Abstract
Background
Malaria is the leading cause of death among children less than five years of age in sub-Saharan Africa (SSA), however, precise estimates on the burden of malaria are lacking. The aim of this study was to describe temporal trends for malaria and all-cause mortality by combining a series of clinical and intervention studies conducted in Burkina Faso.
Methods
Data from a demographic surveillance system was used to follow-up children under five years who participated in five observational and intervention studies between June 1999 and December 2004 in rural north-western Burkina Faso. Mortality data was analyzed with cause-specific mortality ascertained using the verbal autopsy method. Person-years (PY) of observations were computed and age-standardized mortality rates (MR) for all-causes and malaria (adjusted for missing causes of death) were calculated. Rate ratios to investigate mortality variations over years were calculated using multivariate Poisson regression.
Results
The study followed 6,387 children aged less than five years (mean follow-up: 2.8 years; 16,099 PY). During the study period, 443 deaths were registered with malaria accounting for 49% of all deaths. All-cause and malaria-specific MR were 26.7 (95% CI: 24.2–29.2) and 15.8 (95% CI: 14.217.7) per 1,000 PY. All-cause MR declined over years of follow-up (from 31.2 to 16.3 per 1,000 PY in 1999/2000 to 2004, respectively) but malaria MR remained relatively stable (from 15.8 to 12.1 per 1,000 PY in 1999/2000 to 2004, respectively) resulting in an increasing relative effect of malaria on all-cause mortality. Variations in all-cause and malaria-specific mortality were observed with increasing age and across village town clusters.
Conclusion
The findings of this study support the continuously decreasing trend of all-cause mortality in most of SSA, but call for more efforts to comprehensively address malaria with existing control tools such as insecticide-treated bed nets and effective first-line combination therapies.
Keywords: malaria and all-cause mortality, children under five, Burkina Faso, DSS
In 2004, the World Health Organization ranked malaria as the eighth highest contributor to the global burden of disease and its’ share in the African region was second to HIV/AIDS (1, 2). Current estimates of malaria morbidity cases annually are in the range of 200–650 million episodes worldwide with about 90% of all episodes occurring on the African continent (3). In sub-Saharan Africa (SSA), about one million annual deaths are attributed to malaria and most deaths occur among children under five years (4–6). On the other hand, the much needed data on reported illnesses and disease episodes in SSA is often incomplete, which makes disease-specific morbidity and mortality estimations rather uncertain (7, 8).
Recent developments to address the data deficiency gaps include the establishment of demographic surveillance systems (DSS) in several settings in developing countries where a well-defined cohort is followed up continuously over time and all vital events are recorded during several rounds of visits to households (9). In poor countries, many deaths often occur without previous visits to a health center where proper documentation is available and therefore a possible diagnosis for cause of death can only be achieved by interviewing relatives of the deceased person on symptoms and circumstances surrounding the time prior to death using a structured questionnaire. This approach is commonly used in DSS studies and is referred to as the verbal autopsy (VA) questionnaire method. Several studies have looked at the sensitivity and specificity of this method with varying conclusions, while acknowledging it is the best available option in many resource poor settings (10–12). However, the accuracy of the VA method for post-mortem malaria diagnosis is particularly limited because of the disease interactions with other infections, non-specific symptoms, and the indirect effects of nutrition (13, 14).
Other methodological developments to address data deficiencies have drawn on meta-analysis approaches where the results of selected studies that fulfill a set criterion are summarized into a few effect measures to address specific research questions. Recent estimates for the burden of malaria morbidity and mortality have relied on this methodology, often with strict inclusion and exclusion criteria especially those aimed at addressing disease-specific seasonal effect biases (6, 15). Critics of meta-analysis methods cite the publication bias in overall effects since published studies often show some significant results. Therefore, methods for including results from unpublished studies need further exploration.
Many DSS sites have on-going epidemiological studies such as drug efficacy trials that cover very short periods and are often linked to hospital-based research centers where morbidity histories are available. Such studies may not fulfill all standard criteria set for inclusion in the meta-analysis and yet they have baseline morbidity information such as parasitological data that could be useful in predicting future outcomes. However, if the follow-up periods of these studies can be extended especially through a DSS follow-up, then it is possible to gain this information that would otherwise be excluded.
This approach is explored in this article and standard analysis methods applied for cohort studies are used for assessing trends and risk factors for all-cause and malaria mortality. The study uses data from several clinical and intervention studies conducted in a holoendemic area in rural Burkina Faso and provides estimates of trends in malaria mortality for a typical rural African population by examining all-cause mortality, cause-specific mortality, and the relative effect of malaria on all-cause mortality.
Materials and methods
Study area
The Centre de Recherché en Sante de Nouna (CRSN) is located in Nouna Health District in the Northwest of Burkina Faso. The CRSN research zone largely overlaps with the Nouna DSS, which covered Nouna town and 41 rural villages in 2004. This area is a dry orchard savannah, populated mainly by subsistence farmers of different ethnic groups. The total population of the CRSN research zone is about 60,000 inhabitants, with about 40% living in Nouna town. Health services are provided through the district hospital in Nouna town and through a number of village-based small health centers staffed by nurses and which care for the surrounding villages through outreach activities. Vaccination coverage has been steadily increasing in recent years and is now around 90% for all standard childhood vaccinations (16).
