Abstract
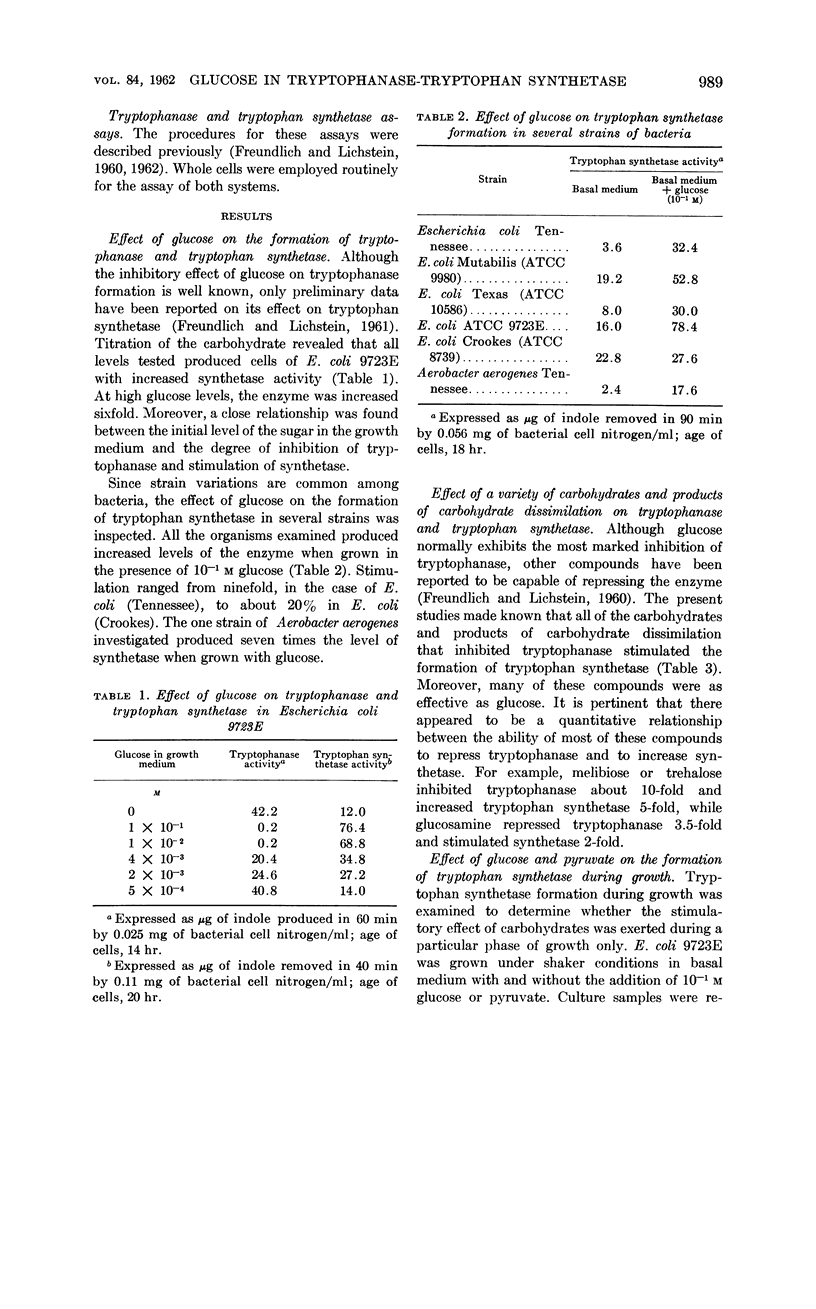
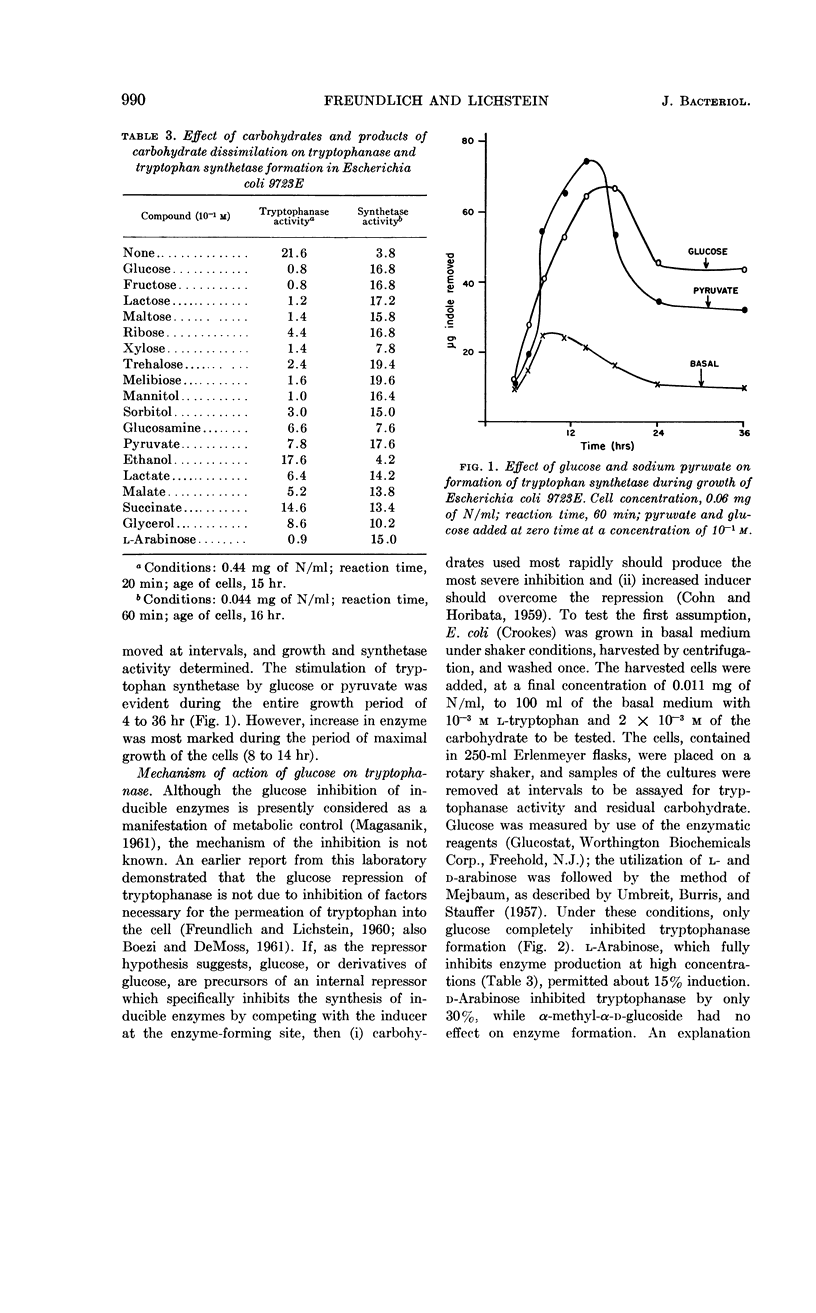
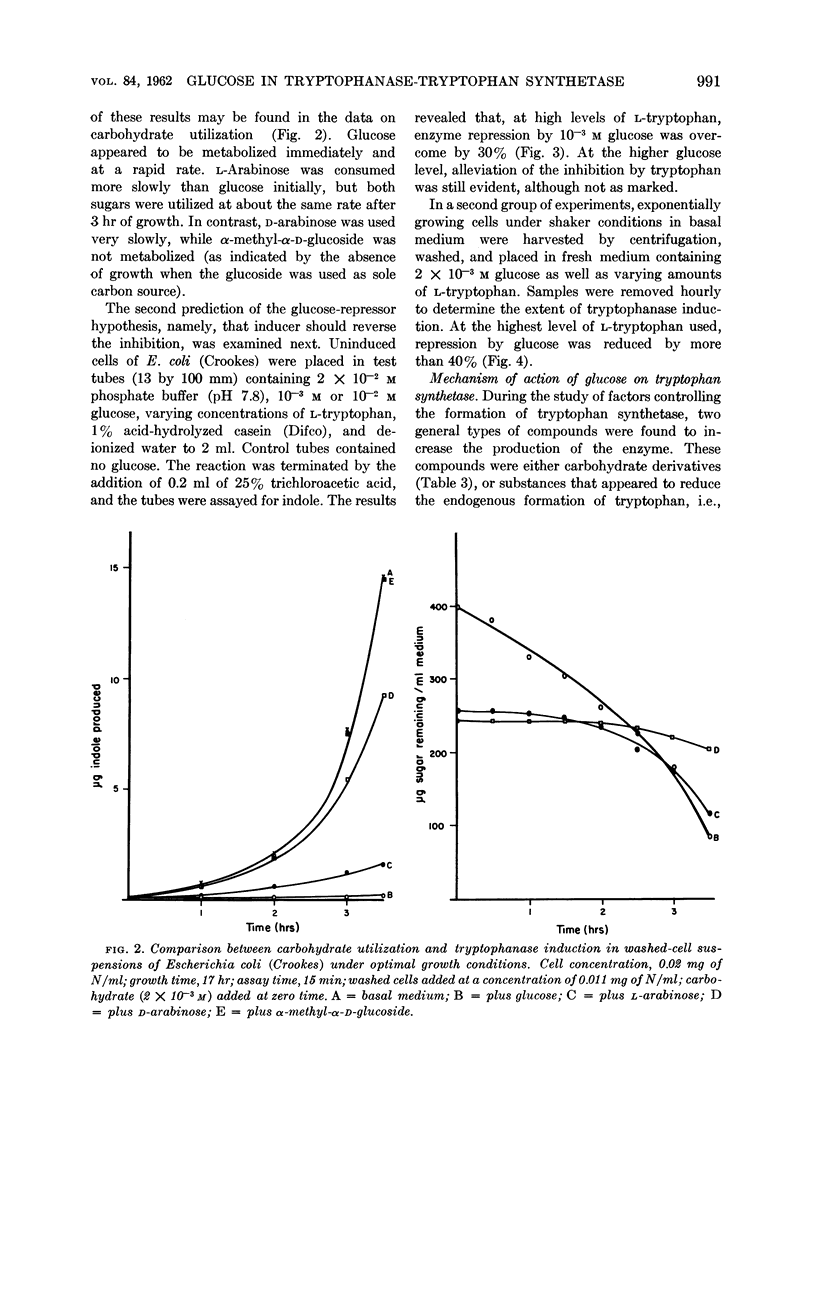
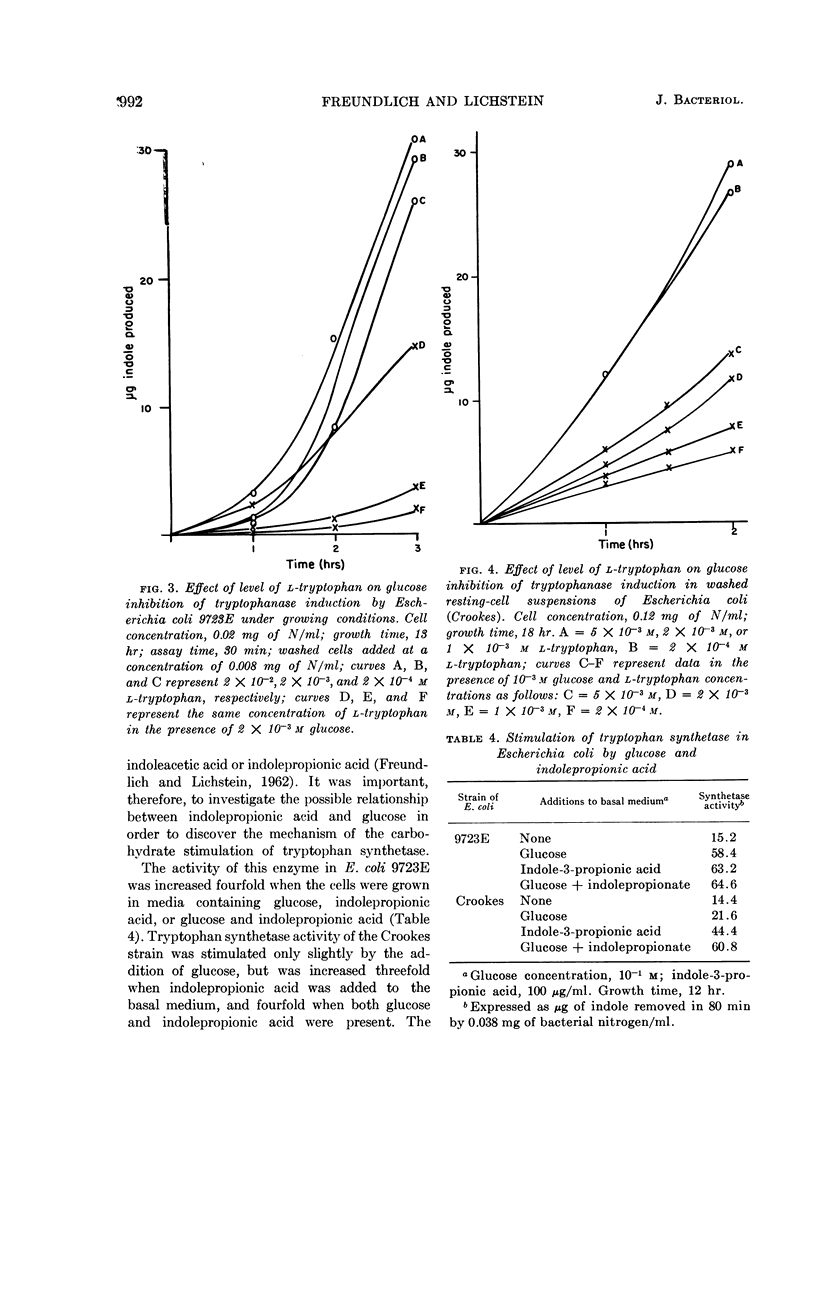
Freundlich, Martin (University of Minnesota, Minneapolis) and Herman C. Lichstein. Tryptophanase-tryptophan synthetase systems in Escherichia coli. II. Effect of glucose. J. Bacteriol. 84:988–995. 1962.—The effect of glucose and other compounds on the formation of tryptophanase and tryptophan synthetase in Escherichia coli was examined. Although most of these compounds were potent inhibitors of the synthesis of tryptophanase, they invariably increased the formation