Abstract
Aims:
To summarise the metabolic responses to niacin that can lead to flushing and to critically evaluate flushing mitigation research.
Methods and results:
This comprehensive review of the mechanism of action of niacin-induced flushing critically evaluates research regarding flushing mitigating formulations and agents. Niacin induces flushing through dermal Langerhans cells where the activation of G protein-coupled receptor 109A (GPR109A) increases arachidonic acid and prostaglandins, such as prostaglandin D2 (PGD2) and prostaglandin E2 (PGE2), subsequently activating prostaglandin D2 receptor (DP1), prostaglandin E2 receptor (EP2) and prostaglandin E receptor 4 (EP4) in capillaries and causing cutaneous vasodilatation. Controlling niacin absorption rates, inhibiting prostaglandin production, or blocking DP1, EP2 and EP4 receptors can inhibit flushing. Niacin extended-release (NER) formulations have reduced flushing incidence, duration and severity relative to crystalline immediate-release niacin with similar lipid efficacy. Non-steroidal anti-inflammatory drugs (NSAIDs), notably aspirin given 30 min before NER at bedtime, further reduce flushing. An antagonist to the DP1 receptor (laropiprant) combined with an ER niacin formulation can reduce flushing; however, significant residual flushing occurs with clinically-relevant dosages.
Conclusions:
Niacin is an attractive option for treating dyslipidemic patients, and tolerance to niacin-induced flushing develops rapidly. Healthcare professionals should particularly address flushing during niacin dose titration.
Review Criteria
Research regarding the mechanism of action of niacin and the formulations and agents used in the mitigation of flushing were systematically reviewed and summarised. PubMed was searched from 1960 to 2008 using the terms niacin, flushing, laropiprant, prostaglandins and aspirin. All hits were reviewed for inclusion of mechanism of action, and pertinent articles were included, excluding results which had been subsequently disproven.
Message for the Clinic
Niacin, an attractive option for treating dyslipidaemic patients, substantially improves most lipid parameters associated with atherosclerosis. However, flushing is a common non-allergenic response to niacin that reduces medication compliance. Several options to mitigate flushing symptoms prior to the development of tolerance are discussed. Clinical trials and practical experience indicate that a high level of medication compliance can be achieved if healthcare providers counsel their patients prior to starting niacin therapy.
Introduction
Niacin, either alone or in combination with a statin, safely and effectively addresses most lipid abnormalities in patients with mixed dyslipidaemia. Therapeutically used for more than 50 years, niacin is the most effective clinically available agent for increasing high-density lipoprotein cholesterol (HDL-C) levels. In most patients, niacin increases HDL-C by 20–40% (1–5). Niacin also has beneficial effects on all known pro-atherogenic lipid parameters, including lowering low-density lipoprotein cholesterol (LDL-C), non-HDL-C and triglycerides. It is the only current lipid therapy that decreases Lp(a), an independent risk factor for atherosclerosis (2,6). Niacin also has favourable effects on lipid particle size; it reduces small, dense LDL (7) while increasing cardio-protective HDL, as measured by either particle size (HDL2) (8) or by apolipoprotein profile (HDL containing apolipoprotein A-I without apolipoprotein A-II) (9). These alterations in lipids are clinically meaningful, as treatment with niacin has been associated with significant reductions in cardiovascular events and morbidity (10) and, in combination with statins, with regression of atherosclerotic cardiovascular disease (11).
Despite niacin’s numerous beneficial lipid effects, patient compliance to long-term therapy is challenged by flushing, a common side effect of niacin. A significant portion of the effects of niacin on flushing results from activation of the niacin receptor G protein-coupled receptor 109A (GPR109A) in dermal Langerhans cells (12,13), leading to the production of prostaglandins, including prostaglandin D2 (PGD2) and prostaglandin E2 (PGE2), which act on receptors in the capillaries. Flushing is characterised by cutaneous vasodilatation and manifests itself as redness or warmth of the skin, sometimes accompanied by tingling or itching. The onset of flushing can occur rapidly and usually lasts about 1 h. It is a transient, non-allergic response, but it may result in patient discomfort. In a randomised dose escalation trial, the mean incidence of flushing episodes decreased from the highest (2.7 per patient per month) with a 500-mg dose, and decreased to 1.1 with a 2000-mg dose (14). The incidence of flushing decreases with time as quickly as 1 week (15), because tolerance develops via decreased prostanoid (PGD2, a major mediator of flushing) secretion with repeated doses of niacin (15). This review summarises the metabolic responses to niacin that can lead to flushing and examines the current strategies to manage the effects of flushing in patients.
Niacin: mechanism of action
Physiologically, niacin influences lipoprotein metabolism by decreasing triglyceride synthesis via multiple pathways. In adipocytes, it inhibits the lipolysis of triglycerides and retards the mobilisation of free fatty acids (FFAs) to the plasma. As the liver uses plasma FFAs as substrates to form triglycerides, hepatic triglyceride production is decreased. Niacin can also reduce de novo synthesis of triglycerides in the liver by inhibiting the enzyme that catalyses the terminal reaction in cellular triglyceride synthesis, diacylglycerol acyltransferase 2 (DGAT2) (16). A reduction in hepatic triglyceride synthesis has important downstream effects on other lipoproteins. The production of very low density lipoprotein (VLDL) particles and thus VLDL-C is dependent on triglyceride synthesis in the liver, and IDL-C and LDL-C are derived from VLDL-C. Therefore, by decreasing hepatic triglyceride synthesis, niacin impairs synthesis of VLDL and thus decreases circulating levels of VLDL-C and subsequently IDL-C and LDL-C.
