoto O, Uchida K, Watanabe M, Saito J, Takeshita M, Hirabayashi Y, et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2005;171:1142–1149. doi: 10.1164/rccm.200406-716OC. [DOI] [PubMed] [Google Scholar]
- 50. Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. This groundbreaking report identified the critical role of GM-CSF in surfactant homeostasis and alveolar macrophage function.