Abstract
Delta wave deficits during sleep have been observed in patients with schizophrenia. Decreased slow-wave sleep is reported to be associated with negative symptoms. Frontal lobe dysfunction is also believed to underlie negative symptoms of schizophrenia. This study was designed to identify functional abnormalities in schizophrenia manifested on patients' electroencephalograms. Polysomnograph examinations were performed in 12 healthy male volunteers and 11 male outpatients with schizophrenia. We investigated the laterality of frontal cortical delta waves in patients with schizophrenia and in healthy control subjects. Laterality of frontal cortex delta wave counts during all-night sleep was investigated by computer analysis. Total delta wave counts were lower in patients with schizophrenia than in control subjects. Control subjects showed significantly higher delta wave counts in the right frontal cortex than in the left. This asymmetry was not observed in patients with schizophrenia. These findings suggest that reduced right frontal delta wave dominance is involved in the pathophysiology of schizophrenia.
Keywords: schizophrenia, polysomnogram, negative symptom, laterality, pathophysiology, period-amplitude analysis
Introduction
Traumatically and surgically induced frontal lobe lesions in humans can result in apathy, affective flattening, impoverished speech, and lack of spontaneity.1 Similarities between negative symptoms of schizophrenia and sequelae of frontal lobe lesions have been reported since the beginning of the 20th century, and the negative symptom complex of schizophrenia is believed to reflect frontal lobe dysfunction.2,3
Deficits of delta wave sleep have been reported in patients with schizophrenia.4,5 Based on quantitative electroencephalographic analysis, delta wave count during sleep was significantly less in patients with schizophrenia than in healthy volunteers.6 Negative symptoms have been reported to be associated with decreased slow-wave sleep (SWS) and delta wave count during sleep in patients with schizophrenia.7,8 We have reported a significant inverse correlation between the higher amplitude delta wave count in the central cortical region and negative symptom score.9
A few studies have shown functional frontal lobe laterality at rest in patients with schizophrenia.10 However, few studies have examined lateral asymmetry of brain function, such as that reflected by electroencephalography during sleep in patients with schizophrenia. We reported interhemispheric asymmetry of the delta wave count during all-night sleep in normal subjects.11 In the present study, to identify functional abnormalities in patients with schizophrenia shown on the electroencephalogram (EEG), we compared male outpatients with schizophrenia and healthy male volunteers by all-night polysomnography and investigated delta wave laterality in the frontal cortex.
Methods
Subjects
Twelve healthy, right-handed male volunteers (18–39 years of age; mean age, 30.3 years; SD, 7.4) and 11 male, right-handed, medically treated outpatients with schizophrenia (17–39 years of age; mean age, 30.6 years; SD, 7.8) served as the subjects of this study. All subjects showed right-handedness according to the Annett Hand Preference Test.12 The diagnosis of schizophrenia was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition13 criteria; 8 patients showed disorganized-type schizophrenia, and 3 showed undifferentiated-type schizophrenia. Duration of illness ranged from 3 to 23 years (mean duration, 10.7 ± 6.3 years). Subjects with a history of drug or alcohol abuse or of significant medical or neurologic problems were excluded from the study. Of the 11 patients, 3 were drug naive, and 8 were clinically stable and had been taking a fixed dose of a typical neuroleptic drug (such as chlorpromazine, haloperidol, or levomepromazine) without anticholinergic drugs for more than 8 weeks before entering the study. Neuroleptic drug dosage, converted to that of chlorpromazine according to the method of Davis,14 ranged from 0 to 301 mg/d (mean dosage, 172.4 mg/d). Written informed consent was obtained from all subjects prior to the study.
Experimental Procedure
To exclude the “first-night effect,15” each subject participated in an adaptation night in the sleep laboratory before polysomnography. Disc electrodes were attached to the subject at 2000 hours. Polygraphic recording was initiated at a usual bedtime between 2200 hours and 2300 hours and was discontinued at 0700 hours the next morning.