In the Nouna Health District, malaria is holoendemic and markedly seasonal, with most cases occurring during or briefly after the rainy season which usually lasts from June to October (17). Estimates for childhood malaria prevalence are about 30–50% in Nouna town and 75% in the surrounding district, where total annual rainfall varies between 600 and 900 mm (18). In vivo chloroquine resistance was initially reported in the late 1980s and shortly after this period, Day 14 clinical failure rates averaging 5% in children with uncomplicated malaria were observed (17, 19, 20). Results from a number of studies conducted in recent years showed a steep increase in chloroquine resistance in the Nouna area while less resistance was observed for pyrimethamine–sulfadoxine (16, 21). Due to the activities of two large insecticide-treated bed net (ITN) studies, ITN coverage has been continuously increasing since 2000, reaching an overall 28% ITN household coverage in the Nouna Health District by 2007 (22, 23). However, due to the continuously dominating use of chloroquine for fever treatment despite an official change to artemisinin-based combination therapy (ACT), malaria morbidity and mortality have been rather increasing (16, 24), and the rural CRSN study area has remained hyperendemic for malaria (Müller, unpublished data).
The DSS started in 1992 with a census and runs until the present day with routine activities that include quarterly visits to all households in the study zone, upon which all births, deaths, and migrations are routinely registered and updated (25). CRSN research activities include observational studies, as well as community-based and facility-based clinical and public health studies.
Study data and population
Five studies conducted in the Nouna research zone were identified that fulfilled the following criteria: (1) children less than five years included in study; (2) availability of information about malaria morbidity and cause-specific mortality; (3) no overlap in study periods and study location to ensure that children only belonged to one study; and (4) studies conducted during the period 1999–2004. The complete details of the protocol of each study have been described elsewhere (17, 18, 26–28) and shorter summaries are presented here. Briefly, the ITN study was a randomized controlled trial to assess the long-term effects of ITN protection during early infancy among 3,387 children from 41 villages in CRSN (27). The Zinc study assessed the effects of zinc supplementation on malaria and other causes of morbidity and mortality among 712 children from 18 villages in CRSN (17). The Malaria Mothers Providers (MAMOP) study was a cluster-randomized controlled effectiveness trial to evaluate the feasibility and effectiveness of an intervention designed to improve case management of malaria in 1,081 pre-school children through mothers supported by mother-groups and peripheral health workers (26). The Environment and Weather Malaria (EWM) study was an observational study among 867 children to determine the prevalence of malaria among the cohort children, the incidence of malaria, and the effect of temperature, rainfall, and relative humidity on Plasmodium falciparum infection risk (18). The Bourasso study was a cross-sectional study to evaluate the prevalence of malaria infections, chloroquine related resistance, and proportion of moderate to severe anemia in all residents of Bourasso village (including 355 children) near Nouna, Burkina Faso (28).
Child records in each of the studies described above were matched with the child identification numbers used in the Nouna DSS database. Each child was then followed beyond the end of the individual study by utilizing the routine monitoring system of the DSS. Additionally, it was possible to establish the causes of death using the VA information available in the DSS database for children who died.
Statistical methods
Death was considered as the final outcome if it occurred before the child reached the fifth birthday. For person-year (PY) calculations, records for children who had out-migrated were censored at the date of out-migration. In addition, children were censored at their fifth birthday or on the last date of the extended follow-up period depending on whichever came first. Demographic information such as sex, date of birth, ethnicity, vital status indicator, and morbidity information parameters were included in the final database. Age-standardized rates were calculated using the population distribution for under five-year-old children from the nationally representative Burkina Faso 2003 Demographic and Health Survey using the following proportions: 22.4% (0–11 months); 19.5% (12–23 months); 18.6% (24–35 months); 20.9% (36–47 months); and 18.6% (48–59 months). This was done in order to have nationally representative rates since many DSS sites are located in remote locations that tend to be less nationally representative.
All-cause mortality rate (MR) was calculated by dividing the number of deaths by the observed PYs at risk in each age group, and expressed as deaths per 1,000 PYs at risk.
The malaria MRs were adjusted for missing causes of death according to the following formula (29):
| 1 |
where D mal is malaria deaths, PY is person-years, D unk is deaths with unknown causes and D tot is total deaths. This method assumes that the proportion of deaths attributable to malaria for deaths with unknown causes equals the proportion of deaths attributable to malaria for deaths with known causes.
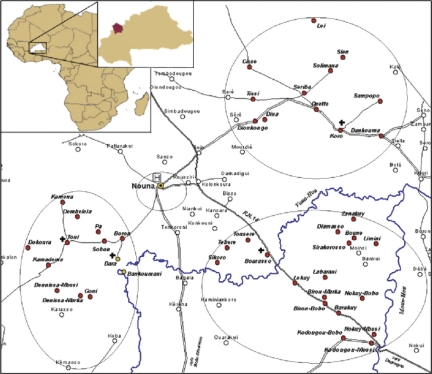
The following variables were used for calculating MRs for all-causes, malaria, and the corresponding rate ratios: child ages (0=[0–1), 1=[1–2), 2=[2–3), 3=[3–4), 4=[4–5)), year as 1999/2000 through 2004; season (1 = rainy, 0 = dry), gender (1 = female, 0 = male); and ethnicities (0 = Dafing, 1 = Bwaba 2 = Mossi 3 = Peulh 4 = Samo 5 = Others). Controlling for rural vs. urban effects was done by grouping the 42 villages of the CRSN research zone into four clusters, namely north-eastern (village = 0), Nouna town (1), south-eastern (2), and south-western (3) (Fig. 1).
