Abstract
Despite advances in antimicrobial chemotherapy, nutritional support, and perioperative critical care, the development of an enterocutaneous fistula continues to represent a major therapeutic challenge, with appreciable morbidity and mortality. Specific problems that must be addressed for the successful management of patients with enterocutaneous fistulas are the control of sepsis, maintenance of adequate fluid and electrolyte balance, provision of adequate and complication-free nutritional support, and skin-stoma care. In addition, many patients with postoperative intestinal fistulation suffer from significant psychological morbidity, which must be addressed during often prolonged periods of rehabilitation. The complex nature of the care required for successful management of patients with enterocutaneous fistulas mandates a multidisciplinary team approach, with specialist nurses, dieticians, pharmacists, radiologists, physicians, and surgeons all having important roles to play.
Keywords: Sepsis, parenteral nutrition, fistuloclysis, laparostomy
The therapeutic goals of management for the patient with an enterocutaneous fistula are to promote spontaneous fistula closure or, where this is not possible, to restore and maintain body composition and physiological function so that the patient is optimally prepared for definitive surgery to resect the fistula and, if possible, restore intestinal continuity.
These goals can be best summarized as resuscitation, restitution, reconstruction, and rehabilitation. In addition, clinical problems faced by patients with enterocutaneous fistulas, like those of patients with major trauma, should be dealt with in the order in which they cause morbidity and mortality. A useful acronym to apply to the management of such patients is “SNAP,” representing management of Sepsis and Skin care, Nutritional support, definition of intestinal Anatomy, and development of a surgical Procedure to deal with the fistula.
INITIAL MANAGEMENT OF THE PATIENT WITH AN ENTEROCUTANEOUS FISTULA
Resuscitation
FLUID AND ELECTROLYTE THERAPY
The development of an enterocutaneous fistula can be associated with profound fluid and electrolyte depletion and disturbances of acid-base balance as a result of massive fistula losses. In patients allowed ad libitum fluid and diet, an enterocutaneous fistula in the proximal jejunum can easily lead to the loss of 6 L of fluid per day. In many cases, the losses of enteric contents are compounded by the loss of the normal inhibitory effect on gastric secretion, and a gastric hypersecretory state coexists with the intestinal fistula, compounding management. The potential for major fluid and electrolyte losses in intestinal fistulas can be appreciated by a review of the normal volume and electrolyte composition of gastrointestinal secretions, as indicated in the following1 (Table 1).
Table 1.
Composition and Volume of Intestinal Secretions
| Source | Volume (mL/day) | Na (mmol/L) | K (mmol/L) | Cl (mmol/L) | HCO3 (mmol/L) |
|---|---|---|---|---|---|
| Saliva | 1500 | 10 | 26 | 15 | 50 |
| Gastric | 1500 | 100 | 10 | 100 | 0 |
| Duodenal | 2000 | 130 | 5 | 90 | 10 |
| Ileal | 3000 | 140 | 5 | 100 | 30 |
| Pancreatic | 800 | 140 | 5 | 75 | 115 |
| Biliary | 800 | 150 | 5 | 100 | 35 |
Initial management of the patient with an enterocutaneous fistula should therefore entail a careful assessment of fluid and electrolyte status. This requires immaculate attention to the fluid balance chart and assiduous collection and charting of fistula and urine output and fluid intake. Signs of dehydration and altered skin turgor should be carefully looked for. All patients with a high-output intestinal fistula (>500 mL/day) should have an indwelling urinary catheter to facilitate careful fluid balance, at least until daily fistula output and fluid requirements are defined. Daily (or even more frequent) estimation of serum electrolytes should be undertaken to detect and correct dehydration and electrolyte depletion, and estimation of urinary electrolyte concentrations may be especially useful in this context2 as it allows detection of minor degrees of sodium depletion (urinary sodium concentration <20 mmol/L) that may not be apparent in blood estimations until total body electrolyte composition has become significantly more deranged. Fluid and electrolyte losses should be replaced with crystalloids, as dictated by urinary output and serum electrolytes. In high-output fistulas, the volumes of fluid required frequently necessitate central venous access. In addition to correction of water, sodium, and potassium depletion, consideration should be given to other electrolytes, particularly in patients with high-output enterocutaneous fistulas. Magnesium is a mainly intracellular ion but may become depleted rapidly in patients with excessive gastrointestinal fluid losses. Magnesium deficiency arises with apathy and nausea and features of neuromuscular hyperexcitability, including carpopedal spasm and stridor. With prolonged mild hypomagnesemia, hypocalcemia and hypokalemia may also develop as a result of resistance of bone to the actions of parathyroid hormone and excessive renal potassium losses, respectively. Intravenous replacement of magnesium is frequently required to correct these electrolyte abnormalities.2
Adjunctive therapies to control fistula output include prohibition of oral fluid or dietary intake, the use of intravenous proton pump inhibitors,3 and occasionally octreotide.4 Where attempts to control gastric acid secretion have been made, most commonly in patients with a high-output proximal small intestinal fistula, repeated estimation of fistula pH may allow estimation of the effectiveness of therapy. The aim of treatment should be to counteract the gastric hypersecretory component of the fistula output (which may render the pH of proximal small intestinal fistula output acidic), and fistula output should be neutral or even alkaline with effective treatment.
