Abstract
Background
The prevalence of abdominal aortic aneurysms (AAA) with a maximal diameter of 3 cm or more is age-dependent; among persons over age 65, it lies between 4% and 8% in men and between 0.5% and 1.5% in women. About 10% of all AAAs have a maximum diameter of 5 cm or more. The prognosis of ruptured AAA (rAAA) is dismal, with an overall mortality of at least 80%. Ultrasonography of the abdominal aorta is a safe and technically simple method of detecting AAAs.
Methods
Evaluation of population-based, randomized studies of ultrasonographic screening for the detection of AAA, based on a selective review of the literature.
Results
A meta-analysis of four randomized controlled studies showed that ultrasonographic screening was associated with a significant lowering of AAA-related mortality in men aged 65 to 80 after it had been performed for 3–5 years (risk reduction 44%, odds ratio [OR] 0.56, 95% confidence interval [CI] 0.44–0.72) and after it had been performed for 7–15 years (risk reduction 53%, OR 0.47, 95% CI 0.25–0.90). AAA screening was also associated with a significant lowering of the overall mortality after 7–15 years, but not in the first 5 years. Ultrasonographic screening led to a significant increase in the number of elective AAA operations performed and to a 50% reduction of the number of emergency operations for rAAA.
Conclusion
Ultrasonographic screening for AAA is a technically simple diagnostic test that is associated with a major reduction of AAA-related mortality. In view of the higher prevalence of AAA among the elderly, it is recommended that all men aged 65 or older and all men and women with a family history of AAA should be systematically screened. A national ultrasound screening program should be urgently implemented in Germany in order to bring about a major reduction in AAA-associated mortality.
Keywords: abdominal aortic aneurysm, ultrasonographic diagnosis, aneurysm, vascular diagnosis, aortic surgery
The total number of patients treated in German hospitals for a non-ruptured abdominal aortic aneurysm (AAA) (I71.4) in 2002 was 11 697; it has since risen slightly to 12 531 in 2007. Women make up 15% of this figure. The number of patients with ruptured AAA (rAAA, I71.3) has also risen, from 1899 in 2000 to 2350 in 2007.
In England and Wales, about 1.9% of all deaths in men and 0.9% of all deaths in women every year are due to a rAAA; in the USA around 9000 persons die every year from this cause (1, 2). According to German Federal mortality figures, in 2007 a total of 1295 persons (936 men, 359 women) died of a rAAA. In view of the 21 691 sudden deaths each year for which no cause is given (R00 to R99), however, it must be assumed that the real figure for AAA-related mortality is much higher.
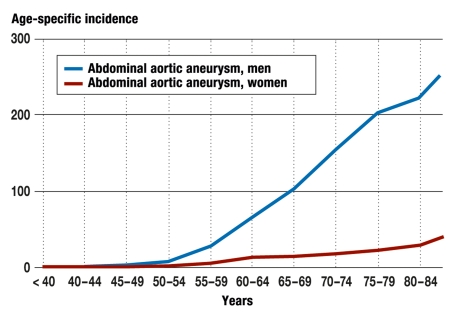
According to data from the German Federal Statistical Office (Statistisches Bundesamt Deutschland) (hospital statistics related to payments per case), in 2007 a total of 8846 open AAA operations and 3966 endovascular stent implantations (endovascular aortic repair, EVAR) were carried out. Thus, about 85% of all patients with a main diagnosis of AAA do in fact undergo operative treatment, about two-thirds of them by open surgery and one-third by implantation of an endovascular prosthetic stent. Analysis of age-specific incidence for all AAA (with or without rupture) shows that AAA larger than 5 cm, particularly in older men, require treatment. The incidence is 283/100 000 in men aged over 85 years, but only 47/100 000 in women over 85 (3) (figure 1).
Figure 1.