The mechanism through which niacin increases HDL-C is under investigation. Niacin does not appear to directly increase hepatic HDL particle or apolipoprotein A-I (the most abundant lipoprotein in HDL) synthesis. Instead, niacin likely prevents the catabolism of circulating HDL through several ways. Niacin decreases HDL catabolism by decreasing the fractional clearance of ApoA-I associated with HDL (17). When liver cells were treated with niacin, their uptake of HDL was inhibited, but the uptake of its cholesteryl ester was not (9). By preventing the hepatic catabolism of HDL, but not the uptake of the cholesteryl ester, niacin can increase the amount of circulating functional HDL, and thus facilitate reverse cholesterol transport. Therefore, the amount of HDL-ApoA-I-containing lipoprotein particles would be increased without increasing the rate of production of these particles. Recent research indicates that niacin decreases hepatocyte surface expression of beta-chain adenosine triphosphate (ATP) synthase (18), a mitochondrial protein reported to mediate hepatic HDL holoparticle endocytosis (19). These findings suggest that niacin, by downregulating hepatocyte surface expression of the beta-chain ATP synthase, reduces hepatic removal of HDL through holoparticle endocytosis, thus implicating a potential cellular receptor site for niacin’s action to raise plasma HDL.
Some of niacin’s effects on FFAs may be because of its properties as a high-affinity agonist for the G-protein coupled receptor, GPR109A protein-upregulated in macrophages by interferon-γ (PUMA-G) in mice (20–22). In humans, GPR109A is expressed in adipocytes, dermal immune cells (Langerhans) and macrophages, but not in the liver (23). Mice that lack the PUMA-G GPR109A receptor do not show improvements in lipid parameters after niacin treatment (21). Whether this mechanism operates in humans is unclear because of rebound lipolysis that occurs after the acute initial reduction (24). There is strong evidence that activation of the GPR109A (12) receptor in Langerhans cells (13) leads to flushing, even though the antilipolytic effects of niacin are likely mediated through GPR109A receptors in the adipocytes. Mice that lack the PUMA-G GPR109A receptor do not flush when administered niacin, but can flush after the receptor is restored in immune cells following a bone marrow transplant from normal mice (12).
In addition to lipid effects, recent research also suggested that niacin beneficially affects vascular inflammatory processes involved in atherogenesis. The findings from these studies indicate for the first time that niacin inhibits vascular inflammation by decreasing endothelial reactive oxygen species (ROS) production resulting in decreased endothelial expression of redox-sensitive genes, vascular cell adhesion molecule-1 (VCAM-1) and monocyte chemotactic protein-1 (MCP-1), and monocyte/macrophage adhesion and accumulation, key events in early atherogenesis (25). These in vitro studies describe a novel mechanistic role for niacin in decreasing atherosclerosis beyond its conventional role as a lipid-regulating agent.
Niacin-induced flushing: basic mechanism and mediators
Flushing symptoms occur following vasodilatation of small capillaries under the skin, a response that can be mediated via histamine/bradykinin or prostaglandins. Flushing is not unique to niacin; it has also been reported frequently by patients taking phosphodiesterase inhibitors, selective serotonin reuptake inhibitors (SSRIs), selective oestrogen receptor modulators (SERMs), adenosine and tretinoin. Topical and oral administration of niacin has not been associated with increases in blood levels of either histamine or bradykinin, suggesting that niacin-induced flushing is not mediated by mast cells (26,27). The release of histamine or bradykinin causes a substantial rise in nitric oxide, which leads to increased intracellular release of cyclic guanosine monophosphate (cGMP) and vasodilatation. Elimination of endothelial nitric oxide synthase (eNOS), an enzyme critical for NO production, did not stop niacin-induced flushing in mice (12), providing further support that the histamine/bradykinin pathway is not involved in niacin-induced flushing.
Prostaglandins (PGs), specifically forms D2 and E2, have been identified as participants in the niacin-induced flushing response (28,29). PGs, prostacylcins, thromboxanes and leukotrienes, collectively considered eicosanoids, are hormone-like chemical messengers derived from arachidonic acid. PGs have numerous biological effects, including essential roles in platelet aggregation, neurotransmitter release, and inflammatory and vasomotor responses. Individual prostaglandins can have both positive and negative effects (i.e. pro-inflammatory or anti-inflammatory, vasodilatory or vasoconstrictive) depending upon their concentration, relative proportion to other prostaglandins and expression of receptor types. As prostaglandins are rapidly metabolised and have short half-lives, their metabolic effects are typically localised and can be variable in different parts of the body.
The arachidonic acid cascade
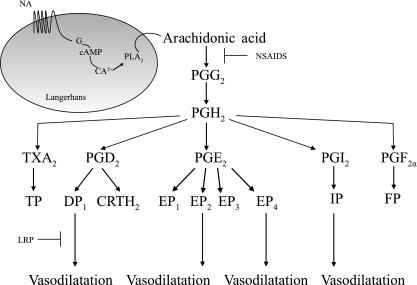
Activation of the GPR109A receptor by niacin initiates a signalling cascade that ultimately results in the production of prostaglandins, and thus, flushing (Figure 1). In Langerhans cells, niacin can activate GPR109A to increase intracellular Ca2+ (13). This Ca2+ increase triggers phospholipases, predominantly Phospholipase A2 (PLA2), to release arachidonic acid from cellular lipid stores (30). Free arachidonic acid serves as a precursor to the production of eicosanoids, including lipoxygenases, thromboxanes and prostaglandins.
Figure 1.