MANAGEMENT OF SEPSIS
Diagnosis
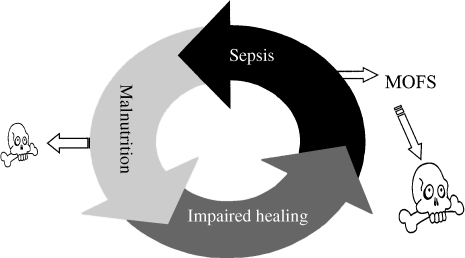
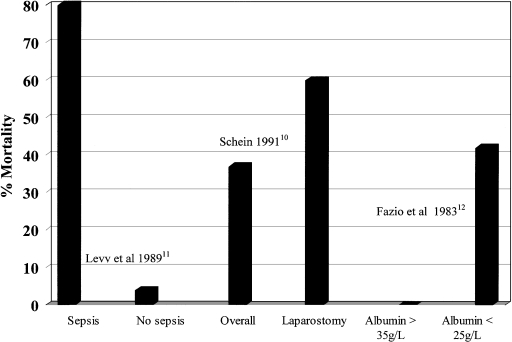
Effective management of abdominal sepsis is the most important single determinant of the outcome of enterocutaneous fistula. Failure to gain control of abdominal sepsis accounts for at least 80% of deaths in patients with intestinal fistulas5 and results in a state of hypercatabolism6 and failure of exogenous nutritional support to restore body composition,7 immune function, and fistula healing.8 These factors lead, in turn, to persistent fistulation and sepsis, resulting in a cachectic, immunocompromised patient9 in whom spontaneous fistula closure is unlikely and who is equally poorly prepared to withstand definitive surgery should this prove necessary (Fig. 1). Studies that have examined the effect of various factors on the mortality rate associated with intestinal fistulas have shown sepsis or surrogate markers, such as the need for laparostomy (see later), or hypoalbuminemia to be strongly associated with increased mortality10,11,12 (Fig. 2).
Figure 1.
Interrelationship between sepsis, nutritional depletion, impaired healing, and death in patients with enterocutaneous fistulas. MOFS, multiple organ failure syndrome.
Figure 2.
Influence of sepsis on mortality from enterocutaneous fistula.
Spontaneous healing of fistulas is unlikely to occur when there is an associated abscess cavity and active infection. Although the principal features of the systemic inflammatory response syndrome have been recognized for more than a decade,13 it is important to recognize that many patients with intestinal fistulation have long-standing low-grade infection and walled-off abscesses within the peritoneal cavity.
The usual manifestations of sepsis (pyrexia, leukocytosis, tachycardia, and tachypnea) are often only very subtly present or may even be completely absent in such patients, who present instead with cachexia, jaundice, hyponatremia, and hypoalbuminemia.8,9 Despite the lack of classical signs of active infection in these patients, surgical intervention is frequently associated with sudden and catastrophic deterioration, with rapid progression to septic shock and death from multiple organ failure, presumably as a consequence of a “second hit.” Prospective identification of such patients is of great importance, as it may allow the strategy for management of intra-abdominal collections in such patients to be based upon percutaneous drainage, which is likely to be better tolerated.14 Because inadequately treated abdominal infection is of such major prognostic importance, all patients with enterocutaneous fistulas should be assumed to have active sepsis, until proved otherwise, by prompt radiological investigation. Even in patients without obvious sepsis, abscess cavities can be detected in up to 50% of patients with enterocutaneous fistulas and more than 80% of these are amenable to percutaneous drainage.14 The investigation of first choice for suspected abdominal infection is contrast-enhanced computed tomography (CT) scanning, which has a diagnostic accuracy in excess of 97% in experienced hands.15 Alternative scanning techniques, including labeled leukocyte scanning, magnetic resonance imaging, and ultrasonography, may be of value as an adjunct to CT but have not been shown to be of greater clinical value.16
Use of Antibiotics
Antibiotics are of value in the management of abdominal sepsis in patients with enterocutaneous fistulas but only as an adjunct to radiological or surgical drainage. In most cases, although decisions taken with respect to antibiotic use are determined by local policy, second-line agents including piperacillin/tazobactam or carbapenems are used. Blood cultures are seldom of value in determining the choice of antibiotic, even in patients with a pyrexia associated with intra-abdominal infection, whereas the incidence of positive cultures following drainage of the primary focus of infection is high.17 It is therefore appropriate to modify initial antibiotic therapy with the results of microbiological culture. Additional consideration should be given to antifungal chemotherapy, especially in patients with long-standing low-grade sepsis associated with an enterocutaneous fistula, because of the potential for secondary infection with fungi associated with prolonged hospitalization and repeated use of broad-spectrum antibiotics.
Radiological Drainage
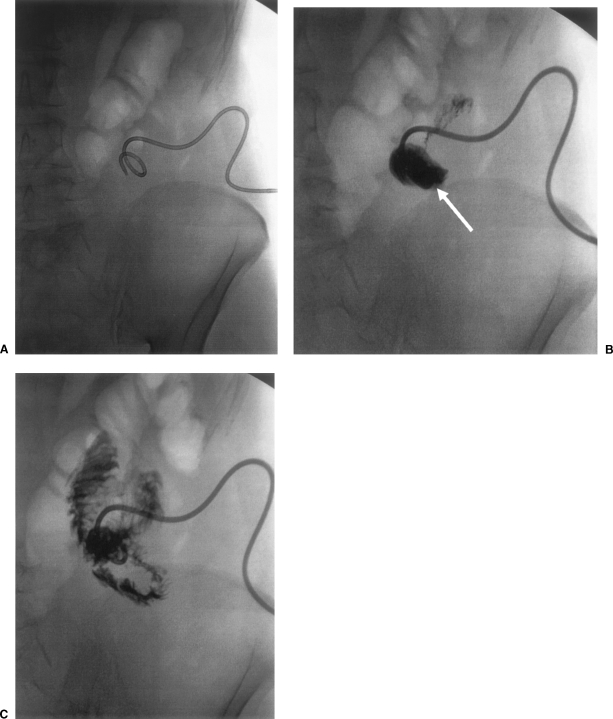
Identification of sepsis associated with the development of an enterocutaneous fistula should lead to a plan for prompt drainage, and, where possible, this should be undertaken radiologically. Radiological drainage avoids the second hit associated with undertaking major surgery in the sick, septic patient and is therefore preferable to surgical drainage, provided that there is no coexisting indication for surgery (see later) and collections can be adequately reached percutaneously. In some cases (Fig. 3), draining an associated intra-abdominal abscess may effectively downstage a complex fistula, allowing a simple fistula track to be created, which may then close spontaneously. Initial percutaneous drainage with a small-bore pigtail catheter can be followed up by dilatation of the drainage track under local anesthetic and subsequent insertion of larger drains and irrigation catheters, allowing abscess cavities to be controlled by twice-daily irrigation with saline. Contrast studies can then be undertaken through the drains to confirm collapse of the abscess cavities and the drains can then subsequently be gradually withdrawn.
