Abstract
Antipsychotic drugs are thought to exert their therapeutic action by antagonizing dopamine receptors but are also known to produce side effects in the heart by inhibiting cardiac ether-a-go-go–related gene (ERG) K+ channels. Recently, it has been discovered that the same channels are present in the brain, including midbrain dopamine neurons. ERG channels are most active after the cessation of intense electrical activity, and blockade of these channels prolongs plateau potentials in bursting dopamine neurons. This change in excitability would be expected to alter dopamine release. Therefore, the therapeutic action of antipsychotic drugs may depend on inhibition of both postsynaptic dopamine receptors and presynaptic ERG K+ channels.
Keywords: schizophrenia, bursting, dopamine, antipsychotic drugs, review
Introduction
Efforts to identify the pharmacological basis for the therapeutic actions of antipsychotic drugs have focused primarily on the interaction of these compounds with G-protein–coupled receptors. Early observations that antipsychotics block dopamine receptors in proportion to their clinical potency contributed significantly to formulation of the dopamine hypothesis of schizophrenia.1,2 Subsequent findings showing that many second-generation antipsychotic drugs potently block serotonin receptors gave rise to the notion that these sites are somehow involved in conferring the atypical properties of these drugs.3 In both instances, the existence of a distinct pharmacological action (eg, dopamine or serotonin receptor blockade), shared by a group of compounds with disparate chemical structures, provided important clues as to how these drugs might exert their therapeutic effects in individuals with schizophrenia.
Recent clinical and experimental evidence suggests that members of the phenothiazine, butyrophenone, dibenzazepine, and benzamide classes of antipsychotics as well as other structurally heterogeneously neuroleptic drugs share another common pharmacological property—specifically, the ability to block ether-a-go-go–related gene (ERG) potassium channels.4 ERG K+ channels (also referred to as Kv11 channels following International Union of Pure and Applied Chemistry nomenclature) belong to a superfamily of voltage-activated K+ channels encoded by 3 distinct gene subfamilies, including ether-a-go-go (eag), ether-a-go-go-like (elk), and ether-a-go-go–related (erg) genes.5 The first member of the family to be identified was discovered in a Drosophila melanogaster mutant and named for the characteristic leg-shaking behavior exhibited when the flies were anesthetized with ether.6 Several years later, a human homolog of eag was identified from low-stringency screening of a human hippocampal cDNA library and subsequently localized to chromosome 7.5 Designated the human-eag–related gene, herg (or KCNH2) shares less than 50% sequence homology with other eag genes and is thus considered to represent a distinct subfamily in humans. Erg genes resembling herg have been identified in mice (merg) and rats (rerg) and the channels they encode share a common set of functional properties that set them apart from other EAG K+ channels. Accordingly, these genes are referred to simply as erg throughout the remainder of the text.
Biophysical Properties of ERG K+ Channels
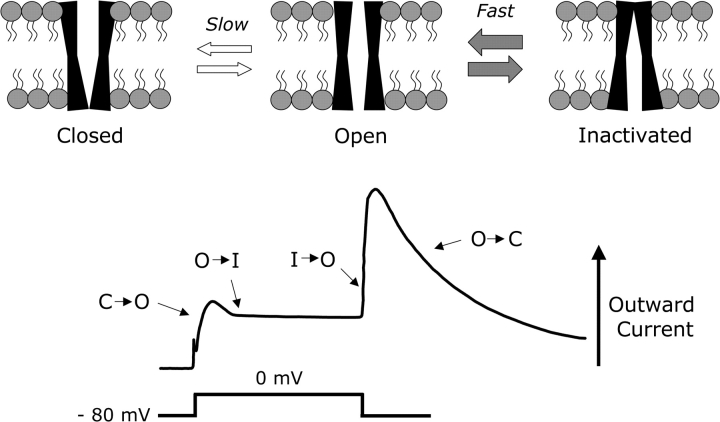
ERG K+ channels, like other ion channels, are macromolecular protein complexes that gate the flow of ions, in this case K+, across cell membranes. Functional ERG channels are comprised of 4 alpha subunits, each consisting of a protein comprised of 6 transmembrane-spanning domains (S1–S6). The first 4 domains of each subunit (S1–S4) comprise the voltage sensor which controls channel gating, while S5 and S6 collectively form the pore region of the channel.7 Voltage-dependent changes in the conformation of the protein complex give rise to 3 conductance states: closed (nonconductive), open (conductive), and inactivated (nonconductive) (see figure 1). ERG channels, which are closed at hyperpolarized membrane potentials, open slowly in response to membrane depolarization but inactivate so quickly that very little outward K+ current flows at the peak of the action potential. As the membrane potential begins to repolarize, the channels rapidly recover from inactivation and must once again enter an open (conductive) state prior to closing. Because the rate of recovery from inactivation greatly exceeds the rate at which the channels deactivate (ie, reenter a close state), a large albeit transient “resurgent” current is generated as the membrane potential repolarizes. Thus, ERG channels act as strong inward rectifiers, preferentially conducting outward currents at relatively hyperpolarized membrane potentials. However, unlike classical inward rectifiers, they must be initially induced to open with depolarization. During repeated spiking or prolonged depolarization, the sum of the fraction of channels in the activated and inactivated states can summate temporally,8,9 enhancing the inward rectification effect. These properties ensure that ERG channels are most active at the cessation of intense activity.
