Abstract
Background
The growth hormone-IGF (insulin-like growth factor) system plays a central role in hormonal growth regulation. Recombinant human (rh) growth hormone (GH) has been available since the late 1980s for replacement therapy in GH-deficient patients and for the stimulation of growth in patients with short stature of various causes. Growth promotion by GH occurs in part indirectly through the induction of IGF-1 synthesis. In primary disturbances of IGF-1 production, short stature can only be treated with recombinant human IGF-1 (rhIGF-1). rhIGF-1 was recently approved for this indication but can also be used to treat other conditions.
Methods
Selective review of the literature on IGF-1 therapy, based on a PubMed search.
Results and conclusion
In children with severe primary IGF-1 deficiency (a rare condition whose prevalence is less than 1:10 000), the prognosis for final height is very poor (ca. 130 cm), and IGF-1 therapy is the appropriate form of pathophysiologically based treatment. There is no alternative treatment at present. The subcutaneous administration of IGF-1 twice daily in doses of 80 to 120 µg/kg accelerates growth and increases final height by 12 to 15 cm, according to current data. There is, however, a risk of hypoglycemia, as IGF-1 has an insulin-like effect. As treatment with IGF-1 is complex, this new medication should only be prescribed, for the time being, by experienced pediatric endocrinologists and diabetologists.
Keywords: dwarfism, growth, hormonal therapy, pediatric disease, developmental disorder
In the past 50 years, a trend has developed in the understanding of growth regulation that is based on the so-called somatomedin hypothesis (e1). This has led to an understanding of the insulin-like growth factor (IGF) system and its different components and multiple effects (1). At the center of the system is IGF-1, an insulin-like peptide that vitally affects the metabolism and diverse cell functions. After IGF-1 had been cloned and became biosynthetically produced subsequently, initial clinical studies aimed to investigate its growth promoting and insulin-like effects (2). The neuroprotective potential of IGF-1 has been investigated experimentally and in clinical studies only recently (3– 5). Table 1 lists the possible therapeutic roles for IGF-1. For most indications, however, these will require further extensive, controlled studies.
Table 1. The therapeutic potential of IGF-1.
| Systemic application | Studies (evidence level) |
| Growth disorders | |
| Severe primary IGF-1 deficiency (for example, Laron syndrome, defects of the intracellular JAK/STAT signal transduction cascade) | T Ib – T IIb |
| Reduced effectiveness of growth hormone (for example, chronic renal failure, wasting syndrome, idiopathic dwarfism) | T IV, T Ib |
| Insulin resistant states | |
| Severe congenital insulin resistance syndromes (for example, Leprechaunism, insulin receptor defects) | T IIb |
| T IV | |
| Type 1 diabetes (as additional treatment in complex cases) | T IIb |
| Type 2 diabetes (as additional treatment in complex cases) | T IIb |
| Neuroprotection | |
| After hypoxic insult | Basic research |
| Neurodegenerative disorders (for example, amyotrophic lateral sclerosis) | T Ib |
| Cardiovascular disorders | T IV |
| Local application | |
| Wound healing impairment | Basic research |
| Tissue reconstruction and repair | Basic research |
| Extracorporal tissue engineering | Basic research |
In 2007 the European Medicines Agency (EMEA) licensed the use of recombinant (rh) IGF-1 (mecasermin) for the treatment of dwarfism in severe primary IGF-1 deficiency. Children affected by this pathology are extremely short (height <3.0 standard deviations), reaching a spontaneous adult height of about 130 cm, with a doll-like appearance comprising a large head, small hands and feet, scarce musculature, and obesity. The appearance resembles that found in severe growth hormone deficiency, even if growth hormone secretion is normal. Severe primary IGF-1 deficiency with mutations in the GH receptor (GHR), with mutations in the post-GHR signaling pathway, and with IGF-1 gene defects is extremely rare (prevalence <1:10 000). Treatment with IGF-1 is the only effective therapeutic option in such cases. Since IGF-1 has only just been licensed for the treatment of primary IGF-1 deficiency, a discussion of the substance with its complex mechanisms of action is of general interest. We conducted a literature search in Medline using the search terms “therapy rhIGF-1”, “insulin resistance rhIGF-1”, “primary IGF deficiency”, “IGF-1 generation test”, “rhIGF-1 safety”, “IGF-1 and malignancies review”.
