Background
Antipsychotic medication is a mainstay of treatment for schizophrenia. Risperidone and olanzapine are popular choices among the new generation drugs.
Objectives
To determine the clinical effects, safety and cost effectiveness of risperidone were compared with those of olanzapine for treating schizophrenia.
Search Strategy
We searched the Cochrane Schizophrenia Group's Register (September 2005), and references of all identified studies were inspected for further trials. We also contacted relevant pharmaceutical companies for additional information.
Selection Criteria
We included all clinical randomized trials comparing risperidone with olanzapine for schizophrenia and schizophrenia-like psychoses.
Data Collection and Analysis
We extracted data independently. For homogenous dichotomous data, we calculated random effects, relative risk (RR), 95% confidence intervals (CIs), and, where appropriate, numbers needed to treat/harm (NNT/H) on an intention-to-treat basis. For continuous data, we calculated weighted mean differences.
Main Results
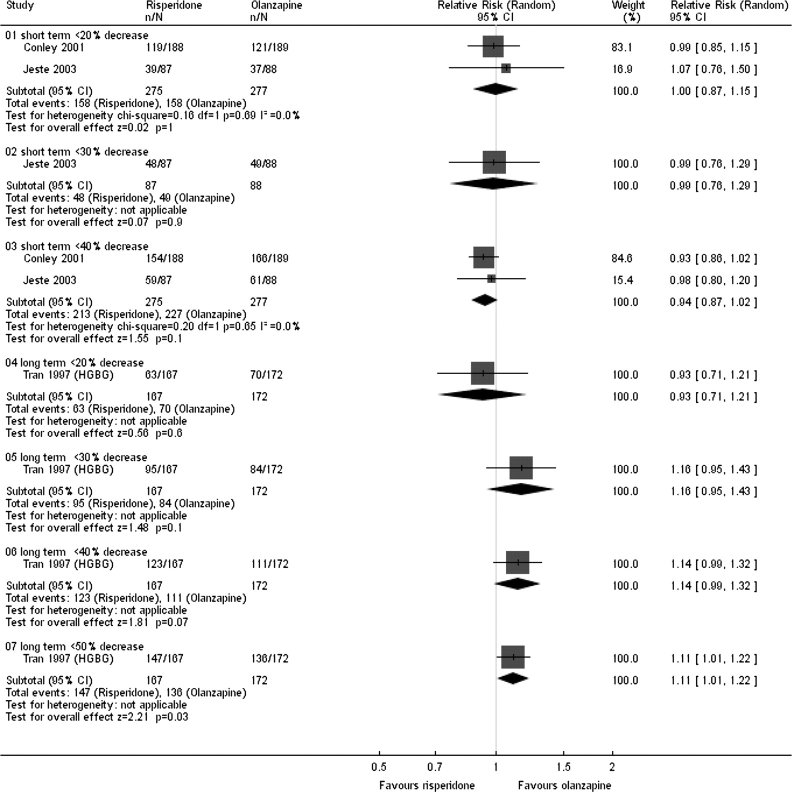
We found no difference for the outcome of unchanged or worse in the short term (n = 548, 2 randomized control trial (RCTs), RR = 1.00, 95% CI = 0.88 to 1.15). One study favored olanzapine for the outcome of relapse/rehospitalization by 12 months (n = 279, 1 RCT, RR = 2.16, 95% CI = 1.31 to 3.54, NNH = 7, 95% CI = 3 to 25). Most mental state data showed the 2 drugs to be as effective as each other (n = 552, 2 RCTs, RR ‘no <20% decrease Positive and Negative Syndrome Scale by 8 weeks’ 1.00, 95% CI = 0.87 to 1.15) (Figure 1). Both drugs commonly cause adverse events: 75% given either drug experienced an adverse event; 20% experienced anticholinergic symptoms; both groups experienced insomnia although it was more frequent with risperidone (n = 1588, 5 RCTs, RR = 1.41, 95% CI = 1.15 to 1.72, NNH = 15, 95% CI = 9 to 41); and about 30% experienced sleepiness (n = 1713, 6 RCTs, RR = 0.92, 95% CI = 0.79 to 1.07). People given either drug often experienced extrapyramidal symptoms (n = 893, 3 RCTs, RR = 1.18, 95% CI = 0.75 to 1.88); 25% of people using risperidone and 18% of people using olanzapine required medication to alleviate these symptoms (n = 419, 2 RCTs, RR = 1.76, 95% CI = 1.25 to 2.48, NNH = 8, 95% CI = 4 to 25). People allocated to risperidone (13%) were less likely to gain more than 7% of their baseline weight compared with those given olanzapine (28%), and the weight gain was often considerable and of quick onset (n = 984, 2 RCTs, RR gain more than 7% of their baseline weight in the short term 0.47 95% CI = 0.36 to 0.61, NNH = 7, 95% CI = 6 to 10). Risperidone participants were less likely to leave the study due to metabolic side effects and weight gain compared with olanzapine participants (n = 667, 1 RCT, RR = 0.19, 95% CI = 0.08 to 0.45). Patients on risperidone were more likely to experience abnormal ejaculation (n = 370, 2 RCTs, RR = 4.36, 95% CI = 1.38 to 13.76, NNH = 20, 95% CI = 6 to 176). Both drugs are associated with high attrition rates; in the long term, 66% of those allocated risperidone left the study early compared with 56% given olanzapine (n = 1440, 5 RCTs, RR = 1.17, 95% CI = 1.08 to 1.27, NNH = 11, 95% CI = 7 to 23) (Figure 2).