Fig. 1. .
Village clusters in CRSN, Nouna, Burkina Faso. The four clusters namely; Nouna town, north-eastern, south-eastern, and south-western village clusters are marked using circular boundaries.
Rate ratios to investigate variations over several years on mortality among children were calculated with a multivariate Poisson regression analysis using observed PYs as an offset and adjusted for child age, seasonal effects, gender, ethnicity, and village clusters. The model was fitted as follows:
where the total PYs were adjusted for missing causes of death.
A similar model for all-causes of death which included the same covariates was used to estimate the rate ratios with the offset as log of total PYs.
In addition, the relative effect of malaria on all-cause mortality over years of the study and child ages was investigated, adjusted for the co-variables mentioned above. This was possible by modeling the observed malaria deaths in a Poisson regression using the log of all known observed deaths as an offset. The test for linear trend in the relative effect of malaria on all-cause mortality was carried out by including years of study as a continuous variable while adjusting for child ages and seasonal effects. All analyses were performed using SAS 9.1 statistical software.
Results
Description of the cohort
A total of 6,387 under five-year-old children (50.5% males and 49.5% females) were included in the pooled cohort covering the period 1999–2004. After a mean follow-up period of 2.8 years, 443 child deaths were registered with 16,098.6 accumulated PYs. About 49% of all deaths were attributed to malaria as compared to other causes that accounted for about 39% (Table 1). Causes of death were unknown for about 12% of all registered deaths. This included deaths where the available VA information could not determine the cause of death and deaths whose information for the underlying cause was still missing at the time of analysis. Ultimately, most recent deaths accounted for the largest percentage of deaths due to unknown causes.
Table 1.
Characteristics of the studies included in the pooled analysis of child mortality in Burkina Faso
| Age groupsb (months) | Number of deathsc | Age-standardized mortality rate (per 1,000 PY) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studya | Number of children | 0–11 | 12–23 | 24–35 | 36–47 | 48–60 | Total PY | Malaria | Otherd | All | Malaria | All-cause |
| Zinc (17) | 699 | 143 | 349 | 155 | 33 | 19 | 2,205.9 | 21 | 22 | 43 | 23.0 | 42.3 |
| ITN (22) | 3,387 | 3,387 | 0 | 0 | 0 | 0 | 10,287.4 | 158 | 182 | 340 | 14.8 | 25.1 |
| Bourasso (28) | 355 | 87 | 123 | 59 | 49 | 37 | 779.2 | 3 | 3 | 6 | 6.0 | 14.7 |
| MAMOP (26) | 1,081 | 29 | 79 | 69 | 54 | 33 | 2,062.8 | 15 | 21 | 36 | 7.2 | 11.6 |
| EWM (18) | 865 | 80 | 200 | 202 | 211 | 174 | 754.3 | 6 | 12 | 18 | 9.1 | 19.9 |
| Total | 6,387 | 3,726 | 751 | 485 | 347 | 263 | 16,098.6 | 201 | 242 | 443 | 16.0 | 26.7 |
aZinc, Zinc intervention study (1999–2003); ITN, insecticide-treated bed net intervention study (2000–2003); Bourasso, cross-sectional study on malaria burden (2001–2003); MAMOP, home-based malaria treatment intervention study (2002–2004); EWM, observational study on environment, climate and malaria (2003–2004).
bAge at the start of observation.
cCause of death is based on verbal autopsy analysis.
dOther causes of death include 111 unknown causes of death at the time of analysis.
An age-standardized all-cause MR of 26.7 (95% CI: 24.2–29.2) per 1,000 PY and a malaria-specific MR of 15.8 (95% CI: 14.2–17.7) per 1,000 PY were estimated for the period of the pooled analysis after weighting by the PYs of each study. Variations in all-cause and malaria-specific MRs by study types and year of study were observed.
Analysis of mortality rates (MRs)
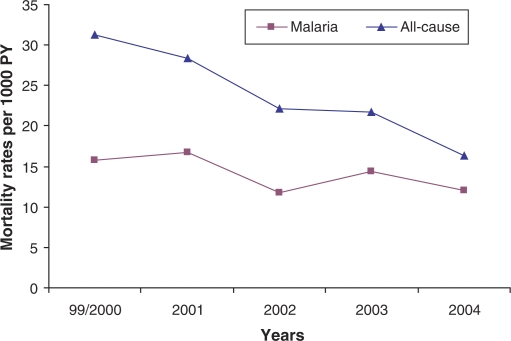
Table 2 shows the results for the analysis of mortality by age, sex, year of death, season of death, season of birth, and village clusters. While there was no association of mortality with sex, age was strongly associated both with all-cause and malaria mortality. All-cause MRs were highest for infants (0–1 years) and steadily decreased thereafter. A strong decreasing trend for all-cause mortality was observed over the years 2000–2004, however, malaria MRs remained rather stable after the year 2000 (Fig. 2). Observed percentages of malaria deaths from all deaths varied between 54% in 1999/2000 and 34% in 2004. However, majority of the unknown causes of death were observed in 2004 (25.3%). Malaria MRs were nearly twice as high in the rainy, than in the dry season (19.8 vs. 13.4 per 1,000 PY), whereas the seasonal differences in overall MRs were less strong. Significant differences in mortality between village clusters (Fig. 1) were also observed. In contrast to Nouna town, higher all-cause and malaria MRs were found in the rural village clusters (Table 2). No major differences in all-cause or malaria MRs were observed by season of birth.