The IGF-GH system
In 1957 Salmon and Daughaday found a growth hormone (GH) dependent factor that had growth promoting effects on the epiphyseal cartilage. Because of its stimulating effect in the uptake of sulfate in the cartilage, this factor was called “sulfation factor.” After more had been found out about its multiple metabolic effects, the factor became known as somatomedin (e1). Somatomedin consists of two proteins (e2, e3) that were termed “insulin-like growth factors” (IGF-1 and IGF-2) owing to their chemical structure. Binding studies and molecular studies found that there are specific cell receptors for these proteins (IGF-1-R; IGF-2-R). Both IGF-1 and IGF-2 are able to bind to the insulin receptor; however, the affinity of IGF-1 for the insulin receptor is only one-hundredth that of insulin itself (e4). These circumstances provide the basis for the insulin-like effects of the IGFs, which affect cellular uptake of glucose and amino acids, glycogen synthesis, lipogenesis, and cellular reproduction (e5, e6). Effects that are more specific for the IGFs, however, are effects of cell differentiation, cell proliferation, and apoptosis.
The complexity of the IGF system is increased by the fact that specific IGF binding proteins exist for IGFs (IGFBP-1 to IGFBP-6) (e7). These are all structurally similar but can be changed in terms of their binding behavior for IGFs by means of chemical modifications such as phosphorylation, glycosylation, or proteolysis. IGFs and IGFBPs can develop in almost all types of tissue (e8). The IGFs and IGFBPs in blood are synthesized primarily in the liver. The secretion of growth hormone, insulin, and sexual steroids, as well as liver function and nutritional status are the main determinants of their synthesis (e9). Through association to binding proteins and the acid-labile subunit (ALS), the breakdown of IGFs is inhibited and their biological half life is prolonged from a few minutes to several hours (e10, e11). Only a minute proportion of IGF-1 (1–2%) in the blood is unbound according to the law of mass action. Mediated by binding protein cascades, circulating IGF is transported to different peripheral target organs. The IGFBPs thus have different biological functions (e8).
Growth hormone (GH) is a peptide hormone that is produced in the somatotropic cells of the frontal lobe of the pituitary gland. They are released in a pulsatile manner (e12). The growth promoting effect of GH occurs indirectly as a result of stimulation of IGF-1 formation in the liver (endocrine) and in the epiphysis (growth plate) (paracrine/autocrine) as well as directly on the epiphysis as a result of IGF independent effects. In the liver, GH stimulates the formation of IGF-1, IGFBP-3, and ALS; hepatically produced IGF-1 reaches the epiphysis—among other locations—through the circulation. On the epiphysis, GH stimulates the formation of prechondrocytes and local synthesis of IGF-1 (e13). GH as well as IGF-1 are thus prerequisites for the optimal longitudinal growth of the bones. IGF-1 and GH synergistically affect growth and the anabolic metabolism. They have antagonistic effects on the glucose and fat metabolisms, since GH has anti-insulin effects (e14) whereas IGF-1 has insulin-like effects (e15).
IGF-1 deficiency
The concentrations of IGF-1 in the circulation and the release of GH are quantitatively linked to one another in a typical endocrine regulatory circuit (e16). If the secretion of GH is reduced (GH deficiency), the IGFs that are dependent on GH (IGF-1, IGFBP-3, ALS) are also reduced. In such a scenario, administration of GH results in normalization of IGF-1, IGFBP-3, and ALS and in growth stimulation. If the synthesis of IGF-1 is inhibited while hypophyseal function is normal, growth is also inhibited but GH secretion remains normal or is even raised. This is the case, for example, in GH receptor defects (Laron syndrome) or in defects of the intracellular JAK/STAT signal transduction cascade (e17, e18). In such cases, the result is pronounced dwarfism with a phenotype that corresponds to that of severe GH deficiency (figure 1). A lack of circulating IGF-1 can also be caused by disorders that affect the formation of IGF-1, such as malnutrition, hypothyreosis, renal failure, or hepatic failure. The terminology with regard to such situations is currently not consistent (e19, e20).
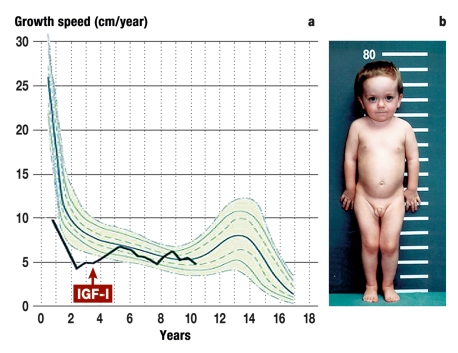
Figure 1.