Polygraphic Recording
Polysomnograms (PSGs) were recorded according to the method of Rechtscahaffen and Kales.16 Polygraphic recordings included an EEG, an electro-oculogram (EOG), and a submental muscle electromyogram (EMG). EEGs were recorded from disc electrodes placed at bilateral frontal (F3, F4) and central (C3) (10–20 electrode system) sites with the use of A1 and A2 as reference sites. Monopolar EOGs were recorded from both canthi, and bipolar EMGs were recorded from the chin. EEGs and EOGs were recorded with a time constant of 0.3 seconds to allow for measurement of electroencephalographic delta components, sensitivity of 10 μV/mm, and a high-cut filter of 120Hz; Electromyographic conditions were 0.003 seconds, 3.5 μV/mm, and 500 Hz, respectively. Electrical impedances were kept below 3 kΩ. PSGs were recorded on paper as well as by analog tape recorder for further computerized analysis. All sleep was scored visually on the C3 EEG for each 20-second time-code PSGs delimited epoch according to Rechtscahaffen and Kales.16 Polygraphic data were scored by trained raters blinded to diagnosis by removal of subjects' names. The sleep variables monitored in this study were total sleep time (excluding periods of waking and movement), sleep latency (latency of initial stage 2), rapid eye movement (REM) latency, and percentage of each sleep stage relative to the total sleep time (% stage).
Delta Half-Wave Analysis
Period-amplitude analysis was performed by the zero-crossing method using the Medilog Sleep Analyzing Computer (SAC: DEE-1100, Oxford Instruments, Oxford, UK).17 The delta wave count from the beginning of sleep (initial stage 2) to the end of sleep (the last awakening), exception of awake times of more than 3 minutes after sleep onset, was analyzed as the half-wave count used for SWS in the SAC sleep staging algorithm (0.5–2.0 Hz ≧31 μV).
Clinical Assessments
Patients were rated on the 18-item Brief Psychiatric Rating Scale (BPRS)18 by the same 2 psychiatrists each time. The psychiatrists rated patients' symptoms independently, and the scores were later determined by mutual agreement. Positive symptom severity was assessed by the sum of the scores of the following 4 BPRS items: conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content. Negative symptoms were assessed by the sum of the scores of the following 3 items: emotional withdrawal, motor retardation, and blunted affect.19
Statistical Analysis
Student t test (2 tailed) was used for statistical analysis of the PSG results (sleep variables). To reduce the possibility of type I errors, we used analysis of variance (ANOVA) with repeated measures for frontal cortex delta half-wave results. This was a 2-way ANOVA for independent groups (patients with schizophrenia and healthy subjects) and repeated measures for hemisphere (right and left). Where group-by-hemisphere interaction was significant, paired t test was used to identify the effect. The asymmetry index (AI) in the frontal region was taken as a (right – left)/(right + left) ratio of delta half waves during all-night sleep. Student t test (2 tailed) was used for statistical analysis of AI results. Spearman rank-order correlation coefficients (2 tailed) were used to test the relation between frontal cortex delta half-wave results and symptom ratings. Data were analyzed with the use of SPSS statistical procedures (SPSS Inc., Chicago, IL).
Results
Sleep Variables
Sleep variables of the healthy volunteers and the patients with schizophrenia are listed in table 1. Patients showed a significant increase in % stage 2 and a marked decrease in the amount of SWS compared with those in the control subjects. There was no significant difference in total sleep time, sleep latency, REM latency, or total awakening time between patients with schizophrenia and control subjects.
Table 1.
Sleep Variables in Control Subjects and Schizophrenia Patients
| Sleep Variable | Control Subjects | Schizophrenia Patients |
| Total sleep time, min | 429.7 ± 22.6 | 423.0 ± 71.1 |
| Sleep latency, min | 17.5 ± 27.6 | 28.1 ± 30.2 |
| REM latency, min | 90.8 ± 41.6 | 129.0 ± 67.4 |
| Total awakening time, min | 16.7 ± 16.0 | 24.2 ± 44.8 |
| Sleep efficiency | 92.7 ± 5.8 | 89.6 ± 14.9 |
| % Stage 1 | 8.5 ± 4.5 | 10.4 ± 5.9 |
| % Stage 2* | 57.4 ± 3.7 | 66.3 ± 8.1 |
| % Stage 3 + 4* | 14.1 ± 5.1 | 6.1 ± 5.7 |
| % Stage REM | 20.1 ± 4.1 | 17.2 ± 4.6 |
Note: REM, rapid eye movement. Each value represents the mean ± SD of the values in subjects. The existence of statistically significant differences between control subjects and patients with schizophrenia is indicated by the asterisks. (*P < .001).