Figure 3.
Radiological downstaging of a complex to a simple jejunocutaneous fistula. (A) Percutaneous drain has been inserted in associated abscess cavity (B, arrow), which subsequently collapses, resulting in a straight track to the jejunum (C).
Surgical Drainage
Surgical management of sepsis associated with the development of an enterocutaneous fistula is likely to be required when there is discontinuity between bowel ends (for example, after anastomotic dehiscence), when radiological investigations have shown multiple infected collections, or when complex fistulas are associated with internal components, including fistulation into adjacent bowel loops and the urinary bladder.18 Studies of the outcome of surgery in the management of abdominal sepsis have clearly shown that control of the source of infection is the best prognostic determinant.17,19 Although there are various surgical strategies for the management of abdominal sepsis associated with intestinal fistulation, the aim of surgical treatment in the management of sepsis associated with enterocutaneous fistula is principally to extirpate the focus of sepsis by exteriorizing the fistulating segment whenever possible. Attempts to restore intestinal continuity or to repair anastomotic leaks in the septic patient are associated with a very low chance of success because of anastomotic failure and should not be undertaken. Likewise, suture lines (e.g., at enterotomies) should not be left within the abdomen if at all possible and, if unavoidable, should be defunctioned by a proximal diverting stoma. Involvement of intrinsically healthy neighboring loops of bowel in the fistulating process is common, especially in Crohn's disease, and resection under these circumstances should be avoided. Studies have shown that short bowel syndrome most commonly arises in Crohn's disease because of emergency resection for the management of anastomotic leakage and fistulation, rather than repeated uncomplicated resection.20
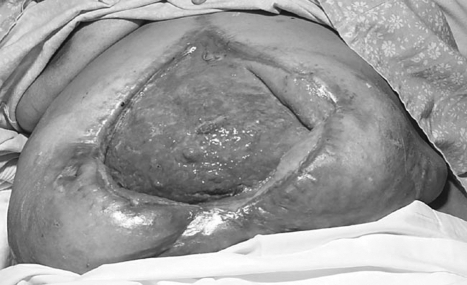
When exteriorizing the fistulating segment would inevitably lead to resection of a substantial amount of bowel or the patient is critically ill and too unstable to tolerate a prolonged surgical procedure, it may be preferable to bring out a proximal loop stoma and drain the fistulating segment externally. In severe sepsis, where enterocutaneous fistulation is associated with widespread peritoneal contamination, or the abdominal wall is rigid because of multiple recent laparotomies, it may prove necessary to leave the abdomen partially or completely open (laparostomy).21 Laparostomy may facilitate the management of abdominal sepsis, allowing regular inspection and toilet of the peritoneal cavity. Once sepsis has been adequately controlled, the wound can be packed with saline-soaked gauze rolls and left open to heal by secondary intention. The exposed small intestine rapidly becomes covered with a sheet of healthy granulation tissue, obscuring individual bowel loops (Fig. 4). Although split skin grafts or pinch grafts can be applied to the granulation tissue, it is usually advisable to delay this for 4 to 6 weeks, allowing the process of wound contraction to occur, thereby reducing the size of the defect that will ultimately need to be closed. Alternative strategies to leaving the abdomen completely open include the use of absorbable mesh or denatured porcine dermal collagen prostheses to cover exposed bowel loops, zip fasteners,22 or staged abdominal reconstruction.23 Fistulas that develop secondarily within the open abdomen can be treated by application of low-grade suction and a large Eakin stoma appliance to the wound.24 In general, a neoperitoneal cavity forms within 6 months after creation of a laparostomy and further surgery can be undertaken to reconstruct the intestinal tract and repair the defect in the abdominal wall at that time.25
Figure 4.
Laparostomy wound at 6 weeks, showing small bowel loops densely covered with healthy granulation tissue.
SKIN CARE
Protection of the skin is a vital early step in the management of the patient with an enterocutaneous fistula.24 Digestive juices may be alkaline or acidic, depending upon the nature of the fistula, and the corrosive enzymes may rapidly digest the abdominal wall. At best this may result in skin destruction and severe pain associated with chemical burns, but in severe cases, rapidly progressive destruction of the abdominal wall may ensue, resulting in spreading secondary infection and death. Inability to control fistula losses is extremely demoralizing for the patient, impairs mobility and rehabilitation, and may greatly complicate the estimation of fluid and electrolyte losses. The specialist nurse and/or enterostomal therapist has an extremely important role to play in the management of the skin around fistula sites, and a variety of devices and techniques have been devised to facilitate the management of the fistula site.24 These include stoma appliances that can be cut to shape, suction catheters, and adhesive paste dressings, to build up or flatten irregularly shaped wounds. In certain cases, where there is mucocutaneous continuity at the fistula site, the use of such devices can be combined with enterostomy tubes, allowing simultaneous collection of fistula effluent and infusion of enteral feed into the bowel (fistuloclysis; see later). In the vast majority of cases, an experienced nurse can provide a comfortable and securely fitting appliance, but where this is not possible, it may be necessary for the surgeon to bring out a defunctioning loop stoma proximal to the fistula. The duodenojejunal flexure is rarely involved in processes of primary or secondary intestinal fistulation and, even in the most hostile abdomen, it is usually possible to exteriorize a proximal loop of jejunum by a left subcostal incision.8,16 A high-output stoma controlled into a well-fitting stoma appliance is vastly preferable to a poorly controlled fistula.