A boy aged 4 years and 4 months with Laron syndrome (compound heterozygote mutation in Exon 4 [C38X8] and Exon 3 [W16X] of the growth receptor gene).
a) Pathologically impaired postnatal growth velocity (<3rd percentile). After treatment with mecasermin was started (maximum dose 2 x 120 µg/kg/day s.c.), a clear acceleration in growth was observed to the 11th year of life, followed by normalization of growth velocity (25th–75th percentile).
b) Classic phenotype with pronounced dwarfism (77.5 cm, <3rd percentile), prominent forehead, snub/saddle nose, and truncal obesity.
According to traditional ideas of endocrine regulatory systems the term “primary disorder” is usually applied if the endocrine organ is affected. A “secondary” disorder means that the regulatory system is disrupted. To use an analogy, looking at the GH-IGF system and regarding IGF-1 as the regulated peripheral hormone, “secondary” IGF-1 deficiency (GH low and IGF-1 therefore also low) can be contrasted with “primary” IGF-1 deficiency (IGF-1 low, therefore GH normal/high) (table 2) (e16).
Table 2. Classification of the causes of IGF-1 deficiency, from (e16).
| Classification | Secondary IGF-1 deficiency | Primary IGF-1 deficiency | Primary IGF-1 deficiency | Functional IGF-1 deficiency |
| Pathogenesis | Defects in growth hormone production | Defects in growth hormone action | Defects in IGF production | Defects in IGF action |
| GH secretion | Low | High/normal | High/normal | High/normal |
| Level of disruption | GH production: | Binding to GHR; | IGF-1 gene: | Lack of binding protein: |
| GHRH/GHRIH defect | Anti-GH-antibodies | Mutation/deletion | IGFBP-3 | |
| GHRH receptor defect | GHBP excess | Other | ALS | |
| GH gene defects | Other | |||
| Functional (aging) | ||||
| Other | ||||
| Level of disruption | Hypophyseal development: | GH receptor: | Cofactors: | Excess of binding proteins: |
| Cellular structures | GHR antibodies | Thyroid hormones | IGF-1 antibodies | |
| Other | GHR gene defects | Insulin | Renal failure | |
| Nutrition | Other | |||
| Liver function | ||||
| Other | ||||
| Level of disruption | Hypophyseal destruction | Post-GHR: | IGF-1 receptor: | |
| JAK-STAT | IGF-1-R | |||
| Other | Post receptor |
IGF-1, insulin-like growth factor 1; GH, growth hormone; GHRH, growth hormone releasing hormone; GHRIH, growth hormone release inhibiting hormone; GHR, growth hormone receptor; GHPB, growth hormone binding protein; ALS, acid-labile subunit; IGF-1-R, IGF-1 receptor; JAK/STAT, “Janus kinases/signal transducers and activators of transcription”
How to confirm severe primary IGF-1 deficiency
IGF-1 is licensed only for the treatment of particularly severe IGF-1 deficiency:
Short height for age (<3.0 standard deviation)
Low blood concentration of IGF-1 (<2.5 percentile).
Further, the recommendation is to confirm the diagnosis by means of an IGF-1 generation test. In such a test, the change in IGF-1 concentration in the blood is quantified after short term administration of recombinant growth hormone, so as to confirm sensitivity to growth hormone. This test also investigates the responsiveness to exogenous administration of GH (normally 3–7 days) at the level of the IGF-1 synthesis. The assumption is that this reflects the improvement in growth achieved by long term GH treatment.
However, the discussion about the standardization and significance of the test has by no means reached its conclusion (6– 11). Often, cases of a gene defect in the GH receptor or in components of the post-receptor signaling cascade can also be verified by molecular genetic methods (12, 13) (figure 2).
