Abstract
In some human cancers, the Notch1 receptor and signaling pathway is upregulated, which increases the oncogenic potential of the cell through prevention of differentiation and inhibition of apoptosis. We sought to evaluate the role of Notch1 in hepatocellular cancer (HCC), and evaluate the therapeutic efficacy of curcumin, a known Notch1 inhibitor. Human liver tumors were compared to normal liver to evaluate for Notch1 and Notch1 Intracellular Domain (NICD). Three human HCC cell lines were exposed to curcumin and evaluated for downstream effectors by Western blot. In addition, standard MTT assays were performed to assess the effect of curcumin in vitro. Finally, a nude mouse xenograft model was utilized to assess the response to curcumin in vivo. High levels of NICD were present in the majority of human HCC samples and all three HCC cell lines. Treatment with curcumin led to a dose-dependent decrease in the expression of NICD associated with the induction of cleaved poly ADP-ribose polymerase (PARP), the degradation of cyclin D1 and increase in cyclin-dependent kinase p21. Curcumin inhibited HCC cell proliferation in vitro. Importantly, transfection of Notch1 small-interfering RNA (siRNA) into HCC cells resulted in cell growth inhibition and apoptosis, recapitulating the effects of curcumin. Finally, treatment with curcumin resulted in a 40% decrease in tumor growth in vivo. These results suggest for the first time that down-regulation of Notch1 signaling with curcumin is an attractive new therapeutic strategy for the treatment of patients with HCC.
Keywords: Hepatocellular carcinoma, curcumin, Notch1, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is a common solid organ malignancy worldwide, with about 600,000 new cases diagnosed each year [1], and it is the third most common cause of cancer-related death worldwide. Surgical resection, in the form of partial hepatectomy or total hepatectomy followed by liver transplantation, is the only curative option, but only 10–30% of patients are candidates for surgery at the time of presentation, due to either poor hepatic reserve or the presence of unresectable or metastatic disease. Attesting to the aggressive nature of this disease, the five-year survival is only 15–40% after curative resection. Conventional chemotherapy is largely ineffective for this disease, with response rates of only 20% and no improvement in survival [2]. Other treatment options including ethanol injection or radiofrequency ablation of small tumors can be utilized with curative intent, but few patients are candidates for these treatments due to the advanced nature of the disease at the time of presentation. Therefore, there is a pressing need for the development of new therapeutic approaches for advanced HCC.
Notch1 has been shown to act as either a tumor suppressor or an oncogene in human cancer [3]. When acting as an oncogene, the Notch1 receptor and signaling pathway are significantly upregulated, which results in increased cellular proliferation, prevention of differentiation, and inhibition of apoptosis [4]. Such a mechanism has been reported in several malignancies including pancreatic cancer, colon cancer, non-small cell lung cancer, cervical cancer, renal cell carcinoma, and several lymphomas [5–7], and this signaling pathway therefore represents a potential therapeutic target.
Curcumin is a phenolic compound from the plant Curcuma longa which is frequently used as a flavoring agent in food and has been shown to have antitumor effects in many cancers, including pancreatic cancer [8–10]. In pancreatic cancer, treatment with curcumin has been found to affect cell growth inhibition and apoptosis, via down regulation of Notch1 [4]. Therefore, these data suggest that inhibition of the Notch1 signaling pathway may be a potential therapeutic target in tumors where this pathway is overexpressed and plays an oncogenic role. We sought to evaluate the expression of Notch1 in human HCC. In addition, we sought to evaluate the therapeutic efficacy of curcumin, a known Notch1 suppressor.
Materials and methods
Cell culture and Curcumin treatment
Human HCC cell lines HEP3B, SK-Hep-1 and SNU449 were obtained from American Type Culture Collection (ATCC, Manassas, VA). HEP3B and SK-Hep-1 cells were cultured in DMEM (Life Technologies, Rockville, MD) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St Louis, MO), 100 IU/mL penicillin and 100 μg/mL streptomycin (Life Technologies) and L-glutamine (Sigma-Aldrich). SNU449 cells were cultured in RPMI 1640 (Life Technologies) containing supplements (10% FBS, penicillin/streptomycin, and L-glutamine). Cells were maintained in humidified air containing 5% CO2 at 37 °C. Curcumin (Sigma Chemical Co., St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) at a concentration of 100 mM and was stored in a dark colored bottle at -20°C. The stock was diluted to the required concentration with DMSO when needed. Prior to curcumin treatment, cells were grown to about 80% confluence, and then exposed to curcumin at varying concentrations (0– 40 μM). Cells grown in a medium containing an equivalent amount of DMSO without curcumin served as control. Experimental findings were confirmed with duplicate experiments.