Niacin activates the arachidonic acid cascade to induce vasodilatation. Niacin activates the G-protein coupled receptor 109A (GPR109A) to increase cAMP and releases arachidonic acid from cell membranes. Arachidonic acid is metabolised to produce prostaglandins, prostacyclin and thromboxane. Activation of the prostaglandin D2 receptor (DP1), prostaglandin E2 receptor (EP2), EP4 and IP receptors can lead to vasodilatation that may contribute to flushing. NSAIDs block the metabolism of arachidonic acid, while LRP blocks DP1-mediated vasodilatation. cAMP, cyclic AMP; PLA2, phospholipase A2; PG, Prostaglandin; CRTH2, chemoattractant receptor homologous-molecule expressed on T helper type 2; NA, nicotinic acid; NSAIDs, non-steroidal anti-inflammatory drugs; LRP, laropiprant
The production of prostaglandins from arachidonic acid involves a complex cascade of enzymes. The first step is the metabolism of arachidonic acid to prostaglandin H2 (PGH2) by PGH synthase, an enzyme that has both cyclooxygenase and endoperoxidase activity, but is commonly referred to as COX. Sequential metabolism of arachidonic acid by COX produces prostaglandin G2 (PGG2), which is then reduced to PGH2, an unstable intermediate. Aspirin and related non-steroidal anti-inflammatory drugs (NSAIDs) can prevent the synthesis of prostaglandins by inhibiting both isoforms of COX (COX-1 and COX-2). The inhibition of COX also eliminates the flushing response to niacin (28,31–33). From PGH2, multiple prostaglandin synthase enzymes synthesise PGD2, PGE2, prostaglandin I2 (PGI2, prostacyclin), thromboxane A2 (TXA2, thromboxane) and prostaglandin F2α (PGF2α).
After their synthesis, prostaglandins exert their effects locally through downstream receptors. There are currently five known prostaglandin receptor families activated by prostaglandins: DP, prostaglandin E receptor (EP), prostacyclin receptor (IP), thromboxane A2 receptor (TP) and F2α receptor (FP). The EP receptors are further divided into four subtypes, prostaglandin E1 receptor (EP1), EP2, EP3 and EP4, and there are two DP receptor subtypes, DP1 and DP2, also called chemoattractant receptor homologous-molecule expressed on T helper type 2 (CRTH2). The receptors are categorised by their affinity for each respective prostaglandin agonist. PGE2 binds to the EP family, PGD2 binds to DP, PGI2 binds to IP and so forth. The downstream effects of the activation of each individual receptor can be dependent on the tissue expression of the receptor as well as the G-protein to which the receptor is coupled. These conditions allow some of the receptors to have opposing actions in the same tissue and result in complicated predictions of receptor activation. Broadly, these receptors can be divided into two groups, ‘relaxant’ or ‘excitatory’, according to their effects on smooth muscle. The relaxant receptors consist of DP, EP2, EP4 and IP, whereas TP, EP1 and FP are categorised as excitatory (34). Based on this classification, the relaxant receptors would be expected to play a role in cutaneous vasodilatation, while the excitatory receptors would act as vasoconstrictors.
Vasodilatory PGs
Through their downstream receptors, PGD2, PGE2 and PGI2 can all exhibit vasodilatory effects on smooth muscle cells in the vasculature, among other effects. Through the DP1 receptor, PGD2 inhibits platelet aggregation and mediates smooth muscle relaxation/contraction. Although PGD2 is known to have vasodilatory properties in the vascular endothelium, it can behave as a vasoconstrictor at higher concentrations and in separate tissues (35). PGD2 can also act through the chemoattractant CRTH2 (DP2) receptor, whose biological role appears to be regulating inflammatory allergic and asthmatic responses (36). PGE2 is perhaps the most widely produced prostaglandin, and as the highest-affinity agonist for the EP receptor family, it exerts the most diverse and versatile effects (37). The EP2 receptors are localised to smooth muscle in the trachea, GI tract and vascular system. They, along with EP4 receptors, are relaxant receptors and induce vasodilatation of various blood vessels through increasing cAMP. The PGE2-EP4 receptor pathway may also mediate some anti-inflammatory effects and facilitate mobilisation, migration and maturation of Langerhans cells in the skin (38). Prostacyclin is the major arachidonic acid product in vascular tissues (39). PGI2 is produced in blood vessels where it is a potent vasodilator and inhibitor of platelet aggregation through the IP receptor (34).
Vasoconstrictory PGs
Thromboxane and PGF2α, acting through the TP and FP receptors, respectively, are both potent vasoconstrictors. Thromboxane A2 (TXA2) plays an extensive role in haemodynamics and cardiovascular function. The majority of thromboxane produced in vivo is made by platelets, where it exhibits opposing actions to PGI2. TXA2 can increase platelet aggregation, and thus deficiencies in the TP receptor can lead to bleeding disorders. TP receptors are expressed in the thymus, spleen and lungs. Increased TXA2 has been linked to cardiovascular diseases including acute myocardial ischaemia and heart failure (40). In humans, PGF2α is a potent constrictor of pulmonary arteries and veins (41,42). It increases blood pressure in experimental animals, but not humans. As PGF2α and TXA2 are potent vasoconstrictors, they are unlikely candidates to induce flushing, but may blunt the actions of vasodilatory prostaglandins.