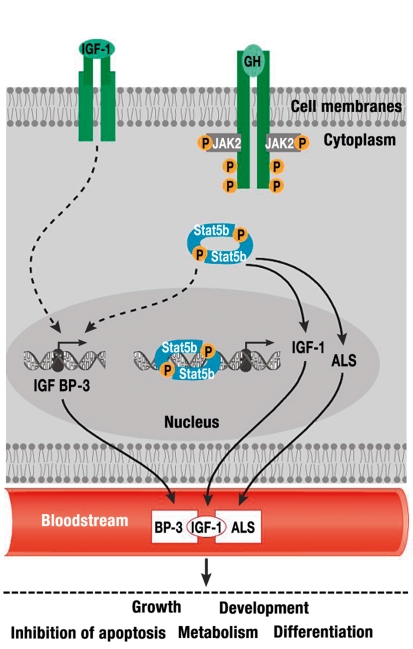
Figure 2.
Growth hormone (GH) binds to the GH receptor, resulting in activation of the transcription factor STAT5b. Signals triggered by STAT5b stimulate directly (IGF-1, ALS) or indirectly (IGFBP-3) the transcription of members of the ternary IGF-1/ALS/BP-3 complex. After binding to the IGF-1 receptor, circulating IGF-1 exerts its effects on proliferation and growth as well as its metabolic and anti-apoptotic effects in a tissue dependent manner (25).
IGF-1, insulin-like growth factor 1; GH, growth hormone; IGFBP-3, insulin-like growth factor binding protein 3; ALS, acid labile subunit; STAT5b, signal transducer and activator of transcription 5B
Treatment of dwarfism in primary IGF-1 deficiency
Experiences with treatment with rhIGF-1 for dwarfism in severe primary IGF-1 deficiency date back to the early 1990s. The pathology described by Laron, with comprehensive phenocopy of severe GH deficiency, normal GH secretion, and GH resistance, has been identified as a GH receptor defect (e21). As rhIGF-1 became available, such patients received treatment (and, in lower numbers, also patients with traditional GH deficiency who developed neutralizing GH antibodies during treatment with GH). Laron and colleagues were the first to report results in 5 children with severe IGF-1 deficiency (Laron syndrome). The children were aged 3.3–14.5 years and received 150 µg rhIGF-1 per kg body weight once daily, which was administered subcutaneously. In the first year of treatment, their growth velocity increased from 2.8–5.8 cm to 8.8–13.6 cm / year. Four study groups have reported further experiences regarding this treatment from Israel (e21, 14), Ecuador (15), Europe (7, 16, 17), and the US (18– 20). Altogether, some 150 children and adolescents were treated with rhIGF-1 from several manufacturers. With the exception of the study from Israel, the patients received 40–120 µg/kg rhIGF-1 twice daily, administered by subcutaneous injection.
The results with regard to growth in the short term and long term (4–7 years) are remarkably consistent (table 3). This is particularly notable when taking into account the heterogeneity of the studies (ethnic origins of patients, geography, preparations) (table 3). Short term studies (15) have shown a positive relation between dose and growth rate. Long term studies (17, 20) have shown an increase in body height by 10–17 cm (table 3). On comparing the results of treatment for severe primary IGF-1 deficiency with IGF-1 and severe GH deficiency with GH with regard to the resulting final height of the participants, the latter has yielded better results. The following reasons are responsible:
Table 3. Growth under IGF-1 therapy at different stages in primary IGF-1 deficiency.
| Studies, first year | N | Age (years) | Height (SDS) at start of treatment | Dose rhIGF-1 (µg/kg body weight) | Manufacturer of rhIGF-1 | Growth rate in first year (cm/y) | Height (SDS) at end of treatment |
| Klinger, Laron (14)*1 | 9 | 7.4 | –5.7 | 150–200; 1× | Fujisawa, Japan | 8.2 | – |
| Backeljauw et al. (18)*1 | 8 | 6.6 | –5.6 | 80–120; 2× | Genentech, USA | 9.3 | – |
| Ranke et al. (16)*1 | 26 | 8.2 | –6.5 | 40–120; 2× | Pharmacia, Sweden | 8.5 | – |
| Guevara et al. (24)*1 | 22 | 9.1 | –8.4 | 80–120; 2× | Genentech, USA | 8.9 | – |
| Chernausek et al. (20)*1 | 59 | 7.8 | –6.5 | 40–120; 2× | Genentech, USA | 8.0 | – |
| Long term studies | Duration of treatment | ||||||
| Ranke et al. (17) | 17 | 9.1 | –6.5 | 40–120; 2× | Pharmacia, Sweden | 4 years | –4.8*3 |
| Chernausek et al. (20) | 6 | 9.9 | –7.3 | 40–120; 2× | Genentech, USA | 7 years (adult) | –4.8*4 |
*1 not randomized, not controlled; *2 dose randomized; *3 gain of 1.7 standard deviation scores (SDS) = approx. 10 cm, *4 gain of 2.5 SDS = approx. 17 cm. Relevant studies were listed; the few individual case reports were not included.