Fig. 1.
Voltage-Dependent Characteristics of ether-a-go-go–Related Gene (ERG) K+ Channels. Upper Panel: Cartoon schematic illustrating the 3 conductance states of ERG K+ channels. Lower Panel: Macroscopic current corresponding to the conductance states as illustrated in the upper panel. At hyperpolarized membrane potentials, ERG channels are closed (C). Depolarizing the membrane potential from −80 to 0 mV (lower trace) slowly opens the channels (C→O) resulting in an initial outward current as K+ ions diffuse out of the cell. However, ERG channels rapidly inactivate (O→I) by entering a second nonconductive state that limits the outward current produced by the initial depolarization. Partial repolarization of the membrane potential induces a rapid transition from an inactive to an open conformation (I→O). Because the rate of deinactivation (I→O) exceeds the rate at which the channels can close, a large resurgent current is generated as the membrane potential repolarizes.
hERG Channels in the Heart
The discovery and characterization of a K+ current with the biophysical properties described above preceded identification of the gene that we now know encodes the channel.10,11 Previously designated IKr, the function of this channel is best understood in the heart where it plays a prominent role in repolarization of cardiac action potentials.7,12 In mammalian heart, native hERG K+ channels are comprised of 2 erg-1 transcripts (ERG1a and ERG1b) that differ slightly in their amino acid composition and kinetic properties.13–15 The unique gating characteristics of hERG K+ channels, including their strong inward rectification, limit the amount of outward current occurring during the initial phase of the cardiac action potential which supports the development of the plateau phase and allows adequate time for Ca2+ entry and proper excitation contraction coupling. On the other hand, the ability of the channels to rapidly recover from inactivation, yet deactivate slowly, results in a large outward current that, together with other voltage-dependent K+ channels, leads to rapid repolarization of the cardiac action potential. The role of hERG K+ channels in normal cardiac function was revealed in studies showing that missense mutations in herg are responsible for long QT syndrome (LQTS), a cardiac repolarization disorder that predisposes affected individuals to life-threatening ventricular arrhythmias such as torsade de pointes.16 (The QT interval of the electrocardiogram is a measure of cardiac action potential duration and thus depends on hERG channel function.) Inherited LQTS can be caused by nearly 200 different mutations in herg, many of which appear to interfere with the normal trafficking of the channel to the cell membrane.7,17
hERG K+ Channels and Antipsychotic Drugs
QT prolongation and an increased risk of torsade de pointes are potential side effects of drugs that block hERG K+ channels. Terfenadine (seldane) and cisapride (propulsid) were both withdrawn from the market because of their propensity to cause fatal arrhythmias particularly when combined with other drugs that decrease cardiac repolarization reserve. A large number of first- and second-generation antipsychotic drugs also potently block hERG K+ channels in vitro18–27 (table 1). Blockade is voltage dependent, suggesting that the binding of these drugs to the channel occurs in the open or inactivated state.21,22,24 Sertindole and pimozide, both potent D2 receptor blockers (Ki ∼ 1.2 and 0.7 nM, respectively), exhibit high affinity for hERG K+ channels expressed in Chinese Hamster ovary (CHO) cells (IC50 3 and 18 nM, respectively). Both drugs are known to prolong QT interval,28,29 although only sertindole was regarded to pose sufficient threat to warrant its temporary removal from the market. Clozapine, ziprasidone, quetiapine, and thioridizine also block hERG K+ channels with nanomolar to low micromolar affinity although they are more potent as D2 dopamine receptor antagonists (table 1). Of these drugs, thioridizine has been shown to exert a disproportionate effect on QT prolongation and as a result carries a black box warning.30 Despite its favorable hERG to D2 receptor affinity ratio, chlorpromazine has been reported to prolong QT interval and increase the risk of ventricular arrhythmia.31 The absence of a direct correlation between the affinity of an antipsychotic drug for hERG K+ channels and its liability for producing QT prolongation is incompletely understood but likely to involve factors such as drug-protein interactions,19 drug metabolism,32 and the degree to which individual drugs accumulate in myocardium.33 While there are clear differences in the propensity of individual