Prostaglandins involved in flushing
Several prostaglandins with vasodilatory properties are influenced by niacin. Specifically, levels of PGD2, PGE2, PGI2 and their metabolites have been shown to be increased as quickly as 12–45 min after niacin treatment (15,26,32,43). Their respective receptors, DP, EP2 and EP4 and IP, can all induce relaxation of blood vessels. After oral niacin treatment, PGD2 levels in the venous blood draining the skin are 14–1200 times higher than the levels in arterial blood (44). The production of PGI2 and PGD2 decreases after repetitive administration of niacin in parallel with the development of flushing tolerance (15). Further, methylnicotinate, which can deliver niacin transdermally, applied to a subject’s arm releases PGD2 only in the exposed arm, with no change in the untreated arm (44). Langerhans cells express PGD2 and PGE2 synthase enzymes (13), indicating they can produce PGD2 and PGE2 to activate receptors on blood vessels that lead to vasodilatation and contribute to flushing side effects. PUMA-G-deficient mice still flush when administered PGD2 (12). Likewise, humans pretreated with the NSAID indomethacin still flush when challenged with PGE (28). Separate deletions of the DP1, EP2 and EP4 in mice result in 40%, 20% and 40% reductions in flushing response, respectively, relative to normal mice after niacin administration (12). In comparison, deletions of COX in mice completely eliminate flushing after niacin treatment (12). Mice that do not express the IP receptor still flush after niacin (12). These experiments indicate that PGD2 and PGE2, signalling through the DP1, EP2 and EP4 receptors, are likely responsible for the flushing side effects of niacin.
Other products of arachidonic acid
Besides COX, arachidonic acid can be metabolised by a family of enzymes called lipoxygenases to produce leukotrienes (LT), a group of inflammatory lipid mediators. They are released from neutrophils, eosinophils, mast cells and macrophages to play a role in innate immunity (45). Leukotrienes mediate asthma effects, mucus secretion and bronchoconstriction/bronchodilation. Leukotriene B4 (LTB4) applied directly to the skin can cause vasodilatation that is not decreased by COX inhibitors, indicating that the vasodilatation is not mediated by prostaglandins (46). The downstream mechanism of LTB4-mediated vasodilatation is currently unknown. Patients treated with niacin have shown evidence of increases in leukotriene E4 (LTE4) but not LTB4 (47). Any potential effects of LTE4 on niacin-induced flushing have not been reported, and its role in vasodilatation is unclear.
Managing patient flushing
Considering niacin’s beneficial effects on nearly all lipid parameters associated with cardiovascular risk, and recent demonstration of its vascular anti-inflammatory properties (25), strategies to minimise or eliminate flushing should be deemed important to increase patient compliance to niacin therapy. As attenuation to flushing rapidly develops (28), dose titration is important in reducing flushing in patients. As a result of niacin’s mechanism of action, flushing can be managed in three ways: (i) by controlling the absorption rate of niacin; (ii) by preventing the production of prostaglandins; or (iii) by simultaneously blocking the DP1, EP2 and EP4 receptors. Educating patients about the benefits of niacin therapy may also increase the likelihood that patients are willing to tolerate any of the minor bothersome effects of flushing (48).
New formulations of niacin
Flushing can persist as long as plasma niacin levels are rising, but abates when constant plasma niacin levels are reached (49). Therefore, flushing is also related to the rate of niacin absorption, as a higher rate of absorption is associated with a higher rate of flushing (50,51). Crystalline, immediate-release (IR) niacin is rapidly absorbed by the body, and peak blood levels can be reached in as quickly as 30–60 min (52). As such, flushing incidence among patients taking IR niacin is close to 100%, and flushing was the major reason for discontinuation of IR niacin in several studies (53–55). To reduce flushing, several alternate formulations of niacin have been made. Sustained-release (SR) niacin formulations were created to delay niacin absorption during treatment. Although SR niacin decreases flushing, it can also cause hepatotoxicity and has shown inconsistent effects on lipids (55,56). Inositol hexanicotinate is commonly referred to as no-flush niacin or flush-free niacin, but this dietary supplement has not been shown to have any beneficial effects on lipid parameters (57,58).
Newer prescription niacin extended-release (NER) formulations (Niaspan®, Abbott, Abbott Park, IL, USA) have shown reduced flushing side effects relative to IR niacin while having equivalent efficacy to alter lipid parameters (5). Among healthy subjects receiving a single 2000 mg dose of a coated formulation of NER, there was a 42% reduction in median flush intensity and a 43% reduction in median flush duration relative to an older formulation of NER (59). In NER clinical trials, not more than 6% of subjects discontinued because of flushing (60). Flushing side effects are less with 1000 mg than 2000 mg of NER, and still retain roughly 70% of the improvement in HDL-C. Once daily dosing of NER in a 59 week-study showed only two subjects out of 723 having aspartate aminotransferase (AST) levels > 3 times the upper limit of normal (ULN) and no subjects with alanine aminotransferase (ALT) > 3 times ULN (1). Trials involving the combination of NER and a statin also show little evidence of hepatotoxicity (61–63).
NER can be safely combined with statins to lower LDL-C, triglycerides and non-HDL-C, while raising HDL-C. This combination therapy does not appear to worsen flushing side effects, and NER has not been shown to potentiate statin-induced myopathies. Combination treatment with NER and simvastatin (NER/S, SIMCOR®, Abbott Laboratories) in recent clinical trials showed this dual therapy was well tolerated (64–66). In a randomised study, comparing combination NER/S to simvastatin alone (SEACOAST I), only 7.5% of subjects receiving 1000/20 mg/day or 2000/20 mg/day NER/S discontinued because of flushing (64). In a similar study (SEACOAST II), discontinuations caused by flushing were not significantly different in subjects receiving NER/S 1000/40 mg/day (4.3%) or 2000/40 mg/day (5.0%) relative to subjects receiving simvastatin monotherapy and 50 mg/day of IR niacin. In a long-term open-label study in subjects with dyslipidaemia (OCEANS) previously treated with simvastatin, the discontinuation rate of subjects receiving 2000/40 mg/day NER/S was only 7% because of flushing (66). As tolerance developed to niacin alone, tolerance similarly developed to NER/S, as > 60% of the subjects who flushed during the first 12-week titration phase did not flush during weeks 41–52 (66).