Age-specific incidence of male and female inpatients with a main diagnosis of abdominal aortic aneurysm with or without rupture (I71.3, I71.4). (Source: Diagnostic data on all hospital patients 2007, German Federal Statistical Office)
Since the proportion of ruptured AAAs has risen slowly but steadily in the past few years (14% in 2000, 18% in 2008), and since in vascular surgery centers only a maximum of 10% of all patients with AAAs are admitted at the ruptured stage, this high proportion of ruptured AAAs could be a sign of regional deficits in care provision and the absence of a systematic screening program (e1).
Materials and methods
Based on a selective literature search on Medline, original publications, meta-analyses, systematic reviews, and Cochrane reviews about screening for abdominal aortic aneurysms were evaluated. The following search terms were used: “epidemiology,” “risk factors for development of an AAA,” “risk factors for rupture of an AAA,” “indications and therapeutic procedures for operative treatment,” and “randomized studies on ultrasound screening.” In addition, data from the German Federal Statistical Office relating to diagnostic data for all hospital patients in 2007 plus hospital statistics relating to payment per case were also included (3).
Epidemiology and risk factors
Abdominal aortic aneurysms develop against a background of chronic deterioration and inflammation of the aortic wall. In up to 20% of all cases of AAA there is an underlying genetic predisposition, with a prevalence of AAAs of 13% to 19% among first-degree relatives of patients with an AAA (e2).
In an arterial aneurysm, the artery is 1.5 times wider than normal. Since the infrarenal aortic diameter is usually about 2 cm, in epidemiological studies an aortic aneurysm is defined by a diameter of 3.0 cm or more. It needs to be remembered, however, that the aortic diameter increases with age, and is larger in men than in women. In everyday clinical practice, therefore, a diameter of 3 to 4 cm is often referred to as aneurysmal widening of the infrarenal aorta, or ectatic abdominal aorta.
Prevalence figures for abdominal aortic aneurysm with a maximum diameter of at least 3.0 cm in screening programs are 5.5% (4% to 8%) for men over 65 and 1.3% (0.5% to 1.5%) for women over 65. In a quarter of all AAA cases, the diameter is 4 cm or more, and in about 10% of cases it is 5 cm or more (5– 8). In 2007 in Germany, 16.3 million people were over the age of 65 (around 7 million men and 9 million women). Extrapolating from these prevalence figures, this would imply that around 100 000 of all men aged over 65 have an AAA with a diameter of 4 cm or more, and around 35 000 of all men over 65 have an AAA with a diameter of 5 cm or more (4– 7). Among women aged over 65, there would be a total of around 30 000 AAA measuring 4 cm or more and 10 000 AAA of 5 cm or more.
The following clinical risk factors for development of an AAA have been identified:
Increasing age
Family history of AAA
Current or previous history of smoking
Coronary heart disease
Arterial hypertension.
Female sex, African ethnic origin, and diabetes mellitus all have a protective effect, probably via a genetic mechanism (8) (figure 2).
Figure 2.
Clinical risk factors for the development of an abdominal aortic aneurysm. Evaluation of eight different population-based studies including more than 110 000 probands (8)
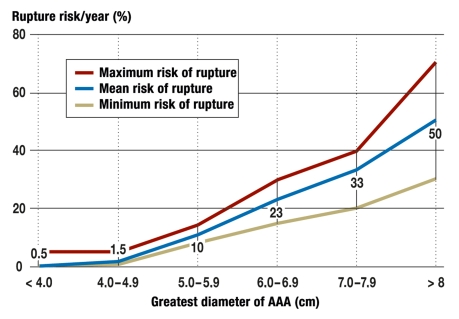
The average rate of growth of an AAA is 2 to 3 mm per year; it is higher in smokers, and can vary greatly between individuals (9). The risk of rupture of an AAA smaller than 4 cm is below 2% per year, but increases exponentially once a diameter of 5 cm is passed (figure 3). Although women have a lower prevalence of AAA, when they have an AAA with a diameter of 5 to 6 cm the risk of rupture is three times as high as in men (10).
Figure 3.