antipsychotics to cause LQTS,34 it has also been difficult to establish a causal relationship between drug-induced QT prolongation and torsade de pointes or sudden cardiac death.35 Approximately 8%–10% of individuals treated with atypical antipsychotic drugs exhibit clinical evidence of QT prolongation, while the incidence of arrhythmogenic disorders in the same population occurs with a frequency of only 1 in 10 000 individuals.30,34 Deaths attributed to drug-induced torsade de pointes amount to less than 1 in 100 000 patients.30 With the possible exception of thioridizine, most instances of antipsychotic drug-induced torsade de pointes are associated with high dosing regimens, intentional drug overdose, synergistic drug interactions, or other predisposing factors, including diabetes, hypokalemia, or cardiac ischemia.4,30,35,36
Table 1.
Comparative Affinity of Antipsychotic Drugs for Dopamine D2 Receptors and hERG K+ Channels
| Drug | D2 Receptora, Ki nmol/l | hERG K+ Channel,b IC50, nmol/l | Relative Potency, hERG IC50/D2 Ki |
| Pimozide | 0.7c | 18d–55e | 26–78 |
| Haloperidol | 0.7f | 27e–1000g | 38–1429 |
| Sertindole | 1.2f | 3h,i–15e | 2–12 |
| Chlorpromazine | 1.3f | 21 600j | 16 615 |
| Risperidone | 1.7c | 148e–167h | 87–98 |
| Thioridizine | 2.3c | 33e–191h | 14–83 |
| Olanzapine | 6.4f | 231e–6013h | 36–940 |
| Ziprasidone | 8.4k | 125e–169h | 15–20 |
| Clozapine | 82f | 320e–2500l | 4–30 |
| Quetiapine | 155f | 5765h | 37 |
[3H]-raclopride or [3H]-spiroperidol.
Displacement from cloned human dopamine D2 receptors, hERG channel current.
Malmberg et al. (1993).25
CHO cells, Kang et al.22
HEK293 cells, Ekins et al.18
Kapur and Seeman (2000).26
Xenopus oocytes, Suessbrich et al.21
CHO cells, Kongsamut et al.19
HEK293 cells, Rampe et al.24
Xenopus oocytes, Thomas et al.20
Seeger et al. (1995).27
HEK293 cells, Lee et al.23
hERG K+ Channels in the Brain
In addition to being ubiquitously distributed in cardiac tissue, the gene encoding Kv11.1 K+ channels in the heart (erg-1) is also strongly expressed in mammalian brain.37 Two additional erg genes (erg-2 and erg-3) and their respective K+ channels (Kv11.2 and Kv11.3) have recently been identified and shown to be distributed exclusively within the central nervous system (CNS).38 Individual members of the erg family are expressed in different levels and patterns throughout the CNS. mRNA encoding erg-1, erg-2, and erg-3 is distributed in the cerebral cortex, hippocampus, reticular nucleus of the thalamus, paraventricular nucleus of the hypothalamus, cerebellum, and several brain stem nuclei.39,40 Moderate to high levels of the erg-1 transcript and all 3 ERG proteins are also expressed in neurons within the pars compacta of the substantia nigra.41 In most regions, the level of expression of erg-1 and erg-3 exceeds that of erg-2. While all 3 transcripts appear to be weakly expressed in pyramidal neurons, in the rat, erg-1 is expressed alone and in high levels in parvalbumin-labeled interneurons in the cingulate and retrosplenial cortex, selective interneuronal populations in the hippocampus, and in aspiny interneurons in the caudate.39,40 The spatial distribution of ERG K+ channels in the CNS generally parallels the distribution of erg transcripts.41 Notably, ERG proteins are not only expressed in neuronal cell bodies but also in dendritic and axonal compartments,41 and the high degree of overlap in the expression of erg-1, erg-2, and erg-3 mRNA in the mammalian brain suggests that native channels are likely to be heteromultimeric. This is an important consideration given that the kinetic properties of Kv11.3 channels are appreciably different from those associated with channels encoded by erg-1 and erg-2.38
The contribution of native ERG K+ current (IERG) to the intrinsic electrical properties of CNS neurons that express the conductance is for the most part poorly understood. One exception is the cerebellar cortex, where Purkinje cells have been shown to exhibit an inwardly rectifying K+ current with the biophysical and pharmacological characteristics of an ERG channel.9 IERG in these neurons is characterized by slow deactivation, a fast recovery from inactivation, and is potently suppressed by the ERG-selective blocker WAY-123,398. Blockade of IERG in Purkinje cells increased neuronal excitability and suppressed spike frequency adaptation without altering the duration of individual action potentials. Loss of IERG also prolonged the duration of complex spikes elicited by activation of climbing fiber inputs. These data support the proposition that pharmacological blockade of IERG in the CNS could result in a change in neuronal excitability that would alter synaptic integration.