Conclusions
We know very little of the effects of these drugs regarding service outcomes, general functioning and behaviors, engagement with services, and treatment satisfaction from randomized trials. In the studies, attrition rates are high and there appears to be little to differentiate between risperidone and olanzapine except on issues of their different but frequent, unpleasant adverse effects. The full version of this review is available on the Cochrane Library.1
Fig. 1.
No specific degree of change in mental state (as defined by Positive and Negative Syndrome Scale total).
Fig. 2.
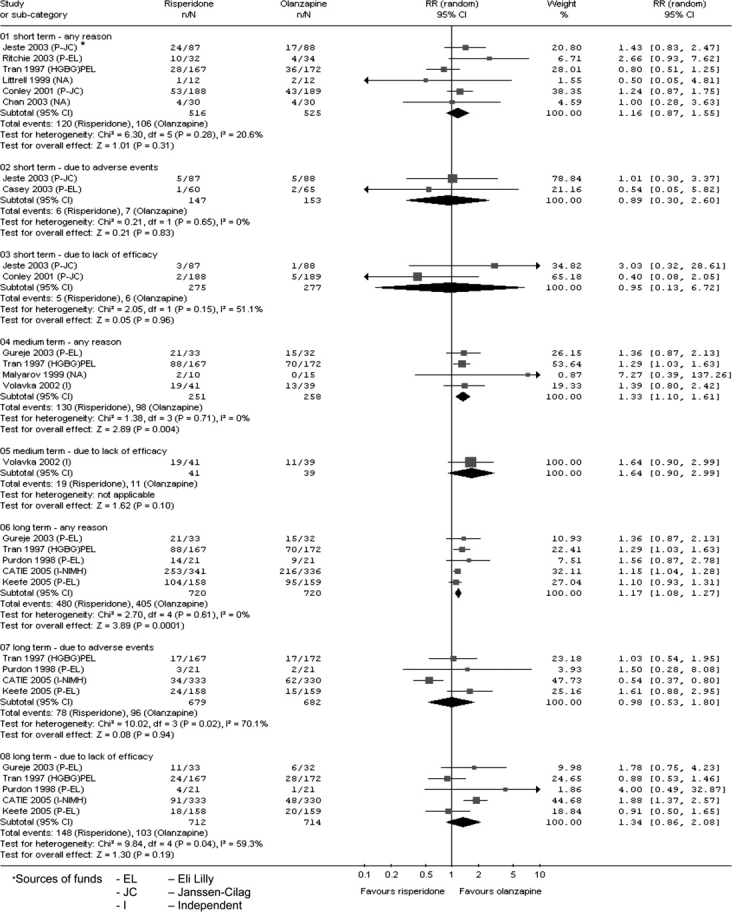
Leaving the study early—for a variety of reasons.
Acknowledgments
Jayaram and Hosalli have attended meetings sponsored by Janssen and Eli Lilly. Stroup has consulted for Janssen and Eli Lilly and has received research funding from Eli Lilly. He was a coprincipal investigator in the Clinical Antipsychotic Trials in Intervention Effectiveness 2005 study.
References
- Jayaram MB, Hosalli P, Stroup S. Risperidone versus olanzapine for schizophrenia. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD005237.pub2. CD005237. [DOI] [PubMed] [Google Scholar]