Cellular proliferation assay
HCC cell proliferation was measured by the MTT (methylthiazolyldiphenyl-tetrazolium bromide, Sigma-Aldrich) rapid colorimetric assay as previously described [11, 12]. Briefly, cells were seeded in quadruplicate on 24-well plates and incubated for 24 hours under standard conditions to allow cell attachment. The cells were then treated with curcumin in concentrations of 0 to 40 μM and incubated for up to 6 days. The MTT assay was performed on days 0, 2, 4, and 6 by replacing the standard medium with 250 μL of serum-free medium containing MTT (0.5 mg/mL) and incubated at 37 °C for 3 hours. After incubation, 750 μL of DMSO (Sigma-Aldrich) was added to each well and mixed thoroughly. The plates were then measured at 540 nm using a spectrophotometer (μQuant; Bio-Tek Instruments, Winooski, VT). Experiments were performed at least twice.
Notch1 RNA interference assays
siRNA against Notch1 or nonspecific siRNA (Santa Cruz Biotechnology, sc-44226 and sc-37007) were transfected into HCC cells using Lipofectamine 2000 (Lipo, Invitrogen, San Diego, CA) per the manufacturer's instructions. The next day, the medium containing the transfection complexes was replaced with fresh medium, and the cells were incubated for another 48 hours. The cells were then harvested and cell lysates were prepared for immunoblotting as described above. To determine the effect of Notch1 on HCC cell proliferation, HCC cells were transfected with Notch1 siRNA or nonspecific siRNA and incubated overnight. The next day, the cells were trypsinized, counted, and plated in equal amounts (30,000 cells per well) onto 24-well plates. The MTT assay was performed on days 0, 2, 4, and 6, as described above. Experiments were performed at least twice.
Western blot analysis
Total tissue or cellular proteins were isolated as previously described [11, 12], and the protein concentrations were determined using a bicinchoninic assay kit (Pierce, Rockford, IL). Tissue or cellular extracts (30–50 μg) were denatured by boiling for 5 minutes and separated by 8%, 10% or 12% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH) by electroblotting. Membranes were blocked in milk (5% nonfat dry milk and 0.1% Tween 20 in phosphate-buffered saline), and exposed to primary and secondary antibodies as described [13]. The following primary antibody dilutions were used: Notch1 (1:1000, Santa Cruz Biotechnology), Glyceraldehyde 3 phosphate dehydrogenase (GAPDH) (1:10,000, Trevigen, Gaithersburg, MD), p21CIP1/WAF1, poly (ADP-ribose) polymerase (PARP) and cyclin D1 (1:1000, Cell Signaling Technology, Beverly, MA). Primary antibody incubations were kept overnight at 4°C and membranes were washed 3 times for 5 minutes or 3 times for 10 minutes in wash buffer (0.1% Tween 20 in phosphate-buffered saline). The membranes were incubated with horseradish peroxidase-conjugated anti-rabbit antibody. Membranes were developed by Immun-Star (Bio-Rad) for PARP and GAPDH or by Super West Femto chemiluminescence substrate (Pierce) for Notch1, p21CIP1/WAF1 and cyclin D1 according to the manufacturers' directions.
Human tumor tissue and normal liver were obtained from patients undergoing surgical resection at our institution under an IRB-approved protocol. Experimental findings were confirmed with duplicate samples.