Antipsychotic Drugs and IERG in the CNS: Therapeutic Implications
The discovery of ERG K+ channels in CNS neurons together with preliminary evidence that they can influence neuronal excitability raises the intriguing question of whether blockade of these channels by antipsychotic drugs contributes in some way to their therapeutic actions or neurological side effects. Although the ERG-blocking capabilities of antipsychotic drugs have focused almost exclusively on Kv11.1 (hERG) channels, there is evidence to suggest that these drugs also block additional ERG channel subtypes in the CNS (Kv11.2 and Kv11.3). Cloned human Kv11.3 channels expressed in CHO cells produce an ERG current that is inhibited by sertindole and pimozide at concentrations comparable to their Ki values at D2 dopamine receptors.42 Rispiridone, haloperidol, thioridazine, and clozapine but not metoclopramide block native ERG currents in cloned pituitary GH3 cells.43 Notably, these channels play a prominent role in controlling burst duration and prolactin release from pituitary lactotrophs.44,45 Haloperidol has also been shown to block IERG in cerebellar Purkinje neurons.9
Midbrain dopamine-containing neurons, cells strongly implicated in both the therapeutic and side effects of antipsychotic drugs, also appear to express functional ERG K+ channels that are blocked by these compounds. Using intracellular recording techniques in conjunction with a brain slice preparation, Steen Nedergaard46 identified an ERG-like slow, voltage-activated K+ current responsible for a long-lasting afterhyperpolarization termed AHPs. AHPs in dopamine neurons is calcium independent, activated by prolonged depolarization, and inhibited by local application of low micromolar concentrations of haloperidol. The effects of haloperidol could not be attributed to blockade of dopamine or sigma receptors as neither (−)sulpiride, (+)SKF10047, nor pentazocine had any effect on AHPs. By contrast, terfenadine, a potent blocker of ERG K+ channels, effectively inhibited AHPs in dopamine neurons.