Delta Half-Wave Analysis
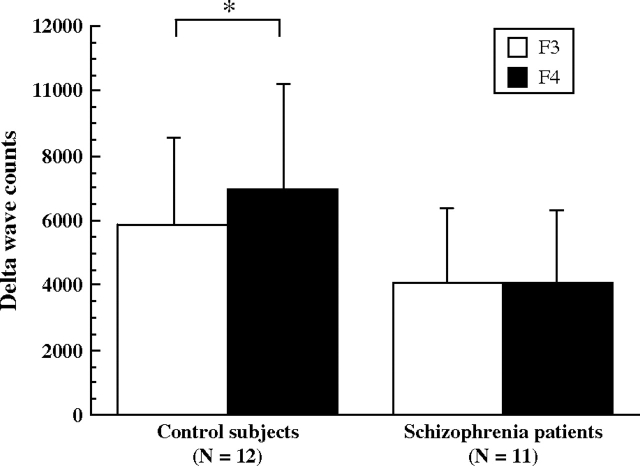
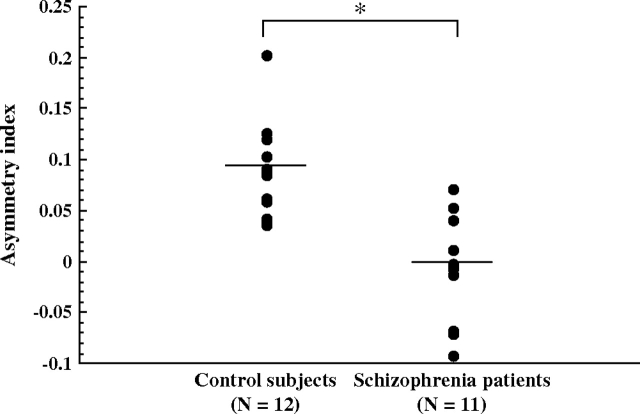
Total delta half-wave counts during all-night sleep in control subjects and in patients with schizophrenia are shown in figure 1. Repeated-measures ANOVA showed schizophrenia to be a significant main effect (F = 4.49, df = 1, 21, P = .04). Patients showed lower total delta half-wave counts during all-night sleep than control subjects. The group-by-hemisphere interaction was significant (F = 17.09, df = 1, 21, P < .001). Control subjects showed significantly higher delta half-wave counts in the right frontal cortical region than in the left. This asymmetry was not identified in patients with schizophrenia. AI results in the frontal cortical region are shown in figure 2. AIs in the control subjects were significantly larger than those in the patients with schizophrenia.
Fig. 1.
Total delta half-wave counts during all-night sleep in control subjects (left) and patients with schizophrenia (right). Statistically significant differences between left (F3) and right (F4) cortical regions is indicated by an asterisk (2-tailed t test. *P < .001).
Fig. 2.
Asymmetry index (AI) of delta wave counts during all-night sleep. Horizontal bars represents means. The AI was taken as (L – R)/(L + R), where L and R were the delta wave counts on the left and right frontal regions, respectively (*P = .008 by Student t test).
Correlation Between BPRS Scores and Delta Wave Counts
Delta half-wave counts in the bilateral frontal cortical regions were inversely correlated with BPRS negative symptom scores (table 2). Delta wave counts in the bilateral frontal cortical regions were not correlated with BPRS total scores or positive symptom scores. AIs of delta wave counts in frontal cortical regions were not related to BPRS total scores, negative symptom scores, or positive symptom scores.
Table 2.
Spearman Rank-Order Correlation Coefficients between Brief Psychiatric Rating Scale positive or negative symptom scores and delta wave counts or asymmetry index in patients with schizophrenia
| Correlation Coefficient | ||||
| Positive Symptom Scores | Negative Symptom Scores | |||
| Spearman | Spearman | |||
| Rho | P< | Rho | P< | |
| Frontal | ||||
| Left | 0.12 | NS | –0.86 | 0.02 |
| Right | 0.01 | NS | –0.77 | 0.04 |
| AI | 0.12 | NS | –0.16 | NS |
Note: NS, not significant.
Discussion
We found that patients with schizophrenia showed lower total frontal cortex delta wave counts (an indicator of SWS) during all-night sleep than did control subjects and that control subjects showed a right-dominant asymmetry of delta wave count, which was reduced in patients with schizophrenia. Several studies of cerebral blood flow and glucose metabolism by positron emission tomography have reported decreased functional activity in the frontal lobes in patients with schizophrenia.20,21 Patients with schizophrenia show a left-dominant asymmetry of glucose metabolism in the medial frontal cortex at rest that is virtually absent in control subjects.10 Patients with predominantly negative symptoms show a lower rate of glucose metabolism in the right hemisphere, particularly in the ventral prefrontal and temporal cortices, compared with patients with predominantly positive symptoms.22 Patients with deficit syndrome show significantly lower regional cerebral blood flow perfusion ratios in the right superior and inferior frontal cortices than did patients without deficit syndrome.23 Wolkin et al24 identified a close relation between negative symptoms and prefrontal hypometabolism, particularly in the right dorsolateral convexity.