Table 2.
Analysis of mortality by age, sex, year of observation, season of birth and death, and village clusters
| Variable | Label | Malaria causes N (%) | Unknown causes N (%) | Other causes N (%) | MMRa | 95% CI for MMR | AMRb | 95% CI for AMR |
|---|---|---|---|---|---|---|---|---|
| Age class (months) | [0– < 12) | 76 (52.4) | 21 (14.5) | 48 (33.1) | 27.1 | (24.0, 30.2) | 49.6 | (45.8, 53.5) |
| [12– < 24) | 77 (58.8) | 10 (7.6) | 44 (33.6) | 21.4 | (18.9, 23.8) | 35.1 | (32.1, 38.1) | |
| [24– < 36) | 47 (52.8) | 8 (9.0) | 34 (38.2) | 13.5 | (11.5, 15.4) | 20.8 | (18.5, 23.1) | |
| [36– < 48) | 22 (56.4) | 5 (12.8) | 12 (30.8) | 8.40 | (6.60, 10.2) | 11.7 | (9.70, 13.6) | |
| [48– < 60) | 10 (37.0) | 8 (29.6) | 9 (33.3) | 9.10 | (6.50, 11.7) | 12.7 | (10.1, 15.3) | |
| Sex | Male | 124 (52.5) | 29 (12.3) | 83 (35.2) | 17.3 | (15.7, 18.8) | 28.3 | (26.5, 30.2) |
| Female | 110 (53.9) | 24 (11.8) | 70 (34.3) | 16.2 | (14.7, 17.8) | 25.0 | (23.3, 26.7) | |
| Year of death | 1999/2000c | 25 (50.0) | 2 (4.0) | 23 (46.0) | 15.8 | (12.6, 18.9) | 31.2 | (26.8, 35.6) |
| 2001 | 49 (55.7) | 14 (15.9) | 25 (28.4) | 16.8 | (14.4, 19.2) | 28.3 | (25.4, 31.2) | |
| 2002 | 51 (45.1) | 11 (9.7) | 51 (45.1) | 11.8 | (10.2, 13.4) | 22.1 | (20.0, 24.2) | |
| 2003 | 63 (64.3) | 3 (3.1) | 32 (32.7) | 14.4 | (12.6, 16.3) | 21.7 | (19.6, 23.9) | |
| 2004 | 46 (50.5) | 23 (25.3) | 22 (24.2) | 12.1 | (10.3, 13.9) | 16.3 | (14.5, 18.1) | |
| Season of death | Dry | 85 (50.6) | 19 (11.3) | 64 (38.1) | 13.4 | (12.1, 14.8) | 24.9 | (23.3, 26.5) |
| Rainy | 149 (54.8) | 34 (12.5) | 89 (32.7) | 19.8 | (18.1, 21.6) | 29.2 | (27.1, 31.3) | |
| Season of birth | Dry | 84 (59.6) | 14 (9.9) | 43 (30.5) | 14.7 | (12.6, 16.7) | 25.6 | (23.6, 27.5) |
| Rainy | 150 (50.2) | 39 (13.0) | 110 (36.8) | 17.8 | (16.4, 19.3) | 27.3 | (25.7, 29.0) | |
| Village clusters | Northeast | 65 (57.5) | 15 (13.3) | 33 (29.2) | 23.3 | (20.1, 26.5) | 34.6 | (31.3, 38.0) |
| Nouna | 1 (33.3) | 0 (0.0) | 2 (66.7) | 2.40 | (0.00, 4.80) | 8.00 | (3.40, 12.7) | |
| Southeast | 89 (47.6) | 25 (13.4) | 73 (39.0) | 12.4 | (11.0, 13.8) | 22.2 | (20.6, 23.8) | |
| Southwest | 79 (57.7) | 13 (9.5) | 45 (32.8) | 19.6 | (17.2, 22.0) | 31.7 | (29.1, 34.4) |
Note: CI, Confidence interval; N, total number of deaths.
aMMR, age-standardized malaria-specific mortality rates. Malaria figures are verbal autopsy diagnosed deaths adjusted for missing causes of death.
bAMR, age-standardized all-cause mortality rates.
cResults presented for the period June 1999–December 2000.
Fig. 2. .
Trends for all-cause and malaria-specific childhood mortality rates for the years 2000–2004.