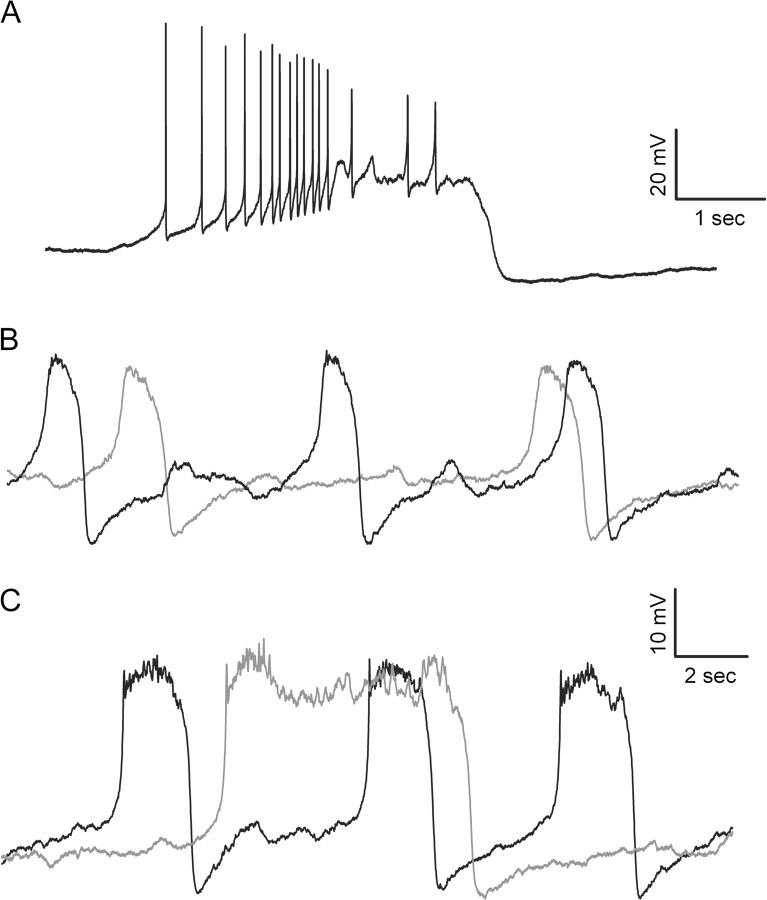
The most prominent physiological effect associated with blockade of the putative ERG current in dopamine neurons was a reduction in the duration of a pause in spontaneous firing that occurs at the end of a train of stimulus-evoked spiking.46 This “poststimulus inhibitory period,” resembles the characteristic pause in spontaneous activity that follows a burst of spikes.47,48 Bursting activity in dopamine neurons has been implicated in a variety of physiological processes, including the encoding of reward, modulation of dopamine release, and the induction of “depolarization block” following chronic administration of antipsychotic drugs.49–51 Although the neurobiological basis of bursting activity in dopamine neurons is not yet understood, it is likely to involve an interaction between afferent inputs and the intrinsic properties of the cell, including the voltage and ligand-gated ion channels expressed in the soma and dendrites of these neurons.52,53 The ability of haloperidol and terfenadine, both potent ERG K+ channel blockers, to reduce the inhibition in activity following episodes of high-frequency firing, suggested that these channels could contribute to the process underlying burst termination. In support of this hypothesis, Canavier et al.8 demonstrated that addition of an ERG K+ conductance to a computational model of oscillatory activity in dopamine neurons faithfully reproduced the time course of membrane potential changes and somatic calcium oscillations observed during plateau potentials induced experimentally by the calcium chelator 1,2-bis(2-aminophenoxy)ethane N,N,N',N'-tetraacetic acid (BAPTA). These data suggested that IERG is capable of terminating plateau potentials in dopamine neurons and implied that the current may be important in the generation of bursting activity by preventing induction of acute depolarization inactivation.8 The predictions of the computational model were tested experimentally by obtaining intracellular recordings from spontaneously active dopamine neurons under conditions similar to those used to simulate plateau potential oscillations in the model. As illustrated in figure 2A, and in accordance with previous studies,54,55 bursting was induced by partially blocking an SK-type Ca2+-activated K+ conductance. The plateau potential oscillations underlying this type of bursting activity are clearly visible when tetrodotoxin is applied to block spiking (figure 2B and C, black traces). Addition of haloperidol, at a concentration previously shown to block the putative IERG in dopamine cells (5 μM), prolonged the plateau potentials exhibited by these neurons in the presence of tetrodotoxin (TTX)8 (figure 2C, gray trace). Comparable results were not observed in response to sulpiride (2 μM, figure 2B, gray trace). At these concentrations, both drugs would have effectively antagonized D2 dopamine receptors; however, only haloperidol would have also reduced IERG activated during the prolonged plateau depolarization.
Fig. 2.
Effect of Haloperidol and Sulpiride on Plateau Potentials Exhibited by Nigral Dopamine-Containing Neurons In Vitro. (A) Intracellular recording obtained from a spontaneously active dopamine neuron in vitro in the presence of the negative SK channel modulator, NS8593.64 Note the “burst” of spikes on the rising phase of a depolarizing plateau potential. Addition of TTX blocks spiking but has no effect on the underlying plateau potential (B and C, black traces). Bath application of haloperidol (C, gray trace) but not sulpiride (B, gray trace) prolongs the duration of plateau potentials recorded in the presence of the SK channel pore blocker, apamin. Modified from Canavier et al.8
While it has yet to be determined whether plateau potentials triggered by blockade of SK channels in vitro are involved in “natural” bursting activity exhibited by dopamine neurons in vivo, plateau properties are importantly involved in regulating the activity of a variety of CNS neurons. The unique kinetic properties of ERG channels including their rapid inactivation and large resurgent outward current would appear to make them uniquely suited for limiting the duration of plateau potentials. It is interesting to note in this regard that many of the CNS neurons expressing high levels of erg transcripts, including subthalamic,56 hypothalamic paraventricular,57 cerebellar Purkinje,58 cortical pyramidal,59 and subicular neurons60 also exhibit regenerative plateau potentials.