Relationship between the diameter of an AAA and its annual risk of rupture
Apart from maximum diameter, risk factors for impending rupture include rapid increase in diameter (> 0.5 to 1 cm per year), family history of AAA, eccentric morphology of the AAA, and continued smoking (11, 12). Overexpression of MMP9 and lack of alpha1-antitrypsin have also been associated with an increasing risk of rupture (e3, e4). The prognosis of a ruptured AAA is extremely poor, with a hospital mortality in Germany of 55% (1295 out of 2350 ruptured AAAs in 2007). Overall mortality is more than 80%, because not all patients reach hospital alive. The only valid intervention against impending AAA rupture is therefore elective operative treatment of the AAA.
Treatment indications and operative procedures
Operative treatment of an AAA is indicated when the AAA diameter has reached 5 to 5.5 cm (4.5 to 5 cm in women) (13, 14). Two randomized studies have demonstrated that AAAs smaller than this have a low risk of rupture (< 2%) (table 1) (14), and that over the course of 5 to 8 years preventive surgery of small AAAs (4 to 5 cm) does not significantly reduce AAA-associated mortality (odds ratio [OR]: 0.78; 95% confidence interval [CI]: 0.56 to 1.10) or overall mortality (OR: 1.01; 95% CI: 0.77 to 1.32) (10). However, about 70% of all aneurysms that are initially smaller do require operation later because their diameters increase above 5 cm.
Table 1. Correlation between greatest diameter and annual and 5-year risks of rupture (%) (14).
| Diameter | Annual risk of rupture | Risk of rupture within 5 years |
| < 3 cm | 0% | No data |
| 3–3.9 cm | 0.4% | 1–2% |
| 4–4.9 cm | 1.1% | 5–13% |
| 5–5.9 cm | 3.3% | 25–38% |
| 6–6.9 cm | 9.4% | No data |
| 7–7.9 cm | 24% | No data |
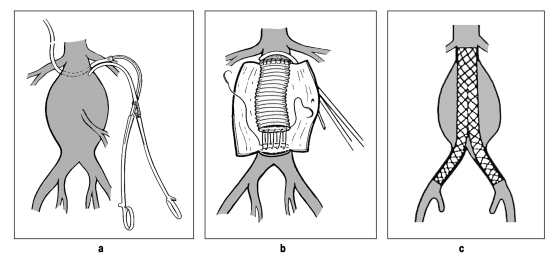
Operative treatment of an AAA may be through open replacement of the abdominal aorta using a tube or bifurcation prosthesis or may be done as a closed procedure by implantation of a prosthetic stent via the femoral arteries (endovascular aortic repair, EVAR) (figure 4). In a systematic review of all randomized studies, the 30-day mortality after EVAR was 1.5%, while after open aortic replacement it was 4.6% (OR: 0.33; 95% CI: 0.17 to 0.64). AAA-related mortality at 2 to 3 years was also significantly reduced by EVAR (OR: 0.53; 95% CI: 0.31–0.92), but overall mortality was not (OR: 0.95; 95% CI: 0.76 to 1.19) (10, 15). The latter observation may be explained, first, by the small number of randomized studies and the numbers of patients included in them, and, second, perhaps also by the effect of concomitant cardiovascular diseases (15). Reliable long-term follow-up data after EVAR are not yet available.
Figure 4.
Open (a, b) and endovascular (c) treatment of an abdominal aortic aneurysm (Source: Vascular International Foundation, Pontresina Manual)
Low complication rates after open and endovascular AAA treatment are achieved by hospitals with expertise in microvascular surgery with high caseloads. In a recent review of more than 450 000 patients, a significant reduction of hospital mortality was achieved by hospitals in which a minimum of 43 elective and 15 emergency operations were carried out annually (16).
Because of the better results, amply demonstrated in the literature, that are achieved in hospitals with good structure and process quality, the Joint Federal Committee (Gemeinsamer Bundesausschuss, GBA) decided from July 2009 to permit vascular reconstructive surgery of AAAs in Germany only in hospitals with at least two specialist microvascular surgeons and where round-the-clock microvascular surgical care is guaranteed. In addition, expertise in conventional and endovascular AAA surgery is also required. A minimum number of cases is not required, in order to avoid false incentivization, e.g., operations on small AAAs. This structural requirement of the GBA will gradually lead to centralization of AAA treatment (17).