Adjusted rate ratios
The results of the multivariate Poisson regression (Table 3) underline the findings presented above. Decreasing rate ratios were observed for increasing child age after adjusting for season, year, gender, ethnicity, and geographical effects. To calculate yearly rate ratios, the years 1999/2000 was selected as the reference year, because Nouna town was included into the DSS region during this year. The findings show that after adjusting for differences in child ages, sex, season of death, village clusters, and ethnicity, all-cause mortality declined strongly over years (p-value = 0.015 for linear trend), while malaria MRs varied without a clear declining trend (p-value = 0.079). Clear higher rates for malaria mortality were observed during the rainy season than in the dry season (RR = 1.7, 95% CI: 1.3–2.3), however, not for all-causes of death (RR = 0.99, 95% CI: 0.82–1.2).
Table 3.
Rate ratios (RR) for malaria, malaria relative to all-known cause, and all-cause mortality for under five-year-old children in rural Burkina Faso from Multivariate Poisson regression model
| Malaria mortality ratesa,c | All-cause mortality ratesa | Malaria relative to known all causea,b | |||||
|---|---|---|---|---|---|---|---|
| Variable | Label | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Age class (months) | [0– < 12) | 1 | – | 1 | – | 1 | – |
| [12– < 24) | 0.98 | (0.68, 1.41) | 0.93 | (0.71, 1.22) | 1.06 | (0.74, 1.52) | |
| [24– < 36) | 0.59 | (0.37, 0.93) | 0.67 | (0.48, 0.93) | 0.90 | (0.59, 1.38) | |
| [36– < 48) | 0.37 | (0.21, 0.67) | 0.41 | (0.27, 0.64) | 0.97 | (0.51, 1.87) | |
| [48– < 60) | 0.38 | (0.18, 0.81) | 0.76 | (0.46, 1.24) | 0.86 | (0.40, 1.86) | |
| Year of death | 1999/2000 | 1 | – | 1 | – | 1 | – |
| 2001 | 0.86 | (0.50, 1.48) | 0.68 | (0.46, 0.99) | 1.13 | (0.61, 2.07) | |
| 2002 | 0.56 | (0.33, 0.96) | 0.53 | (0.37, 0.78) | 0.94 | (0.51, 1.75) | |
| 2003 | 0.71 | (0.41, 1.21) | 0.46 | (0.31, 0.68) | 1.29 | (0.71, 2.36) | |
| 2004 | 0.68 | (0.38, 1.23) | 0.52 | (0.34, 0.80) | 1.47 | (0.81, 2.65) | |
| Season of death | Dry | 1 | – | 1 | – | 1 | – |
| Rainy | 1.74 | (1.34, 2.25) | 0.99 | (0.82, 1.19) | 1.62 | (1.22, 2.15) | |
aAdjusted for gender, ethnicity and village clusters (see Fig. 1).
bLog for all known causes of death used as offset.
cPerson years adjusted for unknown causes of death used as offset.
A steady increase across years in the relative effect of malaria MRs among all known causes of death was observed (Table 3) (Test for linear trend: p-value = 0.091). No trend was observed over age classes. However, as expected, the relative effect of malaria MRs among all known causes of death was greatest during the rainy season in contrast to the dry season (p=0.0025).
Discussion
This analysis was based on a large, multi-study cohort of under five-year-old children followed over a five-year period using a DSS to examine all-cause mortality, cause-specific mortality, and the relative effect of malaria on all-cause mortality over time in Nouna, rural Burkina Faso.
One of the main results from this study is a clear declining trend of all-cause mortality, while there was no evidence for a decrease in malaria mortality. Our findings support the ongoing trends of decreasing childhood mortality in most parts of SSA (30, 31). However, the finding that overall most deaths in this population of young children are due to malaria with an even increasing proportion of deaths being attributed to malaria over the study period are disturbing (32, 33), especially since two of the five studies were malaria intervention studies. The most likely explanation for this is the observed sharp increase in chloroquine resistance during the years of the study (16) which is likely to have a major impact on childhood mortality as observed elsewhere in SSA (12, 34). Chloroquine was the official first-line treatment for uncomplicated malaria in Burkina Faso until 2005 and is still the most frequently used drug to treat fever episodes in children of the Nouna area (16). A second likely cause for the high proportion of malaria deaths might be the tendency of physicians to diagnose fever episodes as malaria together with the known low specificity of the VA method (11, 12, 35). The expected opposite effects of ITN distribution for protection of newborns and pre-school children in the CRSN study area since the year 2000 in the frame of two large ITN trials may not have been strong enough to reverse the likely dramatic effect of the rapidly increasing chloroquine resistance on childhood mortality (22, 23).
Another major finding from this study is the much lower all-cause as well as malaria-specific mortality observed in the Nouna town cluster as compared to the surrounding village clusters. These findings support similar findings on urban–rural comparisons in other areas of SSA (36, 37). Likely explanations for these large differences include better access to quality health care in the urban study area as well as lower malaria transmission intensity in the urban as compared to the rural study.
Both, all-cause and malaria mortality were clearly associated with age which is well known from most of SSA and reflects the vulnerability of very young children to mostly infectious diseases including malaria, often aggravated by the poor nutritional status of pre-school children in such communities (38, 39). Moreover, the data from this paper confirm the strong effect of the rainy season on mortality in this part of SSA, which is mostly but not exclusively attributed to malaria (40–42).