In summary, ERG K+ channels exhibit a unique set of biophysical properties that are well suited for their prominent physiological role in repolarizing plateau potentials both in the heart and the brain. First- and second-generation antipsychotic drugs block ERG channels in the heart resulting in LQTS and an increased liability for developing life-threatening arrhythmias. However, the discovery of ERG K+ channels in brain suggests that central actions of antipsychotic drugs could include alterations in the intrinsic electrical properties of neurons expressing this conductance. Indeed, it seems quite possible that partial block of ERG K+ channels in dopamine neurons by antipsychotic drugs could increase neuronal excitability, facilitate bursting activity, and promote the induction of “depolarization inactivation,” a phenomenon that has been implicated in the therapeutic effects of these agents.50,61,62 Given that Kv11.2 and Kv11.3 channels are expressed solely in the CNS, it should be possible to develop centrally active blockers of IERG that are both regionally selective and devoid of significant cardiotoxicity.63 A more complete understanding of the function role played by ERG K+ channels in CNS neurons and the degree to which clinically relevant doses of antipsychotic drugs modify these effects could provide new insights into the therapeutic mechanism of action of these drugs.
Funding
This work was supported by National Institutes of Health grants R01NS037963 (CCC). and by funds provided by the Office of the Dean and the Department of Psychiatry, University of Maryland School of Medicine (PDS).
References
- 1.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 2.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 4.Calderone V, Testai L, Martinotti E, Del Tacca M, Breschi MC. Drug-induced block of cardiac HERG potassium channels and development of torsade de pointes arrhythmias: the case of antipsychotics. J Pharm Pharmacol. 2005;57:151–161. doi: 10.1211/0022357055272. [DOI] [PubMed] [Google Scholar]
- 5.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan WD, Trout WE., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 8.Canavier CC, Oprisan S, Callaway J, Ji H, Shepard PD. Computational model predicts a role for ERG current in repolarizing plateau potentials in dopamine neurons: implications for modulation of neuronal activity. J Neurophysiol. 2007 doi: 10.1152/jn.00422.2007. doi: 10.1152/jn.00422.2007. [DOI] [PubMed] [Google Scholar]
- 9.Sacco T, Bruno A, Wanke E, Tempia F. Functional roles of an ERG current isolated in cerebellar Purkinje neurons. J Neurophysiol. 2003;90:1817–1828. doi: 10.1152/jn.00104.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 11.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 12.Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lees-Miller JP, Kondo C, Wang L, Duff HJ. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ Res. 1997;81:719–726. doi: 10.1161/01.res.81.5.719. [DOI] [PubMed] [Google Scholar]
- 14.Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem. 2004;279:44690–44694. doi: 10.1074/jbc.M408344200. [DOI] [PubMed] [Google Scholar]
- 15.London B, Trudeau MC, Newton KP, et al. Two isoforms of the mouse ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res. 1997;81:870–878. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- 16.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 17.Delisle BP, Anson BD, Rajamani S, January CT. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res. 2004;94:1418–1428. doi: 10.1161/01.RES.0000128561.28701.ea. [DOI] [PubMed] [Google Scholar]
- 18.Ekins S, Crumb WJ, Sarazan RD, Wikel JH, Wrighton SA. Three-dimensional quantitative structure-activity relationship for inhibition of human ether-a-go-go-related gene potassium channel. J Pharmacol Exp Ther. 2002;301:427–434. doi: 10.1124/jpet.301.2.427. [DOI] [PubMed] [Google Scholar]
- 19.Kongsamut S, Kang J, Chen XL, Roehr J, Rampe D. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol. 2002;450:37–41. doi: 10.1016/s0014-2999(02)02074-5. [DOI] [PubMed] [Google Scholar]