A lack of direct GH effect on the epiphysis
A lack of stimulation by GH of locally produced IGF-1 in the chondrocytes of the epiphysis
rhIGF-1 is not able to normalize IGFBP-3 and ALS; this results in rapid degradation of circulating IGF-1
Owing to the risk of hypoglycemia, no larger (and possible adequate) doses of IGF-1 are given.
Adverse effects
During therapy with IGF-1, a range of typical side effects have been noted (15, 16, 20). These include hypoglycemia, proliferation of lymphatic tissues, and others, as listed in Table 4. Children with primary and secondary IGF-1 deficiency a priori tend to develop hypoglycemia. This risk is reduced in GH deficiency for which GH therapy is given, but probably rises as a result of IGF-1 therapy. This is to do with the genuine insulin-like effects of IGF-1, as a result of which the peripheral glucose uptake is increased and hepatic glucose formation is reduced. In a study reported by Chernausek et al. (20), glucose blood concentrations of <50 mg/dL during IGF-1 therapy were not observed statistically more frequent than in the control without IGF-1. However, during therapy with IGF-1, 49% of patients developed hypoglycemia; in 4 patients this was accompanied by seizures. In the European study, relevant hypoglycemia ceased only once meals were administered before the injections. Further adverse effects include headaches and benign intracranial hypertension as well as papillary edema, which was mostly caused by IGF-1 mediated fluid retention and mostly limited to the early stages of treatment. Proliferation of the lymphatic tissues (tonsils, adenoids, spleen, thymus) is a characteristic side effect of IGF-1 and GH. Antibodies have been observed in about half of the cases (20), especially during the first year of treatment, at low titers (<1:100), but this was thought not to be of physiological relevance. Especially during puberty, facial features coarsened (7).
Table 4. Side effects of IGF-1 therapy as reported in different studies*1.
| Study | Ranke et al. (16) | Guevara et al. (15) | Chernausek et al. (20) | |
| Event | Placebo | IGF-I | ||
| N = 33 | N = 9 | N = 7 | N = 76 | |
| Hypoglycemia | 13 (39%) | 6 (67%) | 6 (87%) | 37 (49%) |
| Tonsillar/adenoidal | 17 (22%) | |||
| hypertrophy | ||||
| Tonsillectomy/ | 2 (9%9 | 17 (22%) | ||
| adenotomy | ||||
| Hypertrophy | 8 (35%) | |||
| of the thymus | ||||
| Sleep apnea | 3 (4%) | |||
| Lipohypertrophy | 7 (21%) | 24 (32%) | ||
| at injection site | ||||
| Headache | 21 (64%) | 2 (22%) | 2 (29%) | |
| Pain at injection site | 16 (48%) | 3 (33%) | 3 (43%) | |
*1 Side effects are documented systematically in these studies
An increase in fatty tissues has been described during long term treatment with IGF-1, (17, e22), which has to be interpreted as an “insulin effect.” Accelerated ossification and inappropriate growth of spleen and kidneys have not been observed (20).
Discussion
Replacement therapy with IGF-1 in children with severe primary IGF-1 deficiency—which is rare, with a prevalence of <1:10 000—is the pathophysiologically correct form of treatment, for which there is currently no alternative (21). The importance of such treatment is obvious from the fact that those affected reach a final height of only 130 cm without treatment. The growth gain of 2–2.5 standard deviation scores (SDS) observed in long term studies, which has thus far been documented in only a very small number of patients, corresponds to a final growth gain of 12–15 cm. It should be borne in mind, however, that patients with a congenital disorder have initially received treatment at inappropriately late stages.