It has been suggested that age-related changes in delta wave amplitude during sleep correlate closely with the rate of glucose metabolism in the cerebral cortex during wakefulness; the greater the metabolic rate during wakefulness, the higher the delta wave amplitude.25 Given these previous results, it is reasonable for us to consider that a decrease in the frontal cortex delta wave count during all-night sleep in our patients may be associated with hypometabolism of frontal cortical regions, and the absence of right delta wave count dominance in our patients may be associated with hypometabolism of the right frontal cortical region. Underactivation of the right frontal cortex may also be involved.
Significant inverse correlations were observed between BPRS negative symptom scores and delta wave counts in the bilateral frontal regions. Several studies have shown that decreased SWS is associated with negative symptoms, larger lateral ventricles, and impaired attention in schizophrenia.26–28 Delta wave activity during SWS was inversely related to the magnitude of the negative symptom; the worse the type 2 schizophrenia, the lower the delta count.7 These findings are in good agreement with the results of the present study.
Although AIs of frontal delta wave counts in patients with schizophrenia differed significantly from those in control subjects, AIs were unrelated to positive or negative BPRS scores in the present study. Laterality theories have been prominent in hypotheses of the pathophysiology of psychotic symptoms.29 Gruzelier and Manchanda30 hypothesized that the balance of left-right cortical activity determines positive and negative symptoms. Changes of interhemispheric EEG power asymmetry in the theta and delta bands have been associated with improvements of affective symptoms such as hostility-suspiciousness.31 However, there are few data regarding laterality of frontal cortical function and negative symptoms. A significantly greater correlation between negative symptoms and right dorsolateral frontal metabolism than between negative symptoms and contralateral regions of interest has been reported.24 Although there was no significant correlation between negative symptoms and laterality of frontal delta wave counts in the present study, it is of note that the ability to express affect is believed to be localized in the right hemisphere.32
Eight patients in the present study took typical neuroleptics throughout the study, and 3 patients were drug naive. There are no reports addressing the role of neuroleptics on delta wave laterality during sleep. It is possible that neuroleptics were a confounding factor in the present study. Neuroleptics affect delta power in spectral electroencephalographic analysis.33 Therefore, delta wave counts during sleep may have been affected by neuroleptics. In addition, medication with typical antipsychotics may have increased negative symptom scores secondary to medication. For this reason, we investigated whether typical antipsychotics affected delta wave counts, AIs of delta wave counts, and negative symptoms. However, no significant correlation was found between typical antipsychotic dosage and the delta wave count, AIs of delta wave counts, or BPRS negative symptom scores. Furthermore, the 3 drug-naive patients showed lower delta counts than did control subjects as well as a left-dominant asymmetry in frontal cortex delta wave counts. These findings support the concept that absence of right frontal cortical dominance of delta wave counts during sleep is associated with the pathophysiology of schizophrenia. Future studies will investigate the relation between interhemispheric asymmetry and regional differences in delta waves and clinical symptoms in larger number of drug-naive patients with schizophrenia.
References
- 1.Fuster JM. The Prefrontal Cortex. New York, NY: Raven Press; 1980. [Google Scholar]
- 2.Andreasen NC, Olsen S. Negative vs positive schizophrenia. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 3.Levin S. Frontal lobe dysfunction in schizophrenia, II: impairments of psychological and brain functions. J Psychiatr Res. 1984;18:57–72. doi: 10.1016/0022-3956(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg I, Braun M, Koresko RL, Gottlieb F. Stage 4 sleep in schizophrenia. Arch Gen Psychiatry. 1969;21:262–266. doi: 10.1001/archpsyc.1969.01740210006002. [DOI] [PubMed] [Google Scholar]
- 5.Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naïve patients with schizophrenia. Schizophr Res. 2003;62:147–153. doi: 10.1016/s0920-9964(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 6.Keshavan MS, Reynolds CF, III, Miewald MJ, et al. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55:443–448. doi: 10.1001/archpsyc.55.5.443. [DOI] [PubMed] [Google Scholar]