Xenograft Studies
Both animal care and treatment were performed in compliance with our animal experiment protocol approved by the University of Wisconsin–Madison Animal Care and Use Committee. Male nude athymic, nu/nu mice (Charles Rivers, Wilmington, Maryland, USA) received subcutaneous (s.c.) injections of SK-Hep-1 cells (1×107) into the right flank. On the next day, mice were randomly divided into two groups for the study. Curcumin was dissolved in DMSO at a stock concentration of 500 mg/mL and stored at room temperature. Fresh dilutions in PBS were made for each injection. The treatment group of mice received 100 mg/kg curcumin every day by intraperitoneal (i.p.) injection for 16 days, starting on the day following cancer cell injection. A control group was injected with vehicle (DMSO) diluted in PBS. Each group consisted of 10 animals. The mice were weighed three times during the experimental period to assess toxicity of the treatments, and the tumors were measured every four days using calipers. Tumor volume was calculated from the two-dimensional caliper measurements using the following formula: tumor volume = [length × (width)2 × π]/6. On the final day of the study, all mice were sacrificed by carbon dioxide inhalation.
Statistical analysis
Analysis of variance (ANOVA) with Bonferroni post hoc testing was performed using a statistical analysis software package (SPSS version 10.0, SPSS, Chicago, IL). Student's t-test was used to compare tumor size in the in vivo model. A p-value of < .05 was considered significant.
Results
Overexpression of NICD in human HCC specimens
To investigate the activation of the Notch1 signaling pathway in HCC, Western blot analysis was utilized to measure levels of NICD proteins using human HCC samples. As shown in a representative tumor sample (Figure 1), high levels of NICD protein was present in 9/11 (81%) of the human HCC samples tested compared to the surrounding normal tissues. This suggests that the Notch1 signaling pathway is constitutively activated in HCC, thus outlining a potential oncogenic role of this pathway.
Figure 1.
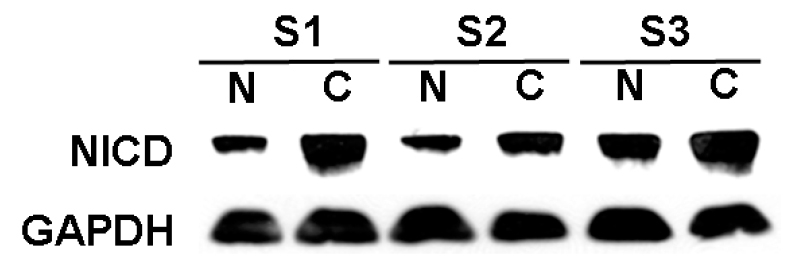
Overexpression of Notch1 in human HCC specimens. Western blot of resected human HCC tumors compared to normal surrounding liver specimens was performed to evaluate levels of active, cleaved Notch1 (NICD). Overexpression of the NICD protein compared to surrounding normal liver tissues occurred in 9/11 (82%) of specimens examined. This Western blot of 3 samples is a representative sample. Note samples are numbered from 1 to 3, N = normal liver tissue; C = HCC tumor tissue. GAPDH was used to confirm equal protein loading.
Pharmacological inhibition of Notch1 suppresses HCC cell proliferation in vitro
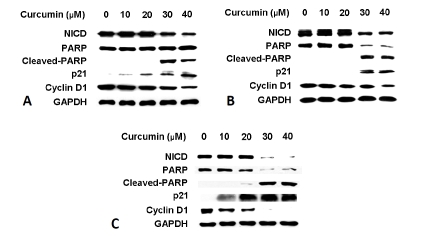
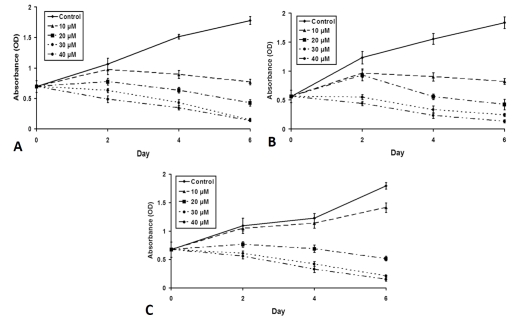
As shown in Figure 2A, high levels of NICD were present in HEP3B cells at baseline. Treatment with curcumin, a known pharmacological inhibitor of Notch1, led to a dose-dependent decrease in the expression of NICD, which was associated with the induction of cleaved poly ADP-ribose polymerase (PARP), the upregulation of cyclin-dependent kinase p21 and decrease in cyclin D1 in a dose-dependent manner. Similar effects of curcumin were observed in SK-Hep-1 (Figure 2B) and SNU449 cells (Figure 2C). To determine how the cell growth was impacted by down-regulation of NICD and associated alteration in the levels of PARP, cyclin D1, and p21, we performed MTT assays. As shown in Figure 3A, HEP3B cells treated with curcumin had a profound dose-dependent inhibition of cell growth. Statistically significant growth inhibition was also seen in SK-Hep-1 cells (Figure 3B) and SNU449 cells (Figure 3C).