Non-steroidal anti-inflammatory drugs
NSAIDs are convenient cotherapies with niacin to reduce flushing. They decrease the production of multiple prostaglandins by preventing COX from metabolising arachidonic acid. In a flush-provocative study, using healthy volunteers, aspirin given 30 min before a 2000 mg dose of NER decreased the incidence, duration and severity of flushing compared with placebo pretreatment (67). Among subjects receiving placebo, 77% of subjects reported flushing with newer formulations of ER niacin. However, only 53% of subjects receiving aspirin 30 min before a dose of a newer formulation of ER niacin flushed (67). Aspirin given concomitantly with NER was also effective at reducing flushing incidence, duration and severity compared with placebo, but not as effective as aspirin 30-min pretreatment (67). In a prospective, randomised, double-blind, placebo-controlled trial, 325 mg aspirin given 30 min before a dose of NER reduced both the number and intensity of flushing episodes, resulting in a lower rate of discontinuation because of flushing in the aspirin group compared with placebo (1.8% vs. 9.4%; p = 0.007) (68). Along with aspirin, indomethacin (33), ibuprofen (69) and naproxen (32) have been shown in subjects to decrease the flushing effects of IR niacin. The most common dose of aspirin used to effectively reduce flushing in IR niacin is 325 mg, with 650 mg offering no further benefit (70). Aspirin use appears to have no negative impact on niacin’s decrease of free fatty acids (33).
DP1 receptor antagonists
Recent efforts to decrease the flushing effects of niacin have focused on eliminating the downstream effects of prostaglandins that play a role in cutaneous vasodilatation. A highly selective DP1 antagonist, laropiprant [LRP, Merck/Merck Sharp & Dohm (MSD)] is currently in clinical trials to be used concomitantly with an alternate formulation of extended-release niacin from NER. In a small clinical trial, using healthy volunteers, LRP administered along with niacin decreased the symptoms of flushing compared with niacin plus placebo (71). However, about 70% of subjects taking a clinically relevant dose (30 mg) of LRP along with 1500 mg of niacin still flushed (71). This high incidence is likely caused by other pathways involved in the niacin flushing response. LRP is highly selective for DP1 and has no affinity for inhibiting the EP2 or EP4 receptors (72), which are also highly likely involved in flushing (12). This situation underscores the difficulty in modulating the downstream actions of prostaglandins for pharmacological effect.
GPR109 antagonists and other agents
Inhibiting the GPR109 receptor would theoretically mitigate the flushing response. Antagonism of the GPR109 receptor to reduce flushing while preserving adipocyte antilipolytic activity would require targeting the Langerhans-specific GPR109A receptor. The potential side effects are unknown. Another potential target would be to inhibit the ability of PLA2 to produce arachidonic acid, thereby eliminating the production of prostaglandins upstream of COX. Glucocorticoids can indirectly inhibit PLA2 (73,74), but there are currently no approved therapies that specifically target this enzyme.
Patient education
One of the easiest ways that medical personnel can help improve their patients compliance with niacin therapy is to provide their patients with a clear understanding of the clinical benefits of niacin as well as what to expect and how to manage flushing (48). While improvements in lipid profiles are meaningful to physicians, patients may be more willing to tolerate the transient flushing symptoms that can occur if they realise that niacin reduces cardiovascular risk, as determined by both mortality and cardiovascular event rates, and that this benefit extends beyond the duration of active therapy. Patients should be counselled to take aspirin (325 mg) 30 min before a snack and extended-release niacin. The importance of continuing to take the final daily maintenance dose of niacin extended-release should be emphasised. If there is a period of discontinuation, the titration procedure has to be followed again, although it maybe possible to accelerate it at the discretion of the prescriber. Patients are also advised to avoid hot beverages, spicy foods and hot showers near the time of taking niacin.
Conclusions
Given niacin’s beneficial effects across a wide spectrum of the lipid profile, proven safety record and ability to be safely combined with statins, it should be regarded as an attractive option for the treatment of dyslipidaemia. The same receptor that is responsible for niacin’s decrease in free fatty acids is also likely the same receptor that is responsible for the flushing side effects. Therefore, it is currently difficult to separate the two effects, but flushing can be effectively managed in patients. As tolerance to flushing develops rapidly, healthcare professionals should particularly address flushing during dose titration of niacin. Clinically, unlike LRP, aspirin is not only an established agent to reduce flushing but it is also indicated for use in most dyslipidemic patients to reduce atherothrombotic complications, which is also the reason to prescribe niacin. As COX is upstream of PGD2, NSAIDs have the ability to block production of PGD2, PGE2 and PGI2. LRP has only the ability to block PGD2-mediated flushing. Initial data indicate that aspirin or LRP combined with extended-release niacin formulations have similar impacts on niacin-induced flushing in patients, although it is difficult to directly compare separate clinical studies. A clinical trial to assess the relative efficacy of these two agents is needed. At least in mice, deletion of the COX enzyme decreased flushing by almost 100%, while elimination of the DP1 receptor only decreased flushing by 40% after niacin treatment. Aspirin has a well-known safety profile, while long-term data on the safety of LRP is awaited. Future therapies that can preserve or even enhance niacin’s important lipid effects while eliminating flushing will likely be important improvements in the treatment of dyslipidaemia.