RESTITUTION
Nutrition and Metabolic Support
After satisfactory resuscitation, control of sepsis, and skin care, attention should be given to provision of nutritional support. Although the presence of incompletely treated sepsis is not a contraindication to nutritional support, administration of nutrients by whatever route, even in supraphysiological amounts, is unlikely to restore body composition or physiological function until sepsis is eradicated.7,8,26,27 Sepsis is associated with a significant increase in energy and nitrogen requirements,26,28 and it may not be possible to meet these except by parenteral nutrition. The presence, however, of insulin resistance associated with sepsis may lead to hyperglycemia during glucose-based feeding, which in turn may be associated with increased infection rates and continued muscle wasting despite aggressive nutritional support.26 In addition, there is evidence that critical illness is associated with enhanced nutrient-induced thermogenesis,29 associated with stimulation of the sympathetic nervous system, which may lead to further increases in energy production rate and urinary catecholamine excretion.30 More recent studies in which the rates of calorie administration have been adjusted to mirror energy requirements have failed to confirm these findings,31,32,33,34 suggesting that inappropriate and excessive glucose-induced thermogenesis, which can also lead to fever and provoke respiratory failure,35 is avoidable.36
Although the ability to provide safe and effective nutritional support by means of parenteral infusion of nutrients has been a major advance in the management of patients with high-output intestinal fistulas and has undoubtedly contributed to the sizable reduction in mortality from this condition, it is important to note that parenteral nutrition is not always required for the maintenance of satisfactory nutritional status in patients with enterocutaneous fistulas. When spontaneous fistula closure is possible, parenteral nutrition allows oral fluids and diet to be withheld to “rest the gut” and promote fistula healing, although, perhaps surprisingly, it remains unclear whether allowing patients whose fistulas might close spontaneously unrestricted access to oral diet and fluid would in fact delay or prevent fistula closure.
Where there is obvious mucocutaneous continuity, spontaneous fistula closure cannot occur and, provided skin care is not compromised, patients can be allowed to eat and drink, which is of great benefit in maintaining morale. Enteral nutrition (with high-calorie, high-protein supplements) may be all that is required for satisfactory nutritional support when there is more than 200 cm of healthy small intestine proximal to an ileocutaneous fistula.
PARENTERAL NUTRITION
In patients with high-output small bowel fistulas, maintenance of satisfactory nutritional and metabolic status is likely to require parenteral nutrition. In the United Kingdom, the concept of management of acute intestinal failure,16 defined as an acute and potentially reversible reduction in functioning intestinal mass below the minimum necessary for the adequate digestion and absorption of nutrients,37 has developed in specialist centers staffed by nursing staff who can ensure that total parenteral nutrition (TPN) can be delivered with minimum associated morbidity and mortality. Surveys of admission problems in such units have shown that almost 50% of patients admitted for treatment in these units, which are characterized by the ability to provide parenteral nutrition with a low rate of complications, were referred because of enterocutaneous fistulas.38
Parenteral nutrition through a tunneled central venous catheter has long been regarded as the “gold standard” for the delivery of intravenous nutrition support, but low complication rates, and in particular low rates of line-related sepsis, are critically dependent upon assiduous attention to detail and a strict aseptic technique when handling the line. Using dedicated nursing staff and strict adherence to aseptic protocols, specialist centers have achieved remarkably low (zero) rates of inpatient line-related sepsis and shown that these can even be extended to patients managing their own parenteral nutrition at home.39 Where nursing expertise is limited and in patients in whom the likelihood of spontaneous closure seems high, intravenous feeding using a peripherally sited catheter may be appropriate and associated with a lower risk of serious morbidity than centrally administered TPN.40 The duration of intravenous feeding by the peripheral route tends to be limited by the development of thrombophlebitis, chiefly as a consequence of the low pH and high osmolality of the feeding solutions.41 This can be minimized by the use of lipid-based feeding regimens (which allow a higher energy density, lower osmolality, and reduced infusion rates); insertion of ultrafine inert catheters (such as 22 gauge polyurethane),40,42 which interfere with venous blood flow less than ordinary devices and permit laminar flow in the vein; ensuring that the feeding solution does not come into direct contact with the delicate venous endothelium; the use of nitrate patches to promote local venodilatation; and hydrocortisone and heparin added to the parenteral feed.43
With high-output fistulas and, in particular, in the catabolic, septic patient, prolonged requirements for nutritional support, large fluid losses, and high energy and nitrogen requirements make peripheral intravenous feeding inappropriate.
Careful and thorough nutritional assessment undertaken by an expert dietician is an important component of the strategy for nutritional support. A detailed assessment of the methods used to determine nutritional requirements is beyond the scope of this article but will rely on anthropometric and biochemical measurements and occasionally indirect calorimetry. Although requirements vary considerably, a good rule of thumb is to aim to provide 30 kcal/kg/day, except for the most septic patients, who may occasionally require up to 40 kcal/kg/day. Nitrogen requirements vary from 1 gN/150 kcal/day for stable patients to 1 gN/120 kcal/day for patients judged to be more catabolic.