In addition, the results show that the CRSN study region, which is a largely rural and highly malaria endemic area, has very high malaria MR when compared to the recent estimate by Rowe and others of 11.4 (95% CI: 9.8–12.9) per 1,000 PY for high transmission areas in rural Africa for the year 2000 (8). All the studies used in our analysis were not included in the review by Rowe and others simply because one or two of their inclusion criteria were not fulfilled. Using the possibility of linking study data to DSS follow-up, the studies were able to meet all the required criteria and provide a direct comparison to estimates provided by Rowe and colleagues. Although, the CRSN estimate is higher than the overall estimate derived by Rowe and others, it fits well in the range of estimates (1.9–24.0) from the studies used by their team (8). Additionally, linking study data to DSS follow-up not only gives the possibility to verify the mortality diagnoses from the DSS database using VA, but provides an opportunity to link morbidity information and mortality outcomes.
Methodologically, the study design utilized here is viable and provides a greater addition to the many other approaches that provide estimates for mortality and morbidity. This approach relies mostly on the existence of a longitudinal study that spans longer periods from which events such as migrations, deaths, etc. that are likely to occur in the long term can be tracked. Earlier results based on the complete DSS analysis for comparable time periods showed very similar results, thereby giving assurance to the results from this study approach and design (43).
This study has some limitations. First of all, comparison of yearly estimates for the DSS area shows that rates for the year 1999 were generally high. Excluding 1999, an overall malaria-specific MR of 13.6 (95% CI: 12.4–14.8) per 1,000 PY was estimated (see Fig. 2). On the other hand, before 2000, the Nouna DSS area did not include the residents of the semi-urban town Nouna, where the district hospital is located. Thus, the observed high rates of 1999 might possibly reflect the purely rural rates arising from poor access to health services and other urban–rural differences that affect health outcomes. Additionally, in 1999 and 2000 only few malaria mortality cases have been observed. Thus, the years 1999 and 2000 were jointly analyzed although this does not completely rule out this bias. Moreover, differences in rainfall between years may also have played a role. Additionally, the process of analyzing time series is much more complicated than it might be done with the existing data here. However, the authors do not have more detailed data or data for longer time intervals.
Secondly, different malaria estimates might possibly arise from the varying sensitivity and specificity of the VA methods (44) as well as the effect of the adjustments for the unknown causes of death (45–47).
Furthermore, data used in this analysis were partly from studies on malaria interventions that were implemented through the CRSN, therefore it is possible that such interventions may potentially confound the mortality risk associations observed here. For instance, in the first ITN study, children were randomized to receive bed nets at either within two weeks after birth or six months after birth (22). However, no significant differences among the two arms were observed. In addition, the number of observations in the various subgroups were sometimes few, which needs to be taken into account when interpreting and drawing conclusions on the overall results.
Despite these methodological limitations, the results provide a characteristic description of the epidemiology of malaria in the area, making them particularly valuable for contributing to the ongoing discussion concerning trends in all-cause and malaria-specific mortality. The Nouna DSS has collected data since 1992, with continuous improvement of data quality over years, giving much confidence on data quality for our study. The possibility of being able to link records from completed studies to child records in the on-going DSS data provides an opportunity not only to monitor future mortality outcomes, but to also monitor linkages between underlying morbidity history and future mortality.
In conclusion, this study provides a better understanding of the pattern of mortality in a malaria-endemic area of SSA by using within-site studies rich in time series for recent periods. The results show evidence of an overall decline in all-cause childhood mortality alongside stagnant malaria-specific MRs, as well as a large urban–rural difference in MRs. As all-cause MRs converge towards the malaria-specific MRs, further declines in all-cause MR might largely be achieved by expanding the efforts towards reducing the malaria burden through programs that have been tested to be effective such as the large-scale use of ITNs and ACTs.
Authors' contributions
RPN coordinated the study, performed the statistical analysis and drafted the manuscript.
HB designed, supervised and participated in coordination of the study and substantially contributed to writing-up of the manuscript.
HR supervised the analysis and coordination of the study and participated in writing-up of the manuscript. AS substantially participated in the conception in particular with regard to the studies in Burkina Faso and writing-up of the manuscript.
BK substantially participated in the conception in particular with regard to the studies in Burkina Faso and writing-up of the manuscript.
YY substantially participated in the conception in particular with regard to the studies in Burkina Faso, in the interpretation of the results, in the discussion and writing-up of the manuscript.
OM substantially participated in the conception, analysis, and discussion and in the interpretation of the results as well as writing-up of the manuscript.
Acknowledgements
This work was supported by the collaborative research grant ‘SFB 544’ of the German Research Foundation (DFG). The authors would like to extend their appreciation to the team of the Centre de Recherché en Santé de Nouna and to the children and families who participated in the various studies.
