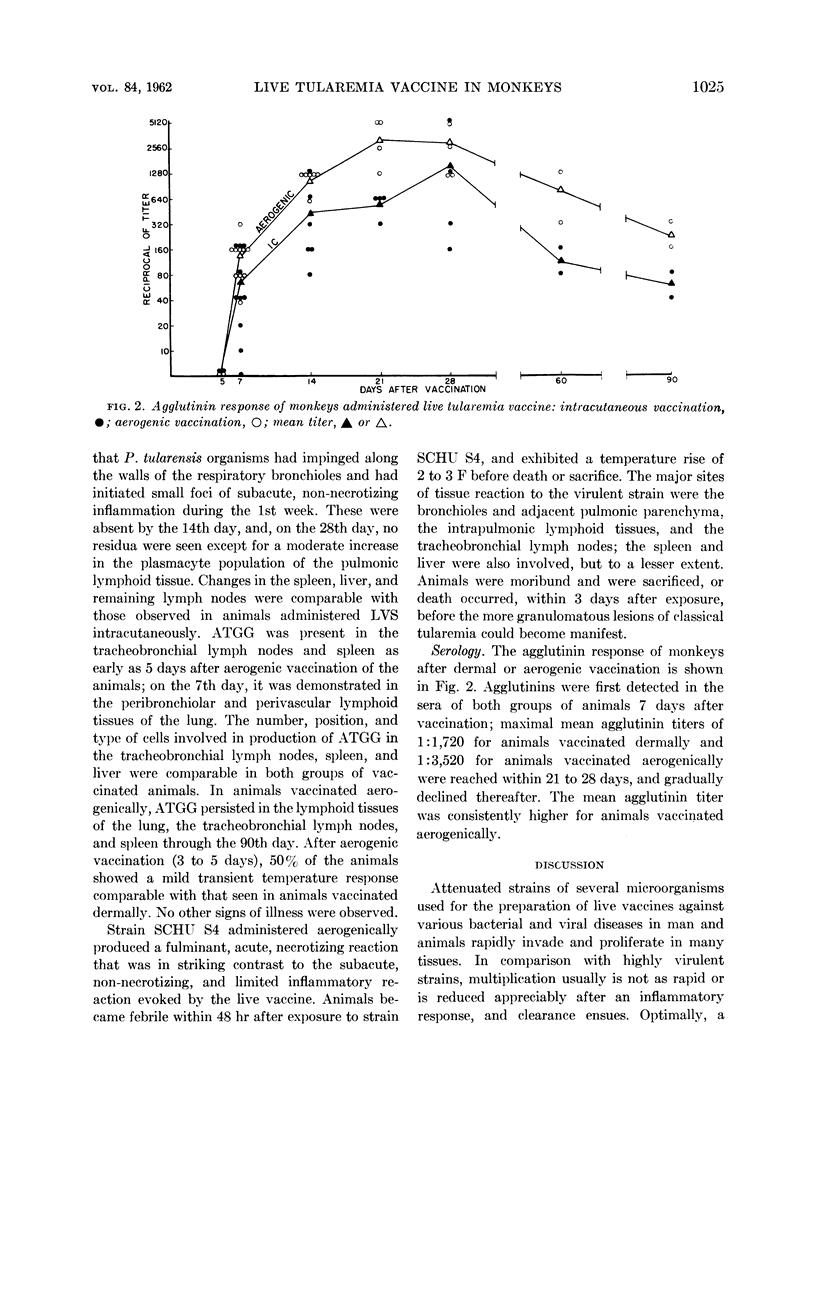
Abstract
Eigelsbach, H. T. (Fort Detrick, Frederick, Md.), J. J. Tulis, M. H. McGavran and J. D. White. Live tularemia vaccine. I. Host-parasite relationship in monkeys vaccinated intracutaneously or aerogenically. J. Bacteriol. 84:1020–1027. 1962.—Bacteriological, histological, immunohistochemical, and serological studies were made on monkeys administered live tularemia vaccine strain LVS by either of two routes. Comparative data are presented on nonvaccinated monkeys exposed via the respiratory route to a highly virulent strain of Pasteurella tularensis. Tissue changes resulting from either aerogenic or intracutaneous vaccination were mild, and consisted primarily of the proliferation of histiocytes without the formation of granulomas. The vaccine strain was isolated from the site of vaccination of animals inoculated dermally, from the lungs of animals vaccinated aerogenically, and from the regional lymph nodes, liver, and spleen of both groups; it was not isolated from the blood or bone marrow. Proliferation of the vaccine strain at the site of dermal inoculation and in the lungs of animals exposed aerogenically was observed within 24 hr; in both groups, the maximal viable population was reached within 3 days and maintained through the 10th day. A reduction in the number of viable vaccine organisms had begun by the 14th day; isolations were obtained only from the regional lymph nodes on the 28th day, and the vaccine strain was not isolated from any of the tissues cultured on the 90th day. Because the monkey is less resistant to tularemia than is man, the benign response of this animal to live tularemia vaccine indicates that the vaccine might also be safe for man when administered by either the dermal or respiratory route.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EIGELSBACH H. T., BRAUN W., HERRING R. D. Studies on the variation of Bacterium tularense. J Bacteriol. 1951 May;61(5):557–569. doi: 10.1128/jb.61.5.557-569.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIGELSBACH H. T., DOWNS C. M. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961 Oct;87:415–425. [PubMed] [Google Scholar]
- EIGELSBACH H. T., TULIS J. J., OVERHOLT E. L., GRIFFITH W. R. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soc Exp Biol Med. 1961 Dec;108:732–734. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- GEFEN N. E., GORDON G. Ia. [Aerosol immunization with dried living vaccines and toxoids. V. A study of morphological changes during aerosol immunization with some dust vaccines]. Zh Mikrobiol Epidemiol Immunobiol. 1961 Jan;32:40–46. [PubMed] [Google Scholar]
- McCrumb F. R. AEROSOL INFECTION OF MAN WITH PASTEURELLA TULARENSIS. Bacteriol Rev. 1961 Sep;25(3):262–267. doi: 10.1128/br.25.3.262-267.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavran M. H., White J. D., Eigelsbach H. T., Kerpsack R. W. Morphologic and Immunohistochemical Studies of the Pathogenesis of Infection and Antibody Formation Subsequent to Vaccination of Macaca irus with an Attenuated Strain of Pasteurella tularensis: I. Intracutaneous Vaccination. Am J Pathol. 1962 Sep;41(3):259–271. [PMC free article] [PubMed] [Google Scholar]
- RIGGS J. L., SEIWALD R. J., BURCKHALTER J. H., DOWNS C. M., METCALF T. G. Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958 Nov-Dec;34(6):1081–1097. [PMC free article] [PubMed] [Google Scholar]
- SASLAW S., EIGELSBACH H. T., PRIOR J. A., WILSON H. E., CARHART S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961 May;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- SASLAW S., EIGELSBACH H. T., WILSON H. E., PRIOR J. A., CARHART S. Tularemia vaccine study. I. Intracutaneous challenge. Arch Intern Med. 1961 May;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- WOLFE E. K., Jr Quantitative characterization of aerosols. Bacteriol Rev. 1961 Sep;25:194–202. doi: 10.1128/br.25.3.194-202.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