FISTULOCLYSIS
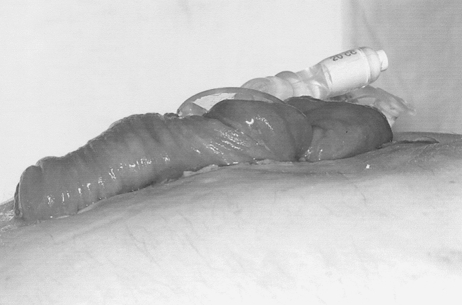
In patients with small bowel fistulas and obvious enterocutaneous continuity, the inability of the fistula to heal spontaneously presents a further option for nutritional support, namely, infusion of feed directly through the fistula into the small intestine distal to the fistula opening (fistuloclysis44). Similar techniques have been employed successfully to provide enteral feeding for patients with very proximal small intestinal mucous fistulas,45 and, provided there is more than 75 cm of healthy small intestine available for absorption of feed distal to the fistula opening, many patients can be fed satisfactorily by placing a feeding catheter through the stoma bag and into the fistula opening (Fig. 5). Feed-associated complications include diarrhea and abdominal pain, but these can be minimized by reducing feed osmolality, using medium-chain triglycerides. Interestingly, polymeric feeds are generally well tolerated, even without infusion of chyme into the distal small intestine, although occasionally peptide or even elemental feeds are required. Fistuloclysis, which is safer and less expensive than parenteral nutrition, is additionally preferable because it prevents atrophy of the small intestine distal to an enterocutaneous fistula, making subsequent reconstructive surgery technically easier.
Figure 5.
Fistuloclysis. The catheter is sited in the distal limb of a small bowel loop prolapsing out of an almost completely healed laparostomy wound.
Definition of Fistula Anatomy and Development of a Plan for Surgical Reconstruction
When sepsis has been eradicated and a stratagem for the delivery of nutritional support established, consideration should be given to delineating fistula anatomy. The most useful initial investigations involve intubation of the external opening(s) and detailed contrast imaging by an experienced radiologist. It is essential that the physician communicate effectively with the radiologist to outline the nature of previous surgery, as this may clarify greatly the understanding of fistula anatomy. If there are several fistulas, thought should be given to the timing of investigations to avoid confusion and, in general, contrast studies of the intestinal tract proximal to the fistula opening should be undertaken first, to avoid contrast-filled loops of defunctioned bowel distal to the fistula, which may degrade image quality and complicate interpretation of subsequent investigations. It is very important not only to clarify the precise location of the fistula with respect to the long axis of the intestinal tract and the course of the fistula but also to determine whether there are distal strictures, associated cavities, or internal communications to other loops of intestine, the biliary or urogenital tracts. This may necessitate intravenous urography, endoscopic retrograde pancreatography, and occasionally transhepatic cholangiography but is important if an accurate assessment is to be made of the likelihood of spontaneous fistula closure or the nature of surgery required. When postoperative fistulation occurs after abdominal surgery for malignant disease, detailed radiological assessment should be undertaken for the presence of metastatic disease because this may have significant implications for further management.
PLAN FOR DEFINITIVE TREATMENT
Once the patient is in a stable metabolic state, with sepsis controlled, nutritional support established, and the anatomy and etiology of the fistula clarified, a plan for further management of the fistula can be made.
Spontaneous closure of enterocutaneous fistulas occurs in up to 60% of patients, in 90% of cases within 4 to 6 weeks of conservative treatment.46 In general, these fistulas are simple lateral fistulas arising from small bowel anastomotic leaks or enterotomies within otherwise normal bowel. During this period oral intake may be restricted, although the role of other strategies, including octreotide, remains controversial. In general, although octreotide may hasten spontaneous fistula closure by reducing the volume of gastrointestinal secretions, it seems unlikely that pharmacological manipulation of gastrointestinal secretion alone will enable a fistula to heal where anatomical factors mitigate against closure.
In contrast, spontaneous healing is unlikely where there is an associated abscess cavity or internal component or in the presence of intrinsic intestinal disease (for example, Crohn's disease or radiation enteritis). In such cases, the aim of further treatment is to obtain fistula closure by surgical means.
Surgical procedures for intestinal reconstruction should be undertaken with due consideration to the scope and timing of surgery. In particular, surgical treatment should be deferred until both local and systemic conditions have been optimized.
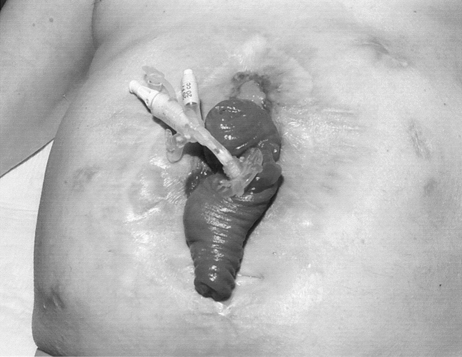
Correction of systemic conditions implies adequate nutritional status and resolution of the systemic inflammatory response. Attempts to restore intestinal continuity should be avoided in the presence of significant (<30g/L) hypoalbuminemia. In addition, attempts should be made to address psychological morbidity before embarking on further surgical treatment. Softening of the abdominal wall and intra-abdominal adhesions is essential for a safe attempt to gain entry to the abdomen without the risk of further enterotomy, which may be disastrous. For these reasons, it is seldom possible to attempt surgical resection of an intestinal fistula within 3 months of the last complete laparotomy. When a fistula occurs within a laparostomy wound, a 6-month period is usually required for the development of a neoperitoneal cavity, which allows separation of adjacent intestinal loops without the risk of enterotomy.25 The most reliable guide to this process is ability to lift the skin away from the rest of the laparostomy wound by gentle traction and prolapse of the fistulating loop of bowel (Fig. 6). In many patients who have recovered from sepsis and malnutrition, a prolonged period of convalescence may therefore be required until conditions within the peritoneal cavity are sufficiently favorable to allow intestinal reconstruction to be safely undertaken. It is frequently appropriate, therefore, to discharge the patient on home parenteral nutrition or fistuloclysis, pending definitive surgical treatment.