- 20.Thomas D, Wu K, Kathofer S, et al. The antipsychotic drug chlorpromazine inhibits HERG potassium channels. Br J Pharmacol. 2003;139:567–574. doi: 10.1038/sj.bjp.0705283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suessbrich H, Schonherr R, Heinemann SH, Attali B, Lang F, Busch AE. The inhibitory effect of the antipsychotic drug haloperidol on HERG potassium channels expressed in Xenopus oocytes. Br J Pharmacol. 1997;120:968–974. doi: 10.1038/sj.bjp.0700989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J, Wang L, Cai F, Rampe D. High affinity blockade of the HERG cardiac K(+) channel by the neuroleptic pimozide. Eur J Pharmacol. 2000;392:137–140. doi: 10.1016/s0014-2999(00)00123-0. [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Kim YJ, Kim KT, Choe H, Jo SH. Blockade of HERG human K+ channels and IKr of guinea-pig cardiomyocytes by the antipsychotic drug clozapine. Br J Pharmacol. 2006;148:499–509. doi: 10.1038/sj.bjp.0706744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rampe D, Murawsky MK, Grau J, Lewis EW. The antipsychotic agent sertindole is a high affinity antagonist of the human cardiac potassium channel HERG. J Pharmacol Exp Ther. 1998;286:788–793. [PubMed] [Google Scholar]
- 25.Malmberg A, Jackson DM, Eriksson A, Mohell N. Unique binding characteristics of antipsychotic agents interacting with human dopamine D2A, D2B, and D3 receptors. Mol Pharmacol. 1993;43:749–754. [PubMed] [Google Scholar]
- 26.Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. Journal of Psychiatry and Neuroscience. 2000;25:161–166. [PMC free article] [PubMed] [Google Scholar]
- 27.Seeger TF, Seymour PA, Schmidt AW, et al. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- 28.Krahenbuhl S, Sauter B, Kupferschmidt H, Krause M, Wyss PA, Meier PJ. Case report: reversible QT prolongation with torsades de pointes in a patient with pimozide intoxication. Am J Med Sci. 1995;309:315–316. doi: 10.1097/00000441-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Mack R, Driscoll R, Silber C. The long term cardiovascularsafety of sertindole. Eur Neuropsychopharmacol. 2002;7:S207. [Google Scholar]
- 30.Titier K, Girodet PO, Verdoux H, et al. Atypical antipsychotics: from potassium channels to torsade de pointes and sudden death. Drug Saf. 2005;28:35–51. doi: 10.2165/00002018-200528010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hoehns JD, Stanford RH, Geraets DR, Skelly KS, Lee HC, Gaul BL. Torsades de pointes associated with chlorpromazine: case report and review of associated ventricular arrhythmias. Pharmacotherapy. 2001;21:871–883. doi: 10.1592/phco.21.9.871.34565. [DOI] [PubMed] [Google Scholar]
- 32.Crumb WJ, Jr, Ekins S, Sarazan RD, et al. Effects of antipsychotic drugs on I(to), I (Na), I (sus), I (K1), and hERG: QT prolongation, structure activity relationship, and network analysis. Pharmacol Res. 2006;23:1133–1143. doi: 10.1007/s11095-006-0070-7. [DOI] [PubMed] [Google Scholar]
- 33.Titier K, Canal M, Deridet E, et al. Determination of myocardium to plasma concentration ratios of five antipsychotic drugs: comparison with their ability to induce arrhythmia and sudden death in clinical practice. Toxicol Appl Pharmacol. 2004;199:52–60. doi: 10.1016/j.taap.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–1052. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 35.Welch R, Chue P. Antipsychotic agents and QT changes. J Psychiatry Neurosci. 2000;25:154–160. [PMC free article] [PubMed] [Google Scholar]
- 36.Warner JP, Barnes TR, Henry JA. Electrocardiographic changes in patients receiving neuroleptic medication. Acta Psychiatr Scand. 1996;93:311–313. doi: 10.1111/j.1600-0447.1996.tb10653.x. [DOI] [PubMed] [Google Scholar]
- 37.Wymore RS, Gintant GA, Wymore RT, Dixon JE, McKinnon D, Cohen IS. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ Res. 1997;80:261–268. doi: 10.1161/01.res.80.2.261. [DOI] [PubMed] [Google Scholar]
- 38.Shi W, Wymore RS, Wang HS, et al. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci. 1997;17:9423–9432. doi: 10.1523/JNEUROSCI.17-24-09423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papa M, Boscia F, Canitano A, et al. Expression pattern of the ether-a-gogo-related (ERG) K+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system. J Comp Neurol. 2003;466:119–135. doi: 10.1002/cne.10886. [DOI] [PubMed] [Google Scholar]