References
- 1.WHO. Geneva/New York: World Health Organization/United Nations Children's Fund; 2002. World Health Report. Reducing risks, promoting healthy life; pp. 120–137. [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–98. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 5.Morris SS, Black RE, Tomaskovic L. Predicting the distribution of under-five deaths by cause in countries without adequate vital registration systems. Int J Epidemiol. 2003;32:1041–51. doi: 10.1093/ije/dyg241. [DOI] [PubMed] [Google Scholar]
- 6.Rowe AK, Rowe SY, Snow RW, Korenromp EL, Armstrong S, Jr, Stein C. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35:691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present and future. Lancet Infect Dis. 2004;4:327–36. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe AK, Rowe SY, Snow RW, Korenromp EL, Schellenberg JR, Stein C, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35:691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.INDEPTH Network. Population, health, and survival at INDEPTH sites. Ottawa: IDRC; 2002. Population and health in developing countries; p. 356. [Google Scholar]
- 10.Alonso PL, Bowman A, Marsh K, Greenwood BM. The accuracy of the clinical histories given by mothers of seriously ill African children. Ann Trop Paediatr. 1987;7:187–9. doi: 10.1080/02724936.1987.11748504. [DOI] [PubMed] [Google Scholar]
- 11.Snow RW, Armstrong JR, Forster D, Winstanley MT, Marsh VM, Newton CR, et al. Childhood deaths in Africa: uses and limitations of verbal autopsies. Lancet. 1992;340:351–5. doi: 10.1016/0140-6736(92)91414-4. [DOI] [PubMed] [Google Scholar]
- 12.Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measurement of trends in childhood malaria mortality in Africa: an assessment of progress toward targets based on verbal autopsy. Lancet Infect Dis. 2003;3:349–58. doi: 10.1016/s1473-3099(03)00657-1. [DOI] [PubMed] [Google Scholar]
- 13.Snow RW, Molyneux CS, Njeru EK, Omumbo J, Nevill CG, Muniu E, et al. The effects of malaria control on nutritional status in infancy. Acta Trop. 1997;65:1–10. doi: 10.1016/s0001-706x(96)00601-8. [DOI] [PubMed] [Google Scholar]
- 14.Muller O, Traore C, Becher H, Kouyate B. Malaria morbidity, treatment seeking behaviour, and mortality in a cohort of young children in rural Burkina Faso. Trop Med Int Health. 2003;8:290–6. doi: 10.1046/j.1365-3156.2003.01030.x. [DOI] [PubMed] [Google Scholar]
- 15.Roca-Feltrer A, Carneiro I, Armstrong Schellenberg JR. Estimates of the burden of malaria morbidity in Africa in children under the age of 5 years. Trop Med Int Health. 2008;13:771–83. doi: 10.1111/j.1365-3156.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- 16.Kouyate B, Sie A, Ye M, De AM, Muller O. The great failure of malaria control in Africa: a district perspective from Burkina Faso. PLoS Med. 2007;4:e127. doi: 10.1371/journal.pmed.0040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller O, Becher H, Baltussen A, Ye Y, Diallo D, Konate M, et al. Effect of zinc supplementation on malaria morbidity among West African children: a randomized double-blind placebo-controlled trial. BMJ. 2001;322:1567–72. doi: 10.1136/bmj.322.7302.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Y, Kyobutungi C, Louis VR, Sauerborn R. Micro-epidemiology of Plasmodium falciparum malaria: Is there any difference in transmission risk between neighbouring villages? Malar J. 2007;6:46. doi: 10.1186/1475-2875-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guigemde TR, Aoba A, Ouedraogo JB, Lamizana L. Ten year surveillance of drug-resistant malaria in Burkina Faso (1982–1991) Am J Trop Med Hyg. 1994;50:699–704. doi: 10.4269/ajtmh.1994.50.699. [DOI] [PubMed] [Google Scholar]
- 20.Muller O, Traore C, Kouyate B. Clinical efficacy of chloroquine in young children with uncomplicated falciparum malaria – a community-based study in rural Burkina Faso. Trop Med Int Health. 2003;8:202–3. doi: 10.1046/j.1365-3156.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- 21.Muller O, Traore C, Kouyate B. Efficacy of pyrimethamine-sulfadoxine in young children with uncomplicated falciparum malaria in rural Burkina Faso. Malar J. 2004;3:10. doi: 10.1186/1475-2875-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller O, Traore C, Kouyate B, Ye Y, Frey C, Coulibaly B, et al. Effects of insecticide-treated bednets during early infancy in an African area of intense malaria transmission: a randomized controlled trial. Bull World Health Organ. 2006;84:120–6. doi: 10.2471/blt.05.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller O, De AM, Becher H, Tiendrebogo J, Beiersmann C, Ye M, et al. Distribution systems of insecticide-treated bed nets for malaria control in rural Burkina Faso: cluster-randomized controlled trial. PloS ONE. 2008;3:e3182. doi: 10.1371/journal.pone.0003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller O, Ye M, Louis VR, Sie A. Malaria in sub-Saharan Africa. Lancet. 2009;373:122. doi: 10.1016/S0140-6736(09)60033-7. [DOI] [PubMed] [Google Scholar]