Figure 6.
Prolapse of a small bowel fistula in an almost completely healed laparostomy wound. This appearance indicates that reconstructive surgery can now be safely undertaken.
Although the precise details of surgical procedures vary, depending upon the anatomy and etiology of the fistula, there are certain key points worthy of consideration.
Fistula surgery is technically demanding and frequently tedious. It is best undertaken by experienced surgeons who are prepared to set aside sufficient time, and it cannot be undertaken both rapidly and safely.
Great care should be taken in getting into the abdomen, and inadvertent enterotomy (which has been shown to predict strongly the subsequent development of postoperative complications47) should be avoided. The use of knife dissection may be safer than blunt or scissor dissection as the majority of bowel injuries in the hostile abdomen are caused by traction or tearing rather than incision.
Although it may be appealing simply to try to isolate the fistulating segment, a careful and meticulous adhesiolysis is preferable, commencing at the duodenojejunal junction, which is rarely involved in even the most dense adhesions. It is very important to have assessed the intestinal tract distal to the fistula, as strictures, internal fistulas, and walled-off leaks may have been overlooked in the earlier radiological assessment. Restoration of intestinal continuity without having established the integrity of the intestine downstream is therefore potentially hazardous and should be avoided.
Fistulating segments of intestine should be resected and not bypassed, to avoid the risk of blind loops, bacterial overgrowth, and compromise of the function of the remaining intestine.
Clearly, anastomotic technique should be meticulous and intestinal anastomoses should not be left within old abscess cavities because of the risks of recurrent fistulation. The greater omentum can occasionally be used to wrap around an anastomosis or to fill an abscess cavity to minimize the risk of this occurrence.
At the conclusion of a long and complex procedure, the abdominal wall must be closed. This may be also be technically demanding, particularly when there has been a large laparostomy defect, but it is important that the abdominal wall be reconstructed with minimal tension, to protect the intestinal tract (including anastomoses) and to offer the patient as a good a functional result as possible. With small laparostomy defects, the abdominal wall can frequently be unpicked from the edges of the wound and rolled back into place. Where this is not possible, the rectus abdominis muscles and their sheaths can sometimes be mobilized back to the midline by making relaxing incisions in the lateral aspect of the external oblique aponeurosis (component separation technique). In larger laparostomy defects, suture techniques including continuous double loop closure48 and the “double near and far” technique49 may allow the approximation of the edges of the abdominal wall, with tension at least distributed evenly across the wound. Suture techniques have, however, been shown to be associated with significant morbidity48 and should be avoided if possible. Nonabsorbable prosthetic mesh can be used to fill defects in the abdominal wall but may be inadvisable when there has been extensive wound contamination in association with an open fistula and in patients with Crohn's disease. Under these circumstances, it may be preferable to use absorbable mesh and undertake delayed closure of an incisional hernia at a later date, although newer prosthetic materials including denatured porcine collagen may offer a promising alternative.
During the closure of large abdominal defects, the surgeon should be aware of the possibility of intra-abdominal hypertension (also referred to as the abdominal compartment syndrome). Sustained increases in intra-abdominal pressure may not only impede respiration by splinting the diaphragm but also impair cardiac return and mesenteric and renal blood flow, leading to organ hypoperfusion.50 With large defects, intra-abdominal pressure can be monitored during and shortly after abdominal closure with a pressure transducer placed within the bladder catheter or directly within the peritoneal cavity through an abdominal drain.
It is usually possible to close the abdominal wall skin at the conclusion of the procedure, although the new skin formed over a laparostomy defect is usually thin and, because it derives a significant proportion of its blood supply from the underlying bowel, frequently ischemic at the conclusion of the operation and therefore best excised.
REHABILITATION
Development of a postoperative enterocutaneous fistula is usually a devastating complication and results in prolonged hospitalization, pain, malaise, further surgery, and major psychological morbidity, frequently compounded by anxiety regarding body image. Not surprisingly, the psychological sequelae, which include depressive illness, anxiety, guilt, and institutionalization, may take many months to resolve. Although many of these problems can be managed by good communication and rapport between the patient, the patient's family, and sympathetic medical and nursing staff, the services of a clinical psychologist with experience of complex surgical care can be very valuable.
REFERENCES
- 1.Gomella L G, Lefor A T. Surgery on Call. Norwalk, CT: Appleton-Lange; 1990.
- 2.Nightingale J MD. In: Nightingale JMD, editor. Intestinal Failure. London: Greenwich Medical Media; 2001. The short bowel. pp. 177–198.
- 3.Nightingale J MD, Walker E R, Farthing M JG, Lennard-Jones J E. Effect of omeprazole on intestinal output in the short bowel syndrome. Aliment Pharmacol Ther. 1991;5:405–412. doi: 10.1111/j.1365-2036.1991.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 4.Paran H, Neufeld D, Kaplan O, Klausner J, Freund U. Octreotide for treatment of postoperative alimentary fistulas. World J Surg. 1995;19:430–433. doi: 10.1007/BF00299182. [DOI] [PubMed] [Google Scholar]
- 5.Soeters P B, Ebeid A M, Fischer J E. Review of 404 patients with gastrointestinal fistulas: impact of parenteral nutrition. Ann Surg. 1979;190:189–202. doi: 10.1097/00000658-197908000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw J HF, Koea J B. Metabolic basis for the management of the septic surgical patient. World J Surg. 1993;17:154–164. doi: 10.1007/BF01658921. [DOI] [PubMed] [Google Scholar]
- 7.Streat S J, Beddoe A H, Hill G L. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987;27:262–266. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Carlson G L. Surgical management of intestinal failure. Proc Nutr Soc. 2003;62:711–718. doi: 10.1079/PNS2003287. [DOI] [PubMed] [Google Scholar]
- 9.Carlson G L, Irving M H. In: Hanson G, editor. Critical Care of the Surgical Patient—A Companion to Bailey and Loves' Surgery. London: Chapman & Hall Medical; 1997. Infection: recognition and management of infection in surgical patients. pp. 273–290.