Acknowledgments
The authors would like to acknowledge Nathan A. Styles, Ph.D., and Janet E. Horton, BGS, Abbott employees, for medical writing and editing assistance in developing this article.
Author contributions
Vaijinath S. Kamanna, Ph.D., Shobha H. Ganji, Ph.D. and Moti L. Kashyap, M.D. developed the concept and content of this review and provided interpretation of the research summarised, critical revision and final approval of this article.
References
- 1.Capuzzi DM, Guyton JR, Morgan JM, et al. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82:74U–81U. doi: 10.1016/s0002-9149(98)00731-0. [DOI] [PubMed] [Google Scholar]
- 2.Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–6. doi: 10.1111/j.1365-2796.1989.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 3.Guyton JR, Goldberg AC, Kreisberg RA, Sprecher DL, Superko HR, O’Connor CM. Effectiveness of once-nightly dosing of extended-release niacin alone and in combination for hypercholesterolemia. Am J Cardiol. 1998;82:737–43. doi: 10.1016/s0002-9149(98)00448-2. [DOI] [PubMed] [Google Scholar]
- 4.Guyton JR, Blazing MA, Hagar J, et al. Extended-release niacin vs gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterol. Niaspan-Gemfibrozil Study Group. Arch Intern Med. 2000;160:1177–84. doi: 10.1001/archinte.160.8.1177. [DOI] [PubMed] [Google Scholar]
- 5.Knopp RH, Alagona P, Davidson M, et al. Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism. 1998;47:1097–104. doi: 10.1016/s0026-0495(98)90284-0. [DOI] [PubMed] [Google Scholar]
- 6.Berglund L, Ramakrishnan R. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol. 2004;24:2219–26. doi: 10.1161/01.ATV.0000144010.55563.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Superko HR, McGovern ME, Raul E, Garrett B. Differential effect of two nicotinic acid preparations on low-density lipoprotein subclass distribution in patients classified as low-density lipoprotein pattern A, B, or I. Am J Cardiol. 2004;94:588–94. doi: 10.1016/j.amjcard.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Wahlberg G, Walldius G, Olsson AG, Kirstein P. Effects of nicotinic acid on serum cholesterol concentrations of high density lipoprotein subfractions HDL2 and HDL3 in hyperlipoproteinaemia. J Intern Med. 1990;228:151–7. doi: 10.1111/j.1365-2796.1990.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Kamanna VS, Kashyap ML. Niacin, but not gemfibrozil, selectively increases LP-AI, a cardioprotective subfraction of HDL, in patients with low HDL-cholesterol. Arterioscler Thromb Vasc Biol. 2001;21:1783–9. doi: 10.1161/hq1001.096624. [DOI] [PubMed] [Google Scholar]
- 10.Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–81. [PubMed] [Google Scholar]
- 11.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 12.Benyó Z, Gille A, Kero J, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–40. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol. 2006;70:1844–9. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg A, Alagona P, Jr, Capuzzi DM, et al. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am J Cardiol. 2000;85:1100–5. doi: 10.1016/s0002-9149(00)00703-7. [DOI] [PubMed] [Google Scholar]
- 15.Stern RH, Spence JD, Freeman DJ, Parbtani A. Tolerance to nicotinic acid flushing. Clin Pharmacol Ther. 1991;50:66–70. doi: 10.1038/clpt.1991.104. [DOI] [PubMed] [Google Scholar]
- 16.Ganji SH, Tavintharan S, Zhu D, Xing Y, Kamanna VS, Kashyap ML. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J Lipid Res. 2004;45:1835–45. doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Blum CB, Levy RI, Eisenberg S, Hall M, III, Goebel RH, Berman M. High density lipoprotein metabolism in man. J Clin Invest. 1977;60:795–807. doi: 10.1172/JCI108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang LH, Kamanna VS, Zhang MC, Kashyap ML. Niacin inhibits surface expression of ATP synthase beta chain in HepG2 cells: implications for raising HDL. J Lipid Res. 2008;49:1195–201. doi: 10.1194/jlr.M700426-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Martinez LO, Jacquet S, Esteve JP, et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzen A, Stannek C, Lang H, Andrianov V, Kalvinsh I, Schwabe U. Characterization of a G protein-coupled receptor for nicotinic acid. Mol Pharmacol. 2001;59:349–57. doi: 10.1124/mol.59.2.349. [DOI] [PubMed] [Google Scholar]
- 21.Tunaru S, Kero J, Schaub A, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–5. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 22.Wise A, Foord SM, Fraser NJ, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–74. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzen A, Stannek C, Burmeister A, Kalvinsh I, Schwabe U. G protein-coupled receptor for nicotinic acid in mouse macrophages. Biochem Pharmacol. 2002;64:645–8. doi: 10.1016/s0006-2952(02)01220-0. [DOI] [PubMed] [Google Scholar]
- 24.Kamanna VS, Kashyap ML. Nicotinic Acid (niacin) receptor agonists: will they be useful therapeutic agents? Am J Cardiol. 2007;100:S53–61. doi: 10.1016/j.amjcard.2007.09.080. [DOI] [PubMed] [Google Scholar]
- 25.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [Epub 2008 May 9.] PMID: 18550065 [PubMed - as supplied by publisher] [DOI] [PubMed] [Google Scholar]