Diagnosis by ultrasound
More than 80% of all AAAs are clinically asymptomatic and are discovered by chance or during an ultrasound examination (figure 5) (e5). A transverse cross-section of the upper abdomen and a paramedian longitudinal section of the aorta are visualized. The longitudinal and transverse extent of the aorta and the aneurysm, the transverse extent of the remaining perfused lumen, and the thickness and distribution of the edge of the thrombus including the branching off of the celiac trunk, the superior mesenteric artery, and the renal arteries can all be individually documented. In addition, the common iliac arteries and the external iliac arteries can also be visualized (18). Ultrasonography is a valid diagnostic technique with a sensitivity and specificity of 98% (19).
Figure 5.
B-mode ultrasonogram of an abdominal aortic anuerysm in cross-section, showing the perfused lumen at the edge of the thrombus
Randomized studies of ultrasound screening
A total of four randomized population-based studies evaluating ultrasound screening for AAAs have been published; together they include more than 135 000 probands (20). With one exception (Chichester), only men between 65 and 83 years of age were examined. In three studies (MASS, Chichester, Western Australia), patients from care homes were excluded; two studies (MASS, Chichester) also excluded patients judged by their primary care physician to be unfit to undergo surgery. Participants in the study were either invited to an ultrasound screening session or remained under the care of their primary care physician in the usual way. Participation rates in the screening groups were between 63% and 80%. Evaluation of the various endpoints was according to the “intention to treat” principle, i.e. on the basis of all those invited to take part (table 2).
Table 2. Overview of the randomized studies of ultrasound screening for abdominal aortic aneurysms (AAA) (1).
| MASS (UK) | Western Australia | Viborg (Denmark) | Chichester (UK), men | Chichester (UK), women | |
| Age (years) | 65–74 | 65–83 | 65–73 | 65–80 | 65–80 |
| Sex | Men | Men | Men | Men | Women |
| Randomized | 67 800 | 38 704 | 12 658 | 6433 | 9342 |
| Follow-up (years) | 7 | 3.6 | 9.6 | 15 | 5 |
| Screening achieved | 80% | 63% | 77% | 73% | 65% |
| Prevalence of AAA >3 cm | 4.9% | 7.2% | 4.0% | 7.6% | 1.3% |
In the MASS trial, in Chichester, and in Viborg (Denmark), patients with an AAA measuring 5 to 6 cm in diameter were referred to a vascular surgeon; probands with smaller aneurysms attended regular follow-up. In all studies, all patients whose AAA progressed to a diameter of more than 5 to 6 cm during the course of the study were also referred to a vascular surgeon; in the MASS and Chichester studies, patients with a smaller aneurysm that increased in diameter at a rate of more than 1 cm per year were also referred. In all studies, probands with an infrarenal aortic diameter of less than 3 cm were not examined again.
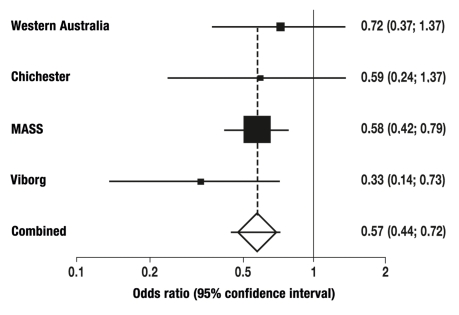
The meta-analysis of all four randomized studies shows a highly significant reduction of AAA-associated mortality in 64- to 83-year-old men in medium-term (3 to 5 years) and long-term follow-up (7 to 15 years), by 44% and 53% respectively (Figure 6, Table 3). A significant reduction in overall mortality was shown after 7 to 15 years, but not during the first 5 years. Ultrasound screening led to a significant increase in elective AAA operations (OR: 3.27 after 5 years and 2.83 after 7 to 15 years), and to a significant reduction by about 50% of emergency operations on ruptured AAAs (table 3).