By exclusive administration of IGF-1, not all components of the IGF systems can be normalized that are not GH dependent and that may be of importance for the effectiveness of circulating IGFs in the target organ. This underlines the pathophysiological complexities in severe primary IGF-1 deficiency. Since neither IGFBP-3 nor ALS is available as therapeutic preparations, there is no other therapeutic option than exclusive administration of IGF-1. The risk of hypoglycemia after administration of IGF-1 is intrinsic on the one hand, but on the other hand it is dose dependent and also depends on the patient’s age and their individual reaction. The risk can be reduced successfully by food intake directly before IGF-1 is administered (16, 20). The treatment has been found to be relatively safe for patients, even though they have to be treated over many years. The importance of the IGF system and especially of concentrations of IGF-1 in the blood in the development and promotion of malignancies is currently unknown (e23). For this reason, raised blood concentrations of IGF-1 owing to exogenous administration should be avoided in the longer term. In any case, the complexity associated with the treatment means that the treatment should be given exclusively by experienced pediatric endocrinologists and pediatric diabetologists. The treatment is a novel therapeutic option, and comprehensive control examinations and detailed documentation are of the essence. Further, at a cost of 22 800 euros/year for a child weighing 20 kg, this therapy is costly (according to Germany’s Red List of approved medications for 2008, 40 mg costs 781.83 euros). In our opinion, dose adjustments, for example, should be made stepwise while monitoring any associated metabolic effects with a great deal of precision. Bearing in mind the multiple potential effects of IGF-1 in addition to its effect on growth, the treatment has to be accompanied by examinations that seek to identify possible changes to the function and composition of the body. The background to this therapeutic option will continue to be the discussion of the role of growth factors in the development and therapy of malignancies (22, 23).
Key Messages.
The growth hormone system IGF (insulin-like growth factor) is central to hormonal growth regulation.
Severe primary IGF-1 deficiency (incidence <1:10 000) is accompanied by extreme dwarfism (adult height <130 cm).
Recombinant human IGF-1 (mecasermin) now enables causal replacement therapy of primary IGF-1 deficiency, for which no alternative exists.
On the basis of the data, which are still limited in terms of long term results, an improvement in the final height can be assumed for primary IGF-1 deficiency.
The insulin-like effect of IGF-1 explains the side effect profile of IGF-1 (for example, hypoglycemia).
The treatment should be given and supervised by experienced pediatric endocrinologists and pediatric diabetologists.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Professor Ranke was in the past and PD Wölfe and Professor Bettendorf are currently members of the advisory board of Ipsen, a manufacturer of IGF-1. Dr Schnabel is a consultant for Ipsen. He has received speaker honoraria from Pfizer, Lilly, Merck Serono, and NovoNordisk. He is in receipt of financial study support from Pfizer.
References
- 1.LeRoith D, Bondy C, Yakar S, Jun-Li Liu J-L, Butler A. The somatomedin hypothesis. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 2.Ranke MB. Insulin-like growth factor-I treatment of growth disorders, diabetes mellitus and insulin resistance. Trends Endocrinol Metab. 2005;16:190–197. doi: 10.1016/j.tem.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Carro E, Trejo JL, Núñez A, Torres-Aleman I. Brain repair and neuroprotection by serum insulin-like growth factor I. Mol Neurobiol. 2003;27:153–162. doi: 10.1385/MN:27:2:153. [DOI] [PubMed] [Google Scholar]
- 4.Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-1 prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Festoff BW. Amyotrophic lateral sclerosis: current and future treatment strategies. Drugs. 1996;51:28–44. doi: 10.2165/00003495-199651010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Blum WF, Cotterill AM, Postel-Vinay MC, Ranke MB, Savage MO, Wilton P. Improvement of diagnostic criteria in growth hormone insensitivity syndrome: solutions and pitfalls. Pharmacia Study Group on insulin-like growth factor I treatment in growth hormone insensitivity syndromes. Acta Paediatr Suppl. 1994;399:117–124. doi: 10.1111/j.1651-2227.1994.tb13303.x. [DOI] [PubMed] [Google Scholar]