- 26.Morrow JD, Parsons WG, III, Roberts LJ., II Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 1989;38:263–74. doi: 10.1016/0090-6980(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 27.Plummer NA, Hensby CN, Black AK, Greaves MW. Prostaglandin activity in sustained inflammation of human skin before and after aspirin. Clin Sci Mol Med. 1977;52:615–20. doi: 10.1042/cs0520615. [DOI] [PubMed] [Google Scholar]
- 28.Andersson RG, Aberg G, Brattsand R, Ericsson E, Lundholm L. Studies on the mechanism of flush induced by nicotinic acid. Acta Pharmacol Toxicol (Copenh) 1977;41:1–10. doi: 10.1111/j.1600-0773.1977.tb02116.x. [DOI] [PubMed] [Google Scholar]
- 29.Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82:29U–34U. doi: 10.1016/s0002-9149(98)00732-2. [DOI] [PubMed] [Google Scholar]
- 30.Lin LL, Lin AY, Knopf JL. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992;89:6147–51. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svedmyr N, Heggelund A, Aberg G. Influence of indomethacin on flush induced by nicotinic acid in man. Acta Pharmacol Toxicol (Copenh) 1977;41:397–400. doi: 10.1111/j.1600-0773.1977.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 32.Eklund B, Kaijser L, Nowak J, Wennmalm A. Prostaglandins contribute to the vasodilation induced by nicotinic acid. Prostaglandins. 1979;17:821–30. doi: 10.1016/0090-6980(79)90055-8. [DOI] [PubMed] [Google Scholar]
- 33.Kaijser L, Eklund B, Olsson AG, Carlson LA. Dissociation of the effects of nicotinic acid on vasodilatation and lipolysis by a prostaglandin synthesis inhibitor, indomethacin, in man. Med Biol. 1979;57:114–7. [PubMed] [Google Scholar]
- 34.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–29. [PubMed] [Google Scholar]
- 35.Lee SL, Levitsky S, Feinberg H. Endogenous vasoconstrictor prostanoids: role in serotonin and vasopressin-induced coronary vasoconstriction. J Pharmacol Exp Ther. 1991;258:292–8. [PubMed] [Google Scholar]
- 36.Nagata K, Hirai H. The second PGD (2) receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:169–77. doi: 10.1016/s0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 38.Kabashima K, Narumiya S. The DP receptor, allergic inflammation and asthma. Prostaglandins Leukot Essent Fatty Acids. 2003;69:187–94. doi: 10.1016/s0952-3278(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 39.Arehart E, Gleim S, Kasza Z, Fetalvero KM, Martin KA, Hwa J. Prostacyclin, atherothrombosis, and cardiovascular disease. Curr Med Chem. 2007;14:2161–9. doi: 10.2174/092986707781389637. [DOI] [PubMed] [Google Scholar]
- 40.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Giles H, Leff P. The biology and pharmacology of PGD2. Prostaglandins. 1988;35:277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 42.Spannhake EW, Hyman AL, Kadowitz PJ. Bronchoactive metabolites of arachidonic acid and their role in airway function. Prostaglandins. 1981;22:1013–26. doi: 10.1016/0090-6980(81)90028-9. [DOI] [PubMed] [Google Scholar]
- 43.Nozaki S, Kihara S, Kubo M, Kameda K, Matsuzawa Y, Tarui S. Increased compliance of niceritrol treatment by addition of aspirin: relationship between changes in prostaglandins and skin flushing. Int J Clin Pharmacol Ther Toxicol. 1987;25:643–7. [PubMed] [Google Scholar]
- 44.Morrow JD, Awad JA, Oates JA, Roberts LJ., II Identification of skin as a major site of prostaglandin D2 release following oral administration of niacin in humans. J Invest Dermatol. 1992;98:812–5. doi: 10.1111/1523-1747.ep12499963. [DOI] [PubMed] [Google Scholar]
- 45.Whatling C, McPheat W, Herslof M. The potential link between atherosclerosis and the 5-lipoxygenase pathway: investigational agents with new implications for the cardiovascular field. Expert Opin Investig Drugs. 2007;16:1879–93. doi: 10.1517/13543784.16.12.1879. [DOI] [PubMed] [Google Scholar]
- 46.Larkin SW, Fraser L, Showell HJ, Williams TJ, Warren JB. Prolonged microvascular vasodilation induced by leukotriene B4 in human skin is cyclooxygenase independent. J Pharmacol Exp Ther. 1995;272:392–8. [PubMed] [Google Scholar]
- 47.Saareks V, Mucha I, Sievi E, Riutta A. Nicotinic acid and pyridoxine modulate arachidonic acid metabolism in vitro and ex vivo in man. Pharmacol Toxicol. 1999;84:274–80. doi: 10.1111/j.1600-0773.1999.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 48.Davidson MH. Niacin therapy: an evolving paradigm for the management of mixed dyslipidemia and low high-density lipoprotein cholesterol. Am J Cardiol. 2008;101(Suppl. 1):S14–9. doi: 10.1016/j.amjcard.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Svedmyr N, Harthon L, Lundholm L. The relationship between the plasma concentration of free nicotinic acid and some of its pharmacologic effects in man. Clin Pharmacol Ther. 1969;10:559–70. doi: 10.1002/cpt1969104559. [DOI] [PubMed] [Google Scholar]
- 50.Pieper JA. Understanding niacin formulations. Am J Manag Care. 2002;8:S308–14. [PubMed] [Google Scholar]
- 51.Piepho RW. The pharmacokinetics and pharmacodynamics of agents proven to raise high-density lipoprotein cholesterol. Am J Cardiol. 2000;86:35L–40L. doi: 10.1016/s0002-9149(00)01468-5. [DOI] [PubMed] [Google Scholar]