- 10.Schein M, Decker G A. Postoperative external alimentary tract fistulas. Am J Surg. 1991;161:435–438. doi: 10.1016/0002-9610(91)91107-t. [DOI] [PubMed] [Google Scholar]
- 11.Levy E, Frileux P, Cugnenc P H, Honiger J, Ollivier J M, Parc R. High-output external fistulae of the small bowel: management with continuous enteral nutrition. Br J Surg. 1989;76:676–679. doi: 10.1002/bjs.1800760708. [DOI] [PubMed] [Google Scholar]
- 12.Fazio V W, Coutsoftides T, Steiger E. Factors influencing the outcome of treatment of small bowel cutaneous fistula. World J Surg. 1983;7:481–488. doi: 10.1007/BF01655937. [DOI] [PubMed] [Google Scholar]
- 13.Bone R C, Balk R A, Cerra F B, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Thomas H A. Radiological investigation and treatment of gastrointestinal fistulas. Surg Clin North Am. 1996;76:1081–1094. doi: 10.1016/s0039-6109(05)70498-9. [DOI] [PubMed] [Google Scholar]
- 15.Gerzof S G, Oates M E. Imaging techniques for infections in the surgical patient. Surg Clin North Am. 1988;68:147–166. doi: 10.1016/s0039-6109(16)44437-3. [DOI] [PubMed] [Google Scholar]
- 16.Carlson G L. In: Nightingale JMD, editor. Intestinal Failure. London: Greenwich Medical Media; 2001. Surgical causes and management. pp. 39–49.
- 17.McLauchlan G J, Anderson I D, Grant I S, Fearon K C. Outcome of patients with abdominal sepsis treated in an intensive care unit. Br J Surg. 1995;82:524–529. doi: 10.1002/bjs.1800820429. [DOI] [PubMed] [Google Scholar]
- 18.Shackley D C, Brew C J, Bryden A A, et al. The staged management of complex entero-urinary fistulae. BJU Int. 2000;86:624–629. doi: 10.1046/j.1464-410x.2000.00871.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson I D, Fearon K C, Grant I S. Laparotomy for abdominal sepsis in the critically ill. Br J Surg. 1996;83:535–539. doi: 10.1002/bjs.1800830434. [DOI] [PubMed] [Google Scholar]
- 20.Agwunobi A O, Carlson G L, Anderson I D, Irving M H, Scott N A. Mechanisms of intestinal failure in Crohn's disease. Dis Colon Rectum. 2001;44:1834–1837. doi: 10.1007/BF02234463. [DOI] [PubMed] [Google Scholar]
- 21.Mughal M M, Bancewicz J, Irving M H. Laparostomy. A technique for the management of intractable abdominal sepsis. Br J Surg. 1986;73:253–259. doi: 10.1002/bjs.1800730405. [DOI] [PubMed] [Google Scholar]
- 22.Hedderich G S, Wexler M J, McLean A P, Meakins J L. The septic abdomen: open management with Marlex mesh with a zipper. Surgery. 1986;99:399–408. [PubMed] [Google Scholar]
- 23.Wittmann D H. Operative and nonoperative therapy of intraabdominal infections. Infection. 1998;26:335–341. doi: 10.1007/BF02962267. [DOI] [PubMed] [Google Scholar]
- 24.Hughes S, Myers A, Carlson G. In: Nightingale JMD, editor. Intestinal Failure. London: Greenwich Medical Media; 2001. Care of intestinal stoma and enterocutaneous fistula. pp. 51–63.
- 25.Scripcariu V, Carlson G, Bancewicz J, Irving M H, Scott N A. Reconstructive abdominal operations after laparostomy and multiple repeat laparotomies for severe intra-abdominal infection. Br J Surg. 1994;81:1475–1478. doi: 10.1002/bjs.1800811024. [DOI] [PubMed] [Google Scholar]
- 26.Carlson G L, Little R A. In: Kinney JM, editor. Organ Metabolism and Nutrition: Ideas for Critical Care. New York: Raven Press; 1994. Insulin resistance and tissue fuels. pp. 49–69.
- 27.Chandran V P, Sim A J. Nutritional support in acute intestinal failure. Bailllieres Clin Gastroenterol. 1991;5:841–860. doi: 10.1016/0950-3528(91)90023-t. [DOI] [PubMed] [Google Scholar]
- 28.Carlson G L, Little R A. In: Boles JM, editor. Endocrine Consequences of Critical Illness. Paris: Arnette; 1992. The pathophysiology and pattern of the hormonal response to severe sepsis. pp. 57–69.