- 25.Yé Y, Sanou A, Gbangou A, Kouyaté B, Nouna DSS, Burkina Faso Population and health in developing countries. Volume 1. Population, health, and survival at INDEPTH sites. INDEPTH Network, Ottawa: IDRC. 2002;356:221–6. [Google Scholar]
- 26.Kouyaté B, Somé F, Jahn A, Coulibaly B, Eriksen J, Sauerborn R, et al. Process and effects of a community intervention on malaria in rural Burkina Faso: randomised controlled trial. Malar J. 2008;7:50. doi: 10.1186/1475-2875-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller O, Traore C, Kouyate B, Becher H. Effects of insecticide-treated mosquito nets (ITN) on malaria morbidity and all-cause mortality in infants of a malaria holoendemic area in rural Burkina Faso. Acta Trap. 2002;83:71–2. [Google Scholar]
- 28.Stich A, Oster N, bdel-Aziz I, Stieglbauer G, Coulibaly B, Wickert H, et al. Malaria in a holoendemic area of Burkina Faso: a cross-sectional study. Parasitol Res. 2006;98:596–9. doi: 10.1007/s00436-005-0104-9. [DOI] [PubMed] [Google Scholar]
- 29.Rowe AK. Analysis of deaths with an unknown cause in epidemiologic analyses of mortality burden. Trop Med Int Health. 2006;11:540–50. doi: 10.1111/j.1365-3156.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 30.Hill AG, MacLeod WB, Joof D, Gomez P, Walraven G. Decline of mortality in children in rural Gambia: the influence of village-level primary health care. Trop Med Int Health. 2000;5:107–18. doi: 10.1046/j.1365-3156.2000.00528.x. [DOI] [PubMed] [Google Scholar]
- 31.Korenromp EL, Arnold F, Williams BG, Nahlen BL, Snow RW. Monitoring trends in under-5 mortality rates through national birth history surveys. Int J Epidemiol. 2004;33:1293–301. doi: 10.1093/ije/dyh182. [DOI] [PubMed] [Google Scholar]
- 32.Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–7. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 33.Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJ, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372:1545–54. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–7. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 35.Todd JE, de FA, O'Dempsey TJ, Greenwood BM. The limitations of verbal autopsy in a malaria-endemic region. Ann Trop Paediatr. 1994;14:31–6. doi: 10.1080/02724936.1994.11747689. [DOI] [PubMed] [Google Scholar]
- 36.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modiano D, Sirima BS, Sawadogo A, Sanou I, Pare J, Konate A, et al. Severe malaria in Burkina Faso: urban and rural environment. Parasitologia. 1999;41:251–4. [PubMed] [Google Scholar]
- 38.Muller O, Garenne M, Kouyate B, Becher H. The association between protein-energy malnutrition, malaria morbidity and all-cause mortality in West African children. Trop Med Int Health. 2003;8:507–11. doi: 10.1046/j.1365-3156.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 39.Becher H, Muller O, Jahn A, Gbangou A, Kynast-Wolf G, Kouyate B. Risk factors of infant and child mortality in rural Burkina Faso. Bull World Health Organ. 2004;82:265–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffar S, Leach A, Greenwood AM, Jepson A, Muller O, Ota MO, et al. Changes in the pattern of infant and childhood mortality in upper river division, The Gambia, from 1989 to 1993. Trop Med Int Health. 1997;2:28–37. doi: 10.1046/j.1365-3156.1997.d01-131.x. [DOI] [PubMed] [Google Scholar]
- 41.Kynast-Wolf G, Hammer GP, Muller O, Kouyate B, Becher H. Season of death and birth predict patterns of mortality in Burkina Faso. Int J Epidemiol. 2006;35:427–35. doi: 10.1093/ije/dyi150. [DOI] [PubMed] [Google Scholar]
- 42.Becher H, Kynast-Wolf G, Sie A, Ndugwa R, Ramroth H, Kouyate B, et al. Patterns of malaria: cause-specific and all-cause mortality in a malaria-endemic area of west Africa. Am J Trop Med Hyg. 2008;78:106–13. [PubMed] [Google Scholar]
- 43.Hammer GP, Some F, Muller O, Kynast-Wolf G, Kouyate B, Becher H. Pattern of cause-specific childhood mortality in a malaria endemic area of Burkina Faso. Malar J. 2006;5:47. doi: 10.1186/1475-2875-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith T. Commentary: malaria death rates remain highly pertinent. Int J Epidemiol. 2006;35:704–5. doi: 10.1093/ije/dyl078. [DOI] [PubMed] [Google Scholar]
- 45.Chandramohan D, Setel P, Quigley M. Effect of misclassification of causes of death in verbal autopsy: can it be adjusted? Int J Epidemiol. 2001;30:509–14. doi: 10.1093/ije/30.3.509. [DOI] [PubMed] [Google Scholar]
- 46.Rowe AK. Should verbal autopsy results for malaria be adjusted to improve validity? Int J Epidemiol. 2005;34:712–3. doi: 10.1093/ije/dyi087. [DOI] [PubMed] [Google Scholar]
- 47.Etard JF, Le Hesran JY, Diallo A, Diallo JP, Ndiaye JL, Delaunay V. Childhood mortality and probable causes of death using verbal autopsy in Niakhar, Senegal, 1989–2000. Int J Epidemiol. 2004;33:1286–92. doi: 10.1093/ije/dyh259. [DOI] [PubMed] [Google Scholar]