Figure 2.
Curcumin inhibits Notch1 activation, and induces apoptosis and cell cycle arrest in HCC cells. High levels of NICD were present in HCC cell lines at baseline. Treatment with curcumin led to a dose-dependent decrease in the expression of NICD associated with the induction of cleaved poly ADP-ribose polymerase (PARP), and the degradation of cyclin D1 and increase in cyclin-dependent kinase p21 in the human HCC cell lines HEP3B (A), SK-Hep-1 (B) and SNU449 (C) cells. GAPDH was used to confirm equal protein loading.
Figure 3.
Curcumin suppresses growth of HCC cells in vitro. HCC cells were treated with curcumin (0–40 μM) for up to 6 days, and cell viability was measured with the MTT assay. The results indicate that curcumin led to the inhibition of cell growth in both a dose-dependent and time-dependent manner in HEP3B (A), SK-Hep-1 (B) and SNU449 (C) cells.
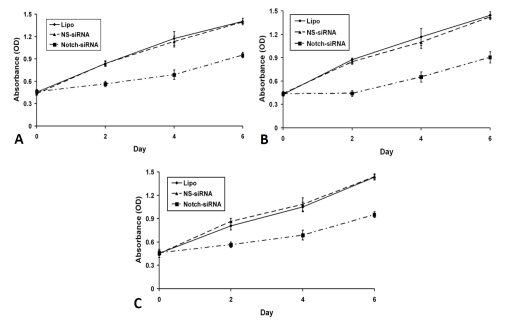
Knock-down of the Notch1 gene by siRNA induces cell cycle arrest and apoptosis in HCC cells
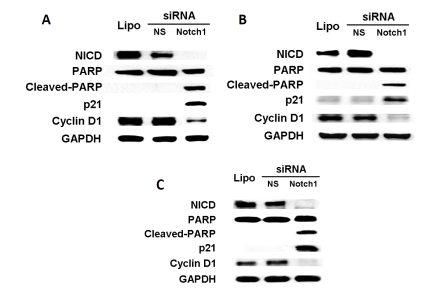
Although curcumin is a known natural inhibitor, the observed growth suppressive effects of this drug on HCC cells could be due to other mechanisms. To confirm the inhibitive effect of Notch1 in HCC cell growth, HCC cells were transiently transfected with lipofectamine (Lipo, vehicle control), nonspecific siRNA (NS siRNA) or siRNA against Notch1 (Notch1 siRNA) alone. MTT assays were utilized to measure the cell viability. HEP3B cells transfected with Notch1 siRNA had a significant growth inhibition compared with those transfected with NS siRNA (Figure 4A). Statistically significant growth inhibition was also seen in SK-Hep-1 cells (Figure 4B) and SNU449 cells (Figure 4C). Furthermore, we utilized Western blot analysis after 2 days of transfection with Notch1 siRNA or NS siRNA (control) to measure the effect of Notch1 down-regulation on the expression of the cell-cycle related genes. As shown in Figure 5A, high levels of NICD protein were present in HEP3B cells transfected with control siRNA. As expected, NICD protein was absent in the cells transfected with Notch1 siRNA. More importantly, the absence of NICD in these cells was associated with the induction of cleaved poly ADP-ribose polymerase (PARP), the degradation of cyclin D1 and an increase in cyclin-dependent kinase p21. Similar results were obtained in SK-Hep-1 (Figure 5B) and SNU449 cells (Figure 5C). These data show clearly that Notch1 siRNA transfection recapitulates the effects of curcumin in HCC cells suggesting that the down-regulation of the Notch1 signaling pathway inhibits HCC cell growth in vitro.