- 29.Allard J P, Jeejheebhoy K N, Whitwell J, Pashutinski L, Peters W J. Factors influencing energy expenditure in patients with burns. J Trauma. 1988;28:199–202. doi: 10.1097/00005373-198802000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Askanazi J, Carpentier Y A, Elwyn D H, et al. Influence of total parenteral nutrition on fuel utilization in injury and sepsis. Ann Surg. 1980;291:40–46. doi: 10.1097/00000658-198001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold J, Shipley K A, Scott N A, Little R A, Irving M H. Thermic effect of parenteral nutrition in septic and nonseptic individuals. Am J Clin Nutr. 1989;50:853–860. doi: 10.1093/ajcn/50.4.853. [DOI] [PubMed] [Google Scholar]
- 32.Arnold J, Shipley K A, Scott N A, Little R A, Irving M H. Lipid infusion increases oxygen consumption similarly in septic and nonseptic patients. Am J Clin Nutr. 1991;53:143–148. doi: 10.1093/ajcn/53.1.143. [DOI] [PubMed] [Google Scholar]
- 33.Arnold J, Leinhardt D, Carlson G, Gray P, Little R A, Irving M H. Thermogenic and hormonal responses to amino acid infusion in septic humans. Am J Physiol. 1992;263:E129–E135. doi: 10.1152/ajpendo.1992.263.1.E129. [DOI] [PubMed] [Google Scholar]
- 34.Carlson G L, Gray P, Arnold J, Little R A, Irving M H. Thermogenic, hormonal and metabolic effects of intravenous glucose infusion in human sepsis. Br J Surg. 1997;84:1454–1459. [PubMed] [Google Scholar]
- 35.Askanazi J, Rosenbaum S H, Hyman A I, Silverberg P A, Milic-Emili J, Kinney J M. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA. 1980;243:1444–1447. [PubMed] [Google Scholar]
- 36.Carlson G L. In: Wernerman J, Little RA, editor. Clinical Endocrinology & Metabolism: Energy Metabolism in Trauma. London: Balliere-Tindall; 1998. Nutrient induced thermogenesis. pp. 603–615. [DOI] [PubMed]
- 37.Fleming C R, Remington M. In: Hill GL, editor. Nutrition and the Surgical Patient. New York: Churchill Livingstone; 1981. Intestinal failure. pp. 219–235.
- 38.Scott N A, Leinhardt D J, O'Hanrahan T, Finnegan S, Shaffer J L, Irving M H. Spectrum of intestinal failure in a specialised unit. Lancet. 1991;337:471–473. doi: 10.1016/0140-6736(91)93403-v. [DOI] [PubMed] [Google Scholar]
- 39.Williams N, Carlson G L, Scott N A, Irving M H. Incidence and management of catheter-related sepsis in patients receiving home parenteral nutrition. Br J Surg. 1994;81:392–394. doi: 10.1002/bjs.1800810324. [DOI] [PubMed] [Google Scholar]
- 40.Williams N, Wales S, Irving M H. Prolonged peripheral parenteral nutrition with an ultrafine cannula and low osmolality feed. Br J Surg. 1996;83:114–116. doi: 10.1002/bjs.1800830137. [DOI] [PubMed] [Google Scholar]
- 41.May J, Murchan P, MacFie J, et al. Prospective study of the aetiology of infusion phlebitis and line failure during peripheral parenteral nutrition. Br J Surg. 1996;83:1091–1094. doi: 10.1002/bjs.1800830817. [DOI] [PubMed] [Google Scholar]
- 42.Plusa S M, Horsman R, Kendall-Smith S, Webster N, Primrose J N. Fine bore cannulas for peripheral intravenous nutrition: polyurethane or silicone? Ann R Coll Surg Engl. 1998;80:154–156. [PMC free article] [PubMed] [Google Scholar]
- 43.Tighe M J, Wong C, Martin I G, McMahon M J. Do heparin, hydrocortisone and glyceryl trinitrate influence thrombophlebitis during full intravenous nutrition via a peripheral vein? J Parenter Enteral Nutr. 1995;19:507–509. doi: 10.1177/0148607195019006507. [DOI] [PubMed] [Google Scholar]
- 44.Teubner A, Farrer K, Ravishankar H, Anderson I, Scott N. Fistuloclysis can successfully replace parenteral feeding in the nutritional support of patients with enterocutaneous fistulas. Br J Surg. 2004;31:625–631. doi: 10.1002/bjs.4520. [DOI] [PubMed] [Google Scholar]
- 45.Levy E, Frileux P, Sandrucci S, et al. Continuous enteral nutrition during the early adaptive stage of the short bowel syndrome. Br J Surg. 1988;75:549–553. doi: 10.1002/bjs.1800750615. [DOI] [PubMed] [Google Scholar]
- 46.Tassiopoulos A K, Baum G, Halverson J D. Small bowel fistulas. Surg Clin North Am. 1996;76:1175–1181. doi: 10.1016/s0039-6109(05)70505-3. [DOI] [PubMed] [Google Scholar]
- 47.Krabben A A Van Der, Dijkstra F R, Nieuwenhuijzen M, Reijnen M M, Schaapveld M, Goor H Van. Morbidity and mortality of inadvertent enterotomy during adhesiotomy. Br J Surg. 2000;87:467–471. doi: 10.1046/j.1365-2168.2000.01394.x. [DOI] [PubMed] [Google Scholar]
- 48.Niggebrugge A H, Trimbos J B, Hermans J, Steup W H, Velde C J Van De. Influence of abdominal-wound closure technique on complications after surgery: a randomised study. Lancet. 1999;353:1563–1567. doi: 10.1016/S0140-6736(98)10181-2. [DOI] [PubMed] [Google Scholar]
- 49.Malik R-A, Scott N A. Double near and far prolene suture closure: a technique for abdominal wall closure after laparostomy. Br J Surg. 2001;88:146–147. doi: 10.1046/j.1365-2168.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 50.Diebel L N, Dulchavsky S A, Wilson R F. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:45–48. doi: 10.1097/00005373-199207000-00010. [DOI] [PubMed] [Google Scholar]