- 40.Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guasti L, Cilia E, Crociani O, et al. Expression pattern of the ether-a-go-go-related (ERG) family proteins in the adult mouse central nervous system: evidence for coassembly of different subunits. J Comp Neurol. 2005;491:157–174. doi: 10.1002/cne.20721. [DOI] [PubMed] [Google Scholar]
- 42.Kang J, Chen XL, Rampe D. The antipsychotic drugs sertindole and pimozide block erg3, a human brain K(+) channel. Biochem Biophys Res Commun. 2001;286:499–504. doi: 10.1006/bbrc.2001.5434. [DOI] [PubMed] [Google Scholar]
- 43.Wu SN, Jan CR, Li HF, Chiang HT. Characterization of inhibition by risperidone of the inwardly rectifying K(+) current in pituitary GH(3) cells. Neuropsychopharmacology. 2000;23:676–689. doi: 10.1016/S0893-133X(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 44.Bauer CK, Schafer R, Schiemann D, Reid G, Hanganu I, Schwarz JR. A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs. Mol Cell Endocrinol. 1999;148:37–45. doi: 10.1016/s0303-7207(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 45.Lecchi M, Redaelli E, Rosati B, et al. Isolation of a long-lasting eag-related gene-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release. J Neurosci. 2002;22:3414–3425. doi: 10.1523/JNEUROSCI.22-09-03414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedergaard S. A Ca2+-independent slow afterhyperpolarization in substantia nigra compacta neurons. Neuroscience. 2004;125:841–852. doi: 10.1016/j.neuroscience.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 47.Shepard PD, Bunney BS. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca2+-activated K+ conductance. Exp Brain Res. 1991;86:141–150. doi: 10.1007/BF00231048. [DOI] [PubMed] [Google Scholar]
- 48.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol Disord Drug Targets. 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- 50.Grace AA, Bunney BS. Induction of depolarization block in midbrain dopamine neuron by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther. 1986;238:1092–1100. [PubMed] [Google Scholar]
- 51.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 52.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 53.Ji H, Shepard PD. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience. 2006;140:623–633. doi: 10.1016/j.neuroscience.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Shepard PD, Bunney BS. Effects of apamin on the discharge properties of putative dopamine-containing neurons in vitro. Brain Res. 1988;463:380–384. doi: 10.1016/0006-8993(88)90414-3. [DOI] [PubMed] [Google Scholar]
- 55.Johnson SW, Wu YN. Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Res. 2004;1019:293–296. doi: 10.1016/j.brainres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Kass JI, Mintz IM. Silent plateau potentials, rhythmic bursts, and pacemaker firing: three patterns of activity that coexist in quadristable subthalamic neurons. Proc Natl Acad Sci U S A. 2006;103:183–188. doi: 10.1073/pnas.0506781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bains JS, Ferguson AV. Hyperpolarizing after-potentials regulate generation of long-duration plateau depolarizations in rat paraventricular nucleus neurons. Eur J Neurosci. 1998;10:1412–1421. doi: 10.1046/j.1460-9568.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez FR, Engbers JD, Turner RW. Firing dynamics of cerebellar Purkinje cells. J Neurophysiol. 2007;98:278–294. doi: 10.1152/jn.00306.2007. [DOI] [PubMed] [Google Scholar]
- 59.Schwindt P, Crill W. Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. J Neurophysiol. 1999;81:1341–1354. doi: 10.1152/jn.1999.81.3.1341. [DOI] [PubMed] [Google Scholar]
- 60.Kawasaki H, Palmieri C, Avoli M. Muscarinic receptor activation induces depolarizing plateau potentials in bursting neurons of the rat subiculum. J Neurophysiol. 1999;82:2590–2601. doi: 10.1152/jn.1999.82.5.2590. [DOI] [PubMed] [Google Scholar]
- 61.White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- 62.Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- 63.Wanke E, Restano-Cassulini R. Toxins interacting with ether-a-go-go-related gene voltage-dependent potassium channels. Toxicon. 2007;49:239–248. doi: 10.1016/j.toxicon.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 64.Strobaek D, Hougaard C, Johansen TH, et al. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol. 2006;70:1771–1782. doi: 10.1124/mol.106.027110. [DOI] [PubMed] [Google Scholar]