Figure 4.
Notch1 RNA interference inhibits HCC cell growth in vitro. HCC cells were transfected with lipofectamine (Lipo, vehicle control), nonspecific siRNA (NS siRNA, Santa Cruz Biotechnology, sc-37007) or Notch1 siRNA alone (Santa Cruz Biotechnology, sc-44226). MTT assays were utilized to measure the cell viability. As shown in (A), HEP3B cells transfected with Notch1 siRNA were found to have significant growth inhibition compared with those transfected with NS siRNA. Statistically significant growth inhibition was also seen in SK-Hep-1 (B) and SNU449 (C) cells.
Figure 5.
Notch1 RNA interference blocks Notch1 expression, and induces apoptosis and cell cycle arrest in HCC cells. HCC cells were transfected with lipofectamine (Lipo, vehicle control), nonspecific siRNA (NS siRNA) or Notch1 siRNA alone, and the total cellular extracts were isolated and analyzed by Western blot after 2 days. As shown in (A), high levels of NICD protein were present in HEP3B cells transfected with Lipo or NS siRNA. As expected, NICD protein was absent in the cells transfected with Notch1 siRNA. Importantly, the absence of NICD in these cells was associated with the induction of cleaved PARP, an increase in cyclin-dependent kinase p21, and a decrease in cyclin D1. Similar results were obtained when SK-Hep-1 (B) and SNU449 (C) cells were evaluated. GAPDH were used to confirm equal protein loading.
Pharmacological inhibitor of Notch1 suppresses growth of HCC xenografts in nude mice
We have demonstrated that curcumin inhibited the growth of HCC cells by down-regulating the Notch1 signaling pathway in vitro. Thus, we sought to investigate if curcumin could inhibit HCC growth in vivo. Therefore, nude mice injected with SK-Hep-1 cells received curcumin (100 mg/kg/d, i.p.) or DMSO alone for 16 days. Animal weight remained stable over the course of the experiments and no adverse effects were observed. All animals survived until the respective study end-points. As shown in Figure 6, tumor growth progressively slowed as the number of injections of curcumin increased. Curcumin-treated animals averaged a 40% inhibition of tumor growth compared to those of DMSO-treated controls (215 ±13 mm3 vs 130 ± 12 mm3 on day 16, p<.05), which demonstrates that curcumin inhibits HCC growth in vivo.
Figure 6.
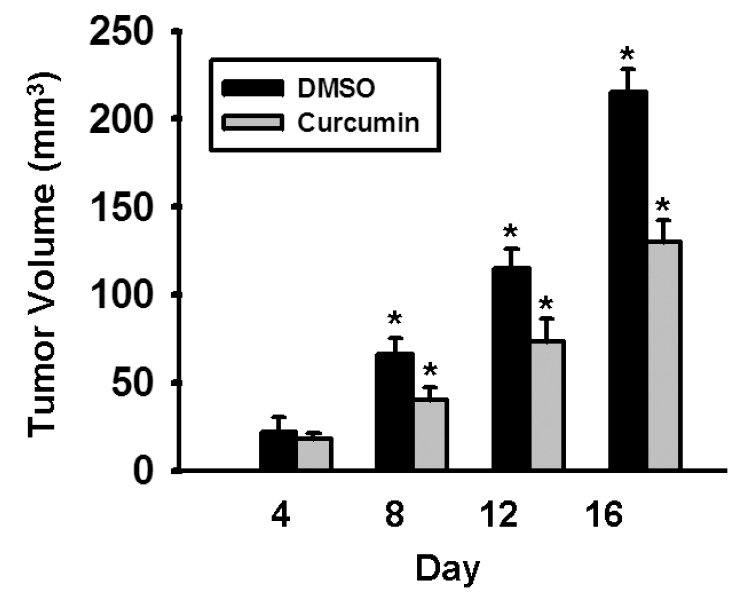
Curcumin inhibits growth of human HCC cells in vivo. Nude mice were injected subcutaneously with SK-Hep-1 cells and then were treated with intraperitoneal (i.p.) curcumin (100 mg/kg) or DMSO every day for 16 days, starting on the day following tumor inoculation. Tumor growth in treated animals was significantly decreased (215±13 mm3 vs 130 ±12 mm3 on day 16, p<.05).