Figure 6.
Relative risk reduction from systematic ultrasound screening: meta-analysis of four randomized studies
Table 3. Middle- and long-term effects of ultrasound screening*1.
| Follow-up at 3–5 years | Screening, n = 62 729 | Control, n = 62 847 | Odds ratio (95% CI) | P |
| AAA-related deaths | 102 | 182 | 0.56 (0.44–0.72) | < 0.0001 |
| Overall deaths | 7 453 | 7 953 | 0.94 (0.86–1.02) | 0.14 |
| Elective AAA ‧operations | 505 | 162 | 3.27 (2.15–5.00) | < 0.0001 |
| Emergency ‧operations | 55 | 101 | 0.55 (0.39–0.76) | 0.0003 |
| Follow-up at 7–15 years | Screening, n = 43 167 | Control, n = 43 312 | Odds ratio (95% CI) | P |
| AAA-related deaths | 123 | 245 | 0.47 (0.25–0.90) | 0.02 |
| Overall deaths*2 | 14 922 | 15 568 | 0.94 (0.91–0.97) | < 0.0001 |
| Elective AAA ‧operations | 567 | 204 | 2.81 (2.40–3.30) | < 0.0001 |
| Emergency ‧operations | 77 | 172 | 0.48 (0.28–0.83) | 0.009 |
*1 Effects in men aged 64 to 83 years in four randomized studies (Chichester, MASS, Viborg, Western Australia).
*2 Includes long-term results from the Australian study, i.e., 62 519 screened patients and 62 664 controls.
Women were invited to screening only in the Chichester study. The prevalence of AAAs more than 3 cm in diameter was 1.3% (7.6% in men). At 5-year follow-up, there was no difference between the group of women examined by ultrasound and the female control group in terms of AAA-related mortality (OR: 1.0; 95% CI: 0.14 to 7.07) or overall mortality (OR: 1.05; 95% CI: 0.92 to 1.19). At 10 years the incidence of AAA rupture was identical in the two groups.
Costs and efficiency
In estimating the cost efficiency of a population-based AAA screening program, in addition to the cost of the screening examinations themselves, all consequential costs also need to be taken into account. These include all the necessary follow-up examinations, personnel and ultrasound equipment, the costs of additional elective operative procedures, and the costs associated with any complications of surgery. In the Viborg study (men, 65 to 74 years), the cost of one ultrasound examination was 11.23 euros. Once all the consequential costs were taken into account, the cost of one prevented AAA-related death was 16 050 euros. The cost of 1 year of life gained was 9057 euros after 5 years, 2708 euros after 10 years, and € 1825 after 15 years (21). In the British MASS study, the sum of 28 400 GBP over 4 years was calculated for 1 year of life gained, going down to an estimated 8000 GBP after 10 years (22).
Table 4 shows how, in comparison to screening for colorectal cancer and breast screening, far fewer probands need to be examined for one disease-specific death to be prevented.
Table 4. Number of patients who need to be screened in order to prevent one disease-specific death.
| NNS* | Time period | |
| Fecal occult blood test | 808 | 8.5 years |
| Colonoscopy | 862 | 13 years |
| Mammography (women aged 50 to 69 years) | Approx 2000 | 10 years |
| Ultrasound of the abdominal aorta (men aged 65 to 80 years) | 350 | 7–15 years |
* numbers needed to screen (24, 25)
Ultrasound screening for AAA thus fulfills all the requirements of a worthwhile screening program. AAA is a frequent disease in men over the age of 65. It generally develops slowly from a small aneurysm to one in danger of rupturing, and can be diagnosed by ultrasound inexpensively, noninvasively, and with high reliability, sensitivity, and specificity. The indications for open or endovascular replacement of the abdominal aorta are clearly defined; the required operations can be performed with a low complication rate in centers with proven expertise and wide experience in vascular surgery. After surgery, patients have a virtually normal life expectancy.