of tryptophan synthetase. The severity of tryptophanase inhibition depended upon the degree of utilization of the compound by the growing bacterial cells. It was found that high levels of tryptophan overcame by 40% the repression caused by glucose. The stimulatory effect of glucose on tryptophan synthetase formation in E. coli 9723E could be duplicated by indole-3-propionic acid. A study of the amino acid pool of E. coli 9723E revealed no free tryptophan in cells harvested from the basal medium containing glucose. In contrast, cells grown in the absence of glucose possessed a measurable amount of this amino acid. The possible mechanisms of the effect of glucose and related compounds on tryptophanase and tryptophan synthetase formation, as well as the relationship of these effects to the metabolic control of tryptophan metabolism, are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., LICHSTEIN H. C. Inhibitory effect of glucose on tryptophanase. J Bacteriol. 1960 Nov;80:633–638. doi: 10.1128/jb.80.5.633-638.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., LICHSTEIN H. C. Tryptophanase-tryptophan synthetase systems in Escherichia coli. I. Effect of tryptophan and related compounds. J Bacteriol. 1962 Nov;84:979–987. doi: 10.1128/jb.84.5.979-987.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN A. P., MAGASANIK B. Enzyme synthesis in guanine-starved cells. J Biol Chem. 1961 Jun;236:1810–1815. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Nutrition of bacteria and fungi. Annu Rev Microbiol. 1957;11:221–252. doi: 10.1146/annurev.mi.11.100157.001253. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K., SIMON E. J. An impaired concentrating mechanism for amino acids in mutants of Escherichia coli resistant to L-canavanine and D-serine. Biochim Biophys Acta. 1959 Apr;32:582–583. doi: 10.1016/0006-3002(59)90650-x. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]