- 7.Ganguli R, Reynolds CF, Kupfer DJ. Electroencephalographic sleep in young, never-medicated schizophrenics: a comparison with delusional and nondelusional depressives and with healthy controls. Arch Gen Psychiatry. 1987;44:36–44. doi: 10.1001/archpsyc.1987.01800130038006. [DOI] [PubMed] [Google Scholar]
- 8.Keshavan MS, Miewald J, Haas G, Sweeney J, Ganguli R, Reynolds CF. Slow-wave sleep and symptomatology in schizophrenia and related psychotic disorders. J Psychiatry Res. 1995;29:303–314. doi: 10.1016/0022-3956(95)00023-x. [DOI] [PubMed] [Google Scholar]
- 9.Kajimura N, Kato M, Okuma T, Sekimoto M, Watanabe T, Takahashi K. Relationship between delta activity during all-night sleep and negative symptoms in schizophrenia: a preliminary study. Biol Psychiatry. 1996;39:451–454. doi: 10.1016/0006-3223(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 10.Siegel BJ, Buchsbaum MS, Bunney WJ, et al. Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. Am J Psychiatry. 1993;150:1325–1336. doi: 10.1176/ajp.150.9.1325. [DOI] [PubMed] [Google Scholar]
- 11.Sekimoto M, Kato M, Kajimura N, Watanabe T, Takahashi K, Okuma T. Asymmetric interhemispheric delta waves during all-night sleep in humans. Clin Neurophysiol. 2000;111:924–928. doi: 10.1016/s1388-2457(00)00258-3. [DOI] [PubMed] [Google Scholar]
- 12.Annett M. A classification of hand preferences by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 14.Davis JM. Organic therapies: antipsychotic drugs. In: Freedman AM, Kaplan HI, Sadock BJ, editors. Comprehensive Textbook of Psychiatry. Baltimore, Md: Williams Wilkins; 1980. pp. 2257–2289. [Google Scholar]
- 15.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 16.Rechtscahaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: Government Printing Office; 1968. [Google Scholar]
- 17.Smith JR, Karakan I, Yang M. Ontogeny of delta activity during human sleep. Electroencephogr Clin Neurophysiol. 1977;43:229–237. doi: 10.1016/0013-4694(77)90130-4. [DOI] [PubMed] [Google Scholar]
- 18.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 19.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Department of Health, Education and Welfare; 1976. [Google Scholar]
- 20.Buchsbaum MS, Ingvar DH, Kessler R, et al. Cerebral glucography with positron tomography. Arch Gen Psychiatry. 1982;39:251–259. doi: 10.1001/archpsyc.1982.04290030001001. [DOI] [PubMed] [Google Scholar]
- 21.Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RSJ. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;60:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Potkin SG, Alva G, Fleming K, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am J Psychiatry. 2002;159:227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- 23.Gonul AS, Kula M, Tutus A, Sofuoglu S. A Tc-99m HMPAO SPECT study of regional cerebral blood flow in drug-free schizophrenic patients with deficit and non-deficit syndrome. Psychiatry Res. 2003;123:199–205. doi: 10.1016/s0925-4927(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 24.Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J. Negative symptoms and hypofrontality in chronic schizophrenia. Arch Gen Psychiatry. 1992;49:959–965. doi: 10.1001/archpsyc.1992.01820120047007. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–161. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 26.van Kammen DP, van Kammen WB, Peters J, Goetz K, Neylan T. Decreased slow wave sleep and enlarged lateral ventricles in schizophrenia. Neuropsychopharmacology. 1988;1:265–271. [PubMed] [Google Scholar]
- 27.Tandon R, Shipley JE, Eiser AS, Greden JF. Association between abnormal REM sleep and negative symptoms in schizophrenia. Psychiatry Res. 1989;27:359–361. doi: 10.1016/0165-1781(89)90151-0. [DOI] [PubMed] [Google Scholar]
- 28.Orzack MH, Hartmann EL, Kornetsky C. The relationship between attention and slow wave sleep in chronic schizophrenia. Psychopharmacol Bull. 1977;13:59–61. [PubMed] [Google Scholar]
- 29.Flor-Henry P. Laterality, shifts of cerebral dominance, and psychosis. In: Gruzelier J, Flor-Henry P, editors. Hemispheric Asymmetries of Function in Psychopathology. Amsterdam, The Netherlands: Elsevier Science Publishers; 1979. [Google Scholar]
- 30.Gruzelier JH, Manchanda R. The syndrome of schizophrenia: relation between electrodermal responses, lateral asymmetries and clinical ratings. Br J Psychiatry. 1982;14:488–495. doi: 10.1192/bjp.141.5.488. [DOI] [PubMed] [Google Scholar]
- 31.Czobor P, Volavka J. Quantitative electroencephalogram examination of effects of risperidone in schizophrenic patients. J Clin Psychopharmacol. 1993;13:332–342. [PubMed] [Google Scholar]
- 32.Cutting J. The Right Cerebral Hemisphere and Psychiatric Disorders. Oxford, England: Oxford University Press; 1990. [Google Scholar]
- 33.Itil TM. Quantitative pharmaco-electroencephalography. In: Itil TM, editor. Modern Problems in Pharmacopsychiarty. Basel, Switzerland: Karger; 1974. pp. 43–57. [Google Scholar]