For these reasons, the Screen for Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act was passed in the USA in January 2007. In this project, new male Medicare patients are invited to a one-off ultrasound examination (part of the “welcome to Medicare” package). In a further project of the US Department of Veterans Affairs (VA), all 65- to 76-year-old veterans who are or ever have been smokers, and all men and women with a family history of AAA, will gradually be screened. Early results show an AAA prevalence of 5.1% (23). The UK Department of Health also announced a national AAA screening program in January 2008. By 2012/2013, 60 screening units are to be built up for a national screening program of around 270 000 men aged 65.
Recommendations
With regard to the introduction of a national ultrasound-based AAA screening program in Germany, the German Society for Vascular Surgery (Deutsche Gesellschaft für Gefäßchirurgie, DGG), following the US Preventive Services Task Force (29), recommends the following procedure:
A one-off ultrasound scan of the abdominal aorta in men over the age of 65, especially if they are or have been smokers
A one-off ultrasound scan of the abdominal aorta in men and women of all ages who have a family history of AAA
A one-off ultrasound scan of the abdominal aorta in women over the age of 65 who are or have been smokers, have a history of cardiovascular disease, and/or have a family history of AAA
A one-off ultrasound scan is enough if it shows an abdominal aorta with a diameter of less than 3 cm.
If the diameter is 3 to 4 cm, a follow-up scan should be performed 12 months later.
If the diameter is 4 to 4.5 cm, a follow-up scan should be performed 6 months later.
If the diameter is 4.5 cm or greater, the patient should be referred to a vascular surgeon and CT angiography be carried out to clarify the findings.
If the diameter is 5 to 5.5 cm, operative treatment should be considered; for women, the threshold diameter is 4.5 to 5 cm.
Conclusions
Ultrasound-based screening for abdominal aortic aneurysm (AAA) is a technically simple, cost-efficient way to bring about a highly significant reduction in AAA-related mortality in older patients. Because of the age-related high prevalence of AAA, men and women with a family history of AAA or with any other arteriosclerotic disease, and all men aged over 65, should be systematically examined. In comparison to other screening programs (breast cancer, colorectal cancer), many fewer probands need to be examined to prevent one disease-related death. Diagnosed AAAs can be treated by open or endovascular surgery with a low perioperative complication rate in centers with good structure and process quality and a large number of cases. A national ultrasound screening program for AAA in Germany should be introduced quickly, because AAA-related mortality can be dramatically reduced.
Key Messages.
Ruptured abdominal aortic aneurysm (AAA) has an associated mortality rate of over 80% in Western industrialized nations, and is the tenth most frequent cause of death in men over 65 years of age.
The risk of an AAA rupturing increases exponentially with its diameter. Once the diameter has reached 5 cm, the risk is 3% per year; at 5 years the cumulative risk is 25%.
More than 80% of patients with an AAA have no symptoms.
For an AAA with a diameter of 5 cm or more, elective surgery is the only certain way to prevent rupture of the aneurysm.
Ultrasound screening for AAA leads to a significant reduction in AAA-related mortality after 3 to 5 years and in overall mortality at 7 to 15 years.
Acknowledgments
The authors thank Dr. Hans-Joachim Florek, Dresden, Prof. Dr. Thomas Hupp, Stuttgart, and Dr. Stefan Nöldeke, Garmisch-Partenkirchen, for valuable suggestions during the writing of this article.