- 7.Savage MO, Blum WF, Ranke MB, et al. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome) J Clin Endocrinol Metab. 1993;77:1465–1471. doi: 10.1210/jcem.77.6.7505286. [DOI] [PubMed] [Google Scholar]
- 8.Schwarze CP, Wollmann HA, Binder G, Ranke MB. Short-term increments of insulin-like growth factor I (IGF-1) and IGF-binding protein-3 predict the growth response to growth hormone (GH) therapy in GH-sensitive children. Acta Paediatr Suppl. 1999;88:200–208. doi: 10.1111/j.1651-2227.1999.tb14392.x. [DOI] [PubMed] [Google Scholar]
- 9.Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-1 generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86:5176–5183. doi: 10.1210/jcem.86.11.8019. [DOI] [PubMed] [Google Scholar]
- 10.Selva KA, Buckway CK, Sexton G, et al. Reproducibility in patterns of IGF generation with special reference to idiopathic short stature. Horm Res. 2003;60:237–246. doi: 10.1159/000074038. [DOI] [PubMed] [Google Scholar]
- 11.Jorge AA, Souza SC, Arnhold IJ, Mendonca BB. Poor reproducibility of IGF-1 and IGF binding protein-3 generation test in children with short stature and normal coding region of the GH receptor gene. J Clin Endocrinol Metab. 2002;87:469–472. doi: 10.1210/jcem.87.2.8191. [DOI] [PubMed] [Google Scholar]
- 12.Goddard AD, Covello R, Luoh SM, et al. The Growth Hormone Insensitivity Study Group. Mutations of the growth hormone receptor in children with idiopathic short stature. N Engl J Med. 1995;333:1093–1098. doi: 10.1056/NEJM199510263331701. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev. 1994;15:369–390. doi: 10.1210/edrv-15-3-369. [DOI] [PubMed] [Google Scholar]
- 14.Klinger B, Laron Z. Three year IGF-1 treatment of children with Laron syndrome. J Ped Endocrinol Metab. 1995;8:149–158. doi: 10.1515/jpem.1995.8.3.149. [DOI] [PubMed] [Google Scholar]
- 15.Guevara A, Vasconez O, Martinez V, et al. A randomized, double blind, placebo-controlled trial on safety and efficacy of recombinant human insulin-like growth factor-I in children with growth hormone receptor deficiency. J Clin Endocrinol Metab. 1995;80:1393–1398. doi: 10.1210/jcem.80.4.7536209. [DOI] [PubMed] [Google Scholar]
- 16.Ranke MB, Savage MO, Chatelain PG, et al. Insulin-like growth factor I improves height in growth hormone insensitivity: two years’ results. Horm Res. 1995;44:253–264. doi: 10.1159/000184637. [DOI] [PubMed] [Google Scholar]
- 17.Ranke MB, Savage MO, Chatelain PG, Preece MA, Rosenfeld RG, Wilton P The Working Group on Growth Hormone Insensitivity Syndromes. Long-term treatment of growth hormone insensitivity syndrome with IGF-1. Results of the European Multicentre Study. Horm Res. 1999;51:128–134. doi: 10.1159/000023345. [DOI] [PubMed] [Google Scholar]
- 18.Backeljauw PF, Underwood LE GHIS Collaborative Group. Prolonged treatment with recombinant insulin-like growth factor-I in children with growth hormone insensitivity syndrome–a clinical research center study. J Clin Endocrinol Metab. 1996;81:3312–3317. doi: 10.1210/jcem.81.9.8784089. [DOI] [PubMed] [Google Scholar]
- 19.Backeljauw PF, Underwood LE. Therapy for 6.5-7.5 years with recombinant insulin-like growth factor I in children with growth hormone insensitivity syndrome: a clinical research center study. J Clin Endocrinol Metab. 2001;86:1504–1510. doi: 10.1210/jcem.86.4.7381. [DOI] [PubMed] [Google Scholar]
- 20.Chernausek SD, Backeljauw FB, Frane J, Kuntze J, Underwood LE for the GH Insensitivity Syndrome Collaborative Group. Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-1 deficiency due to growth hormone insensitivity. J Clin Endocrinol Metab. 2007;92:902–910. doi: 10.1210/jc.2006-1610. [DOI] [PubMed] [Google Scholar]
- 21.Collett-Solberg PF, Misra M on behalf of the Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. The role of recombinant human insulin-like growth factor-I in treating children with short stature. J Clin Endocrinol Metab. 2008;93:10–18. doi: 10.1210/jc.2007-1534. [DOI] [PubMed] [Google Scholar]
- 22.Cohen P, Clemmons DR, Rosenfeld RG. Does the GH-IGF axis play a role in cancer pathogenesis? Growth Hormone IGF Res. 2000;10:297–305. doi: 10.1054/ghir.2000.0171. [DOI] [PubMed] [Google Scholar]