- 52.McKenney J. Niacin for dyslipidemia: considerations in product selection. Am J Health Syst Pharm. 2003;60:995–1005. doi: 10.1093/ajhp/60.10.995. [DOI] [PubMed] [Google Scholar]
- 53.Gibbons LW, Gonzalez V, Gordon N, Grundy S. The prevalence of side effects with regular and sustained-release nicotinic acid. Am J Med. 1995;99:378–85. doi: 10.1016/s0002-9343(99)80185-5. [DOI] [PubMed] [Google Scholar]
- 54.Knopp RH, Ginsberg J, Albers JJ, et al. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: clues to mechanism of action of niacin. Metabolism. 1985;34:642–50. doi: 10.1016/0026-0495(85)90092-7. [DOI] [PubMed] [Google Scholar]
- 55.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271:672–7. [PubMed] [Google Scholar]
- 56.Christensen NA, Achor RW, Berge KG, Mason HL. Nicotinic acid treatment of hypercholesteremia. Comparison of plain and sustained-action preparations and report of two cases of jaundice. JAMA. 1961;177:546–50. doi: 10.1001/jama.1961.03040340010003. [DOI] [PubMed] [Google Scholar]
- 57.Meyers CD, Carr MC, Park S, Brunzell JD. Varying cost and free nicotinic acid content in over-the-counter niacin preparations for dyslipidemia. Ann Intern Med. 2003;139:996–1002. doi: 10.7326/0003-4819-139-12-200312160-00009. [DOI] [PubMed] [Google Scholar]
- 58.Norris RB. “Flush-free niacin”: dietary supplement may be “benefit-free”. Prev Cardiol. 2006;9:64–65. doi: 10.1111/j.1520-037x.2006.04736.x. [DOI] [PubMed] [Google Scholar]
- 59.Cefali EA, Simmons PD, Stanek EJ, Shamp TR. Improved control of niacin-induced flushing using an optimized once-daily, extended-release niacin formulation. Int J Clin Pharmacol Ther. 2006;44:633–40. doi: 10.5414/cpp44633. [DOI] [PubMed] [Google Scholar]
- 60.NIASPAN® [package insert] Abbott Park, IL: Abbott Laboratories; 2007. [Google Scholar]
- 61.Hunninghake DB, McGovern ME, Koren M, et al. A dose-ranging study of a new, once-daily, dual-component drug product containing niacin extended-release and lovastatin. Clin Cardiol. 2003;26:112–8. doi: 10.1002/clc.4960260304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashyap ML, McGovern ME, Berra K, et al. Long-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemia. Am J Cardiol. 2002;89:672–8. doi: 10.1016/s0002-9149(01)02338-4. [DOI] [PubMed] [Google Scholar]
- 63.McKenney JM, Jones PH, Bays HE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study) Atherosclerosis. 2007;192:432–7. doi: 10.1016/j.atherosclerosis.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 64.Ballantyne CM, Davidson MH, McKenney J, Keller LH, Bajorunas DR, Karas RH. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I study) Am J Cardiol. 2008;101:1428–36. doi: 10.1016/j.amjcard.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 65.Ballantyne CM, Davidson MH, McKenney JM, Keller LH, Bajorunas DR, Karas RH. Comparison of the efficacy and safety of a combination tablet of niacin extended-release and simvastatin with simvastatin 80 mg monotherapy: the SEACOAST II (high dose) study. J Clin Lipidol. 2008;2:79–90. doi: 10.1016/j.jacl.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Karas RH, Kashyap ML, Knopp RH, Keller LH, Bajournas DR, Davidson MH. Long-term safety and efficacy of a combination of niacin extended-release and simvastatin in patients with dyslipidemia. Am J Cardiovasc Drugs. 2008;8:69–81. doi: 10.2165/00129784-200808020-00001. [DOI] [PubMed] [Google Scholar]
- 67.Cefali EA, Simmons PD, Stanek EJ, McGovern ME, Kissling CJ. Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation. Int J Clin Pharmacol Ther. 2007;45:78–88. doi: 10.5414/cpp45078. [DOI] [PubMed] [Google Scholar]
- 68.Thakkar RB, Kashyap ML, Lewin AJ, Krause SL, Jiang P, Padley RJ. Acetylsalicylic acid reduces niacin extended-release–induced flushing in patients with dyslipidemia. Am J Cardiovasc Drugs. 2009;9:69–79. doi: 10.1007/BF03256578. [DOI] [PubMed] [Google Scholar]
- 69.Dunn RT, Ford MA, Rindone JP, Kwiecinski FA. Low-dose aspirin and ibuprofen reduce the cutaneous reactions following niacin administration. Am J Ther. 1995;2:478–80. doi: 10.1097/00045391-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Jungnickel PW, Maloley PA, Vander Tuin EL, Peddicord TE, Campbell JR. Effect of two aspirin pretreatment regimens on niacin-induced cutaneous reactions. J Gen Intern Med. 1997;12:591–6. doi: 10.1046/j.1525-1497.1997.07118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai E, De Lepeleire I, Crumley TM, et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin Pharmacol Ther. 2007;81:849–57. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- 72.Cheng K, Wu TJ, Wu KK, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006;103:6682–7. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirata F, Schiffmann E, Venkatasubramanian K, Salomon D, Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980;77:2533–6. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newton R, Kuitert LM, Slater DM, Adcock IM, Barnes PJ. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci. 1997;60:67–78. doi: 10.1016/s0024-3205(96)00590-5. [DOI] [PubMed] [Google Scholar]