Discussion
Notch1 is a multifunctional protein that regulates cellular differentiation, development, proliferation, and survival in a variety of contexts [14]. Both the Notch1 receptor and its ligands are transmembrane proteins with large extracellular domains. Binding of ligand and two proteolytic cleavages in the Notch1 receptor results in the release of NICD. Once released, NICD translocates to the nucleus and associates with transcriptional factors, including one of its main target genes, hairy enhancer of split (HES1), thereby modulating the development and growth of cells [15–18]. Because Notch1 plays an essential role in proliferation, differentiation, and apoptosis, pharmacological inactivation of Notch1-mediated cell growth represents an attractive therapeutic target for cancers in which this signaling pathway is overexpressed.
In the present study, we found that high levels of NICD proteins were present in the human HCC samples compared to the surrounding normal tissues suggesting constitutive activation of the Notch1 and potential oncogenic role of this pathway in human HCC. Importantly, down-regulation of Notch1 expression by curcumin led to a significant growth inhibition in all three HCC cell lines evaluated. A variety of pharmacological effects of curcumin have been reported, including anti-inflammatory, anti-oxidant, and anti-carcinogenic activities [19]. Recently, it has been shown that the anti-cancer property of curcumin is mediated in part by its inhibitive activity of Notch1 [4]. However, curcumin can also suppress the activation of several factors that are implicated in carcinogenesis, including nuclear factor kappa B (NF-kB), activator protein 1 (AP-1), signal transducer and activator of transcription proteins (STAT3, STAT5). Curcumin also modulates the expression of early growth response protein 1 (Erg-1) and peroxisome proliferators-associated receptor gamma (PPAR-g) [20–23]. The observed effects of curcumin in HCC cells may be a result of its activity as an inhibitor of one or several of these proteins.
However, by utilizing siRNA analysis, we investigated the specific role of Notch1 on the anti-proliferation effects of curcumin. Using siRNA to suppress Notch1, the curcumin-induced growth inhibition was recapitulated, demonstrating that the HCC growth inhibition is associated with a reduction in Notch1. The finding that the MTT assay using siRNA resulted in some cell growth at day 6 compared to baseline (vs. the curcumin MTT assay in which there was clear growth inhibition at day 6 compared to baseline) is likely explained by the fact that curcumin modulates cell growth through multiple other pathways other than Notch1 itself, while Notch-siRNA is directed specifically at a single pathway.
Recent results have shown that down-regulation of Notch1 expression regulates cell death through apoptosis [23]. In this study, we observed that levels of cleaved PARP, a well-known apoptotic marker, increased in HCC cells when Notch1 expression was suppressed. Moreover, we found that the reduction in Notch1 by curcumin or siRNA transfection led to an increase in p21 and a decrease in cyclin D1, a pattern indicative of cell cycle arrest. These results suggest that the down-regulation of Notch1 inhibits cell growth through apoptosis and cell cycle arrest in HCC cells. Because of these very promising findings, we evaluated the effect of curcumin on HCC tumor growth in vivo, and found that treatment with curcumin led to a significant suppression of tumor progression in an animal model of HCC.
Other recent series have shown conflicting results regarding Notch expression in HCC. An early work by Qi, et al, showed that overexpression of Notch1 induced cell cycle arrest and apoptosis in a single human HCC cell line, SMC7721, but these results were not correlated with human tumor specimens [24]. Other more recent series have found that Notch3 and Notch4 proteins are upregulated in 78% and 68% of HCC tumors [13]. Notably, Notch1 was not analyzed in this study. However, another recent work demonstrated Notch-1 and HES-1 overexpression in all 15 paired HCC human samples [25]. Furthermore, in a study evaluating 87 resected HCC tumors, Notch 1 protein (cytoplasmic) was upregulated in 89% of tumor specimens, when analyzed using immunohistochemical staining and western blot [26]. Although this data seems at first glance conflicting, the weight of the evidence using human tumor samples, including our own data, would support the finding that Notch is overexpressed in HCC.