- 23.Sachdev D, Yee D. Disrupting insulin-like growth factor signalling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 24.Guevara-Aguirre J, Rosenbloom AL, Vasconez O, et al. Two-year treatment of growth hormone (GH) receptor deficiency with recombinant insulin-like growth factor I in 22 children: comparison of two dosage levels and to GH-treated GH deficiency. J Clin Endocrinol Metab. 1997;82:629–633. doi: 10.1210/jcem.82.2.3743. [DOI] [PubMed] [Google Scholar]
- 25.Woelfle J, Chia DJ, Massart-Schlesinger MB, Moyano P, Rotwein P. Molecular physiology, pathology, and regulation of the growth hormone/insulin-like growth factor-I system. Pediatr Nephrol. 2005;20:295–302. doi: 10.1007/s00467-004-1602-1. [DOI] [PubMed] [Google Scholar]
- e1.Daughaday WH, Hall K, Raben MS, Salmon WD, Jr, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature. 1972;235 doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- e2.Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett. 1978;89:283–286. doi: 10.1016/0014-5793(78)80237-3. [DOI] [PubMed] [Google Scholar]
- e3.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–2776. [PubMed] [Google Scholar]
- e4.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- e5.Ullrich A, Gray A, Tam AW, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Kato H, Faria TN, Stannard B, Roberts CT, LeRoith D. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (alpha IR-3) J Biol Chem. 1993;268:2655–2661. [PubMed] [Google Scholar]
- e7.Ballard J, Baxter R, Binoux M, et al. On the nomenclature of the IGF binding proteins. Acta Endocrinol (Copenh) 1989;121:751–752. doi: 10.1530/acta.0.1210751. [DOI] [PubMed] [Google Scholar]
- e8.Ranke MB, Elmlinger M. Functional role of insulin-like growth factor binding proteins. Horm Res. 1997;48(Suppl 4):9–15. doi: 10.1159/000191304. [DOI] [PubMed] [Google Scholar]
- e9.Clemmons DR, Underwood LE. Nutritional regulation of IGF-I and IGF binding proteins. Annu Rev Nutr. 11:393–412. doi: 10.1146/annurev.nu.11.070191.002141. [DOI] [PubMed] [Google Scholar]
- e10.Baxter RC. Characterization of the acid-labile subunit of the growth hormone-dependent insulin-like growth factor binding protein complex. J Clin Endocrinol Metab. 1988;67:265–272. doi: 10.1210/jcem-67-2-265. [DOI] [PubMed] [Google Scholar]
- e11.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278 E967-76 doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- e12.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- e13.Isaksson O, Lindahl A, Nilsson A, Isgaard J. Mechanisms of the stimulatory effect of growth hormone on longitudinal growth. Endocr Rev. 1987;8:426–438. doi: 10.1210/edrv-8-4-426. [DOI] [PubMed] [Google Scholar]
- e14.Yakar S, Setser J, Zhao H, et al. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113:96–105. doi: 10.1172/JCI200417763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Guler HP, Schmid C, Zapf J, Froesch ER. Effects of insulin-like growth factor I in man. Acta Paediatr Scand (Suppl) 1990;367:52–54. doi: 10.1111/j.1651-2227.1990.tb11633.x. [DOI] [PubMed] [Google Scholar]
- e16.Ranke MB. Defining insulin-like growth factor-I deficiency. Horm Res. 2006;65(Suppl 1):9–14. doi: 10.1159/000090641. [DOI] [PubMed] [Google Scholar]
- e17.Rosenfeld RG. Molecular mechanisms of IGF-I deficiency. Horm Res. 2006;65(Suppl 1):15–20. doi: 10.1159/000090642. [DOI] [PubMed] [Google Scholar]
- e18.Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- e19.Wit JM, Ranke MB, Kelnar CJH. ESPE classification of paediatric endocrine diagnoses. Horm Res. 2008;68(Suppl 2):1–120. [Google Scholar]
- e20.Ranke MB. Diagnosis of growth hormone deficiency and growth hormone stimulation tests. In: Ranke MB, editor. Diagnostics of endocrine function in children and adolescents. Basel: Karger; 2003. pp. 107–128. [Google Scholar]
- e21.Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958-2003. J Clin Endocrinol Metab. 2004;89:1031–1044. doi: 10.1210/jc.2003-031033. [DOI] [PubMed] [Google Scholar]
- e22.Klinger B, Anin S, Silbergeld A, Eshet R, Laron Z. Long-term-IGF-I treatment of children with Laron syndrome increases adiposity. Growth Horm IGF Res. 2006;16:61–64. doi: 10.1016/j.ghir.2005.12.001. [DOI] [PubMed] [Google Scholar]
- e23.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]