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Dr. Flessenkämper took part in an AAA screening project supported by Medtronic, Inc. The other authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Fleming C, Whitlock EP, Beil TL, Lederle FA Preventive Services Task Force. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US. Ann Intern Med. 2005;142:203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 2.UK National Screening Committee ASWG. Standard operating procedures for an abdominal aortic aneurysm (AAA) screening programme. Draft version 7. 2007. [Google Scholar]
- 3.Lindholt JS, Norman P. Screening for abdominal aortic aneurysm reduces overall mortality in men. A meta-analysis of the mid- and long-term effects of screening for abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2008;36:167–171. doi: 10.1016/j.ejvs.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 5.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 6.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329 doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 8.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 9.Powell JT, Brown LC, Forbes JF, Fowkes FG, Greenhalgh RM, Ruckley CV, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg. 2007;94:702–708. doi: 10.1002/bjs.5778. [DOI] [PubMed] [Google Scholar]
- 10.Heider P, Wolf O, Reeps C, Hanke M, Zimmermann A, Berger H, Eckstein HH. Aneurysmen und Dissektionen der thorakalen und abdominellen Aorta. Chirurg. 2007;78:600–610. doi: 10.1007/s00104-007-1370-0. [DOI] [PubMed] [Google Scholar]
- 11.The UK Small Aneurysm Trial Participants. Mortality results for randomized controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 12.Pfeiffer T, Sandmann W. Infrarenales Aortenaneurysma - Diagnostik und Therapie. Chirurg. 2003;74:482–497. doi: 10.1007/s00104-003-0673-z. [DOI] [PubMed] [Google Scholar]
- 13.Law MR, Morris J, Wald NJ. Screening for abdominal aortic aneurysms. J Med Screen. 1994;1:110–115. doi: 10.1177/096914139400100210. [DOI] [PubMed] [Google Scholar]
- 14.Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146:735–741. doi: 10.7326/0003-4819-146-10-200705150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Holt PJ, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg. 2007;94:395–403. doi: 10.1002/bjs.5710. [DOI] [PubMed] [Google Scholar]
- 16.Gemeinsamer Bundesausschuss (GBA) Qualitätssicherungsvereinbarung zum Bauchaortenaneurysma. Bundesanzeiger. 2008;198 www.g-ba.de/informationen/beschluesse/753/ [Google Scholar]
- 17.Arbeitskreis vaskulärer Ultraschall (AvU) der Deutschen Gesellschaft für Ultraschall in der Medizin (DEGUM) Dokumentationsempfehlungen zur Qualitätssicherung in der vaskulären Ultraschalldiagnostik. 2004. [Google Scholar]
- 18.Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472–475. doi: 10.1053/ejvs.1999.0835. [DOI] [PubMed] [Google Scholar]
- 19.Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;2 doi: 10.1002/14651858.CD002945.pub2. CD002945. [DOI] [PubMed] [Google Scholar]
- 20.Lindholt JS, Juul S, Fasting H, Henneberg EW. Cost-effectiveness analysis of screening for abdominal aortic aneurysms based on five year results from a randomised hospital based mass screening trial. Eur J Vasc Endovasc Surg. 2006;32:9–15. doi: 10.1016/j.ejvs.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Multicentre aneurysm screening study (MASS) cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002;325 doi: 10.1136/bmj.325.7373.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee ES, Pickett E, Hedayati N, Dawson DL, Pevec WC. Implementation of an aortic screening program in clinical practice: Implications for the Screen for Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act. J Vasc Surg. 2009 doi: 10.1016/j.jvs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Preventive services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Int Med. 2005;142:198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317:307–312. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Umscheid T, Eckstein HH, Noppeney T, Weber H, Niedermeier HP. Qualitätsmanagement Bauchaortenaneuyrsma der deutschen Gesellschaft für Gefäßchirurgie (DGG) Gefäßchirurgie. 2001;6:194–200. [Google Scholar]
- e2.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- e3.Lindholt JS, Vammen S, Fasting H, Henneberg EW, Heickendorff L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg. 2000;20:281–285. doi: 10.1053/ejvs.2000.1151. [DOI] [PubMed] [Google Scholar]
- e4.Elzouki AN, Ryden AA, Lanne T, Sonesson B, Eriksson S. Is there a relationship between abdominal aortic aneurysms and alpha1-antitrypsin deficiency (PiZ)? Eur J Vasc Endovasc Surg. 1999;17:149–154. doi: 10.1053/ejvs.1998.0740. [DOI] [PubMed] [Google Scholar]
- e5.Beard JD. Screening for abdominal aortic aneurysms. Br J Surg. 2003;19:515–516. doi: 10.1002/bjs.4140. [DOI] [PubMed] [Google Scholar]