In conclusion, our results demonstrate that reduction of Notch1 expression inhibits HCC cell growth in vitro. Treatment of HCC tumor cells with curcumin in vitro and in vivo led to significant growth inhibition due to induction of apoptosis and cell cycle arrest. These findings strongly support that targeting of overexpressed Notch1 signaling pathway is a potential new therapeutic strategy for advanced HCC.
Acknowledgments
This material is the result of work supported with resources and use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, WI.
References
- 1.Hann HW, Lee J, Bussard A, Liu C, Jin YR, Guha K, Clayton MM, Ardlie K, Pellini MJ, Feitelson MA. Preneoplastic markers of hepatitis B virus-associated hepatocellular carcinoma. Cancer Res. 2004;64:7329–7335. doi: 10.1158/0008-5472.CAN-04-1095. [DOI] [PubMed] [Google Scholar]
- 2.Treiber G. Systemic treatment of hepatocellular carcinoma. Dig.Dis. 2001;19:311–323. doi: 10.1159/000050698. [DOI] [PubMed] [Google Scholar]
- 3.Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr.Opin.Genet.Dev. 2007;17:52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol.Cancer Ther. 2006;5:483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 5.Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin.Cancer Biol. 2004;14:357–364. doi: 10.1016/j.semcancer.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Dang TP, Eichenberger S, Gonzalez A, Olson S, Carbone DP. Constitutive activation of Notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene. 2003;22:1988–1997. doi: 10.1038/sj.onc.1206230. [DOI] [PubMed] [Google Scholar]
- 7.Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Olson S, Gonzalez A, Carbone DP, Dang TP. Dominant-negative Notch3 receptor inhibits mitogen-activated protein kinase pathway and the growth of human lung cancers. Cancer Res. 2005;65:3555–3561. doi: 10.1158/0008-5472.CAN-04-3132. [DOI] [PubMed] [Google Scholar]
- 8.Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv.Exp.Med.Biol. 2007;595:173–184. doi: 10.1007/978-0-387-46401-5_6. [DOI] [PubMed] [Google Scholar]
- 9.Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch.Physiol Biochem. 2008;114:127–149. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 10.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr.Probl.Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13:98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 12.Ning L, Jaskula-Sztul R, Kunnimalaiyaan M, Chen H. Suberoyl bishydroxamic acid activates notch1 signaling and suppresses tumor progression in an animal model of medullary thyroid carcinoma. Ann.Surg.Oncol. 2008;15:2600–2605. doi: 10.1245/s10434-008-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gramantieri L, Giovannini C, Lanzi A, Chieco P, Ravaioli M, Venturi A, Grazi GL, Bolondi L. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma. Liver Int. 2007;27:997–1007. doi: 10.1111/j.1478-3231.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 14.Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003;3:203–205. doi: 10.1016/s1535-6108(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 15.Allenspach EJ, Maillard I, Aster JC, Pear WS. Notch signaling in cancer. Cancer Biol.Ther. 2002;1:466–476. doi: 10.4161/cbt.1.5.159. [DOI] [PubMed] [Google Scholar]
- 16.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 17.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat.Rev.Mol.Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc.Natl.Acad.Sci.U.S.A. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sa G, Das T. Anti cancer effects of curcumcycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcum: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 21.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol.Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 22.Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am.J.Physiol Gastrointest.Liver Physiol. 2005;288:G447–G456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 23.Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, Matsuda T, Ohta T, Tanaka Y, Mori N. Curcumin suppresses constitutive activation of AP-1 by downregulation of JunD protein in HTLV-1-infected T-cell lines. Leuk.Res. 2006;30:313–321. doi: 10.1016/j.leukres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323–8329. [PubMed] [Google Scholar]
- 25.Cantarini MC, de la Monte SM, Pang M, Tong M, D'Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Song Z, Chen Y, Xia L, Wang J, Fan R, Du R, Zhang F, Hong L, Song J, Zou X, Xu H, Zheng G, Liu J, Fan D. Deregulated expression of Notch receptors in human hepatocellular carcinoma. Dig.Liver Dis. 2008;40:114–121. doi: 10.1016/j.dld.2007.08.001. [DOI] [PubMed